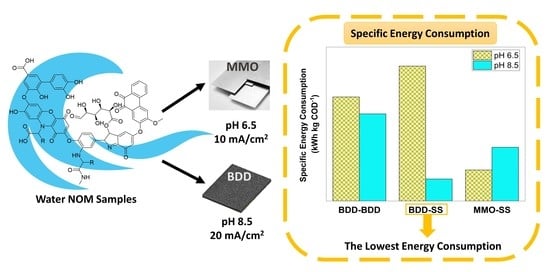
Water Purification and Electrochemical Oxidation: Meeting Different Targets with BDD and MMO Anodes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Electrochemical Setup
2.3. Electrochemical Degradation of Suwannee River NOM
3. Results
3.1. TOC Removal
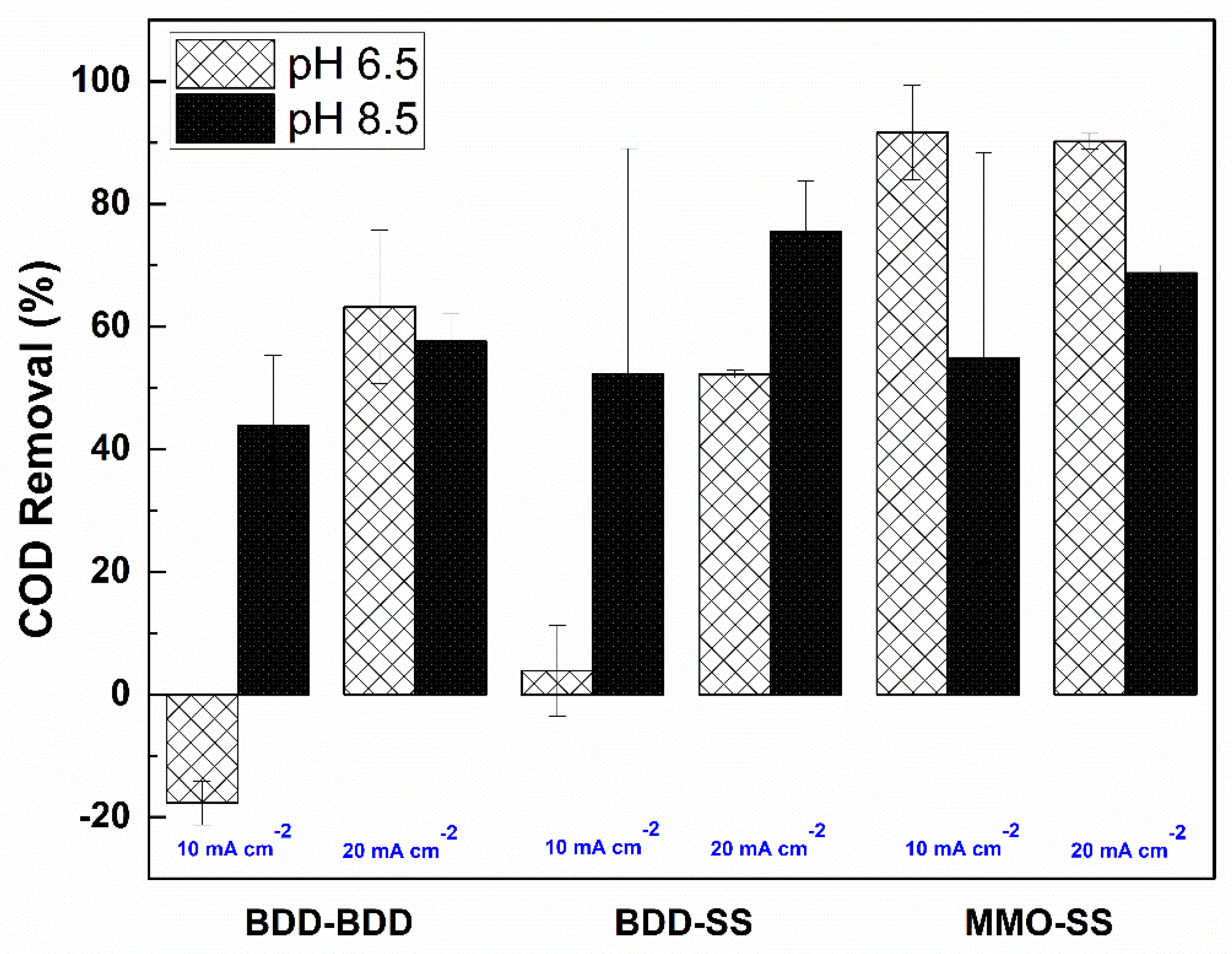
3.2. COD Removal
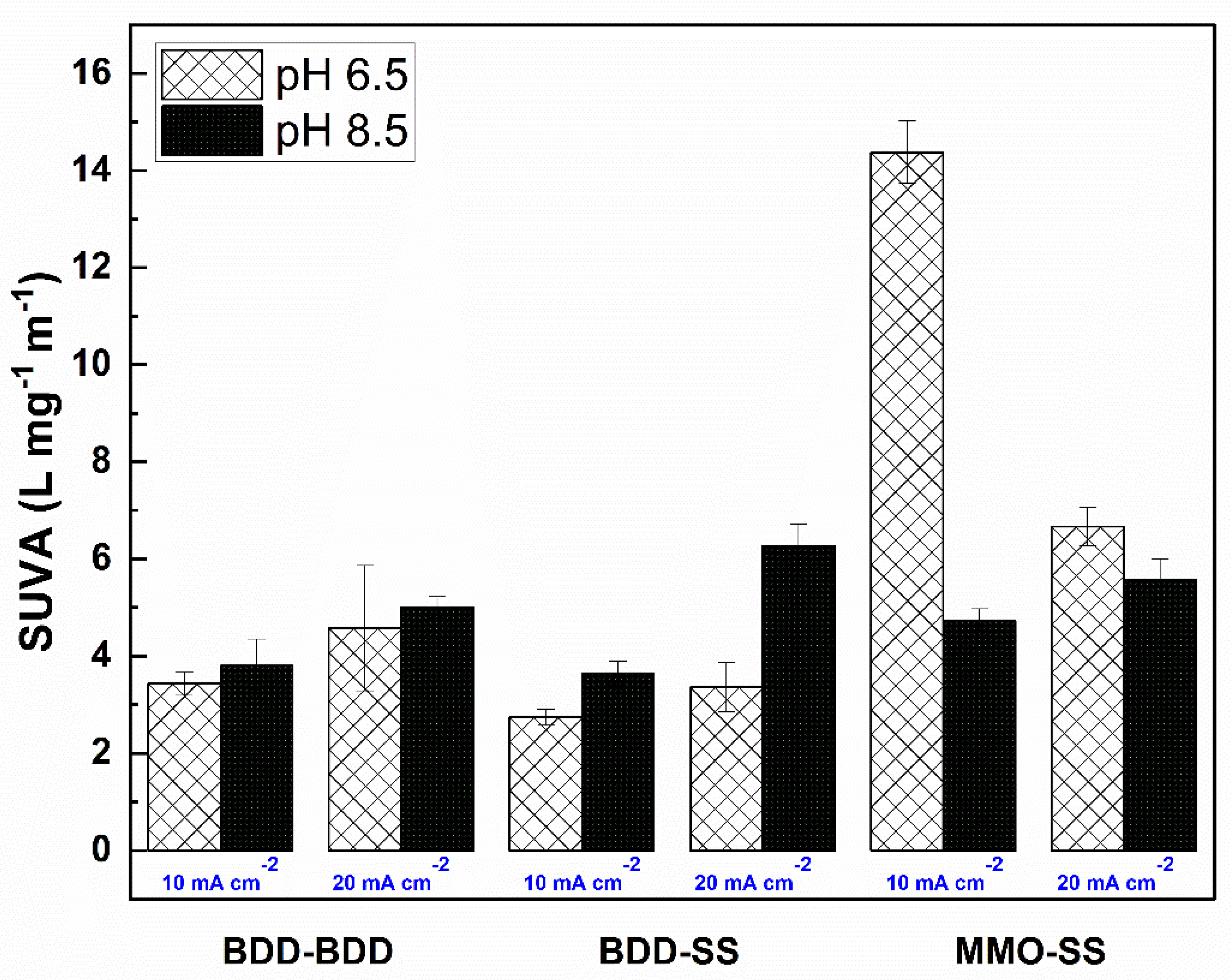
3.3. Specific UV Absorbance
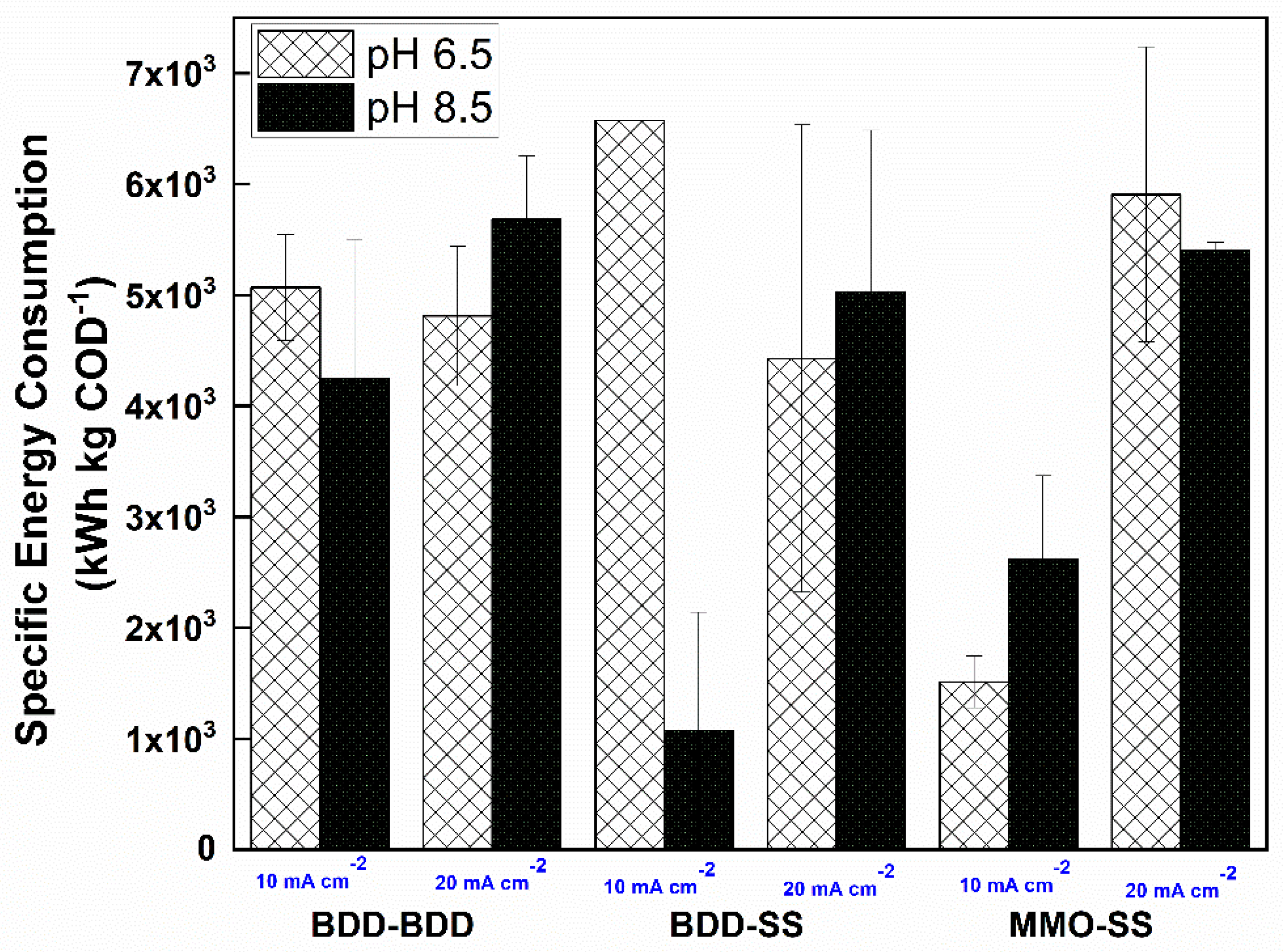
3.4. Estimation of Specific Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goslan, E.H.; Fearing, D.A.; Banks, J.; Wilson, D.; Hills, P.; Campbell, A.T.; Parsons, S.A. Seasonal variations in the disinfection by-product precursor profile of a reservoir water. J. Water Supply Res. Technol. AQUA 2002, 51, 475–482. [Google Scholar] [CrossRef]
- Ates, N.; Kitis, M.; Yetis, U. Formation of chlorination by-products in waters with low SUVA-correlations with SUVA and differential UV spectroscopy. Water Res. 2007, 41, 4139–4148. [Google Scholar] [CrossRef] [PubMed]
- Jarusutthirak, C.; Amy, G.; Croué, J.P. Fouling characteristics of wastewater effluent organic matter (EfOM) isolates on NF and UF membranes. Desalination 2002, 145, 247–255. [Google Scholar] [CrossRef]
- Chowdhury, S.; Champagne, P.; McLellan, P.J. Models for predicting disinfection by-product (DBP) formation in drinking waters: A chronological review. Sci. Total Environ. 2009, 407, 4189–4206. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, M.; Liang, R.; Leeuwen, J.C.V.; Schneider, O.; Khan, A.; Fong, L.C.M.L.C.; Zhou, N.Y.; Servos, M.R. Pharmaceutical Micropollutant Treatment with UV–LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions. Photochem 2022, 2, 503–514. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.C.; Yu, M.J. Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control. Water Res. 2005, 39, 4779–4789. [Google Scholar] [CrossRef]
- MikaSillanpää, M.; Matilainen, A.; Lahtinen, T. Chapter 2—Characterization of NOM. In Natural Organic Matter in Water; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–45. [Google Scholar] [CrossRef]
- Chaplin, B.P. Critical review of electrochemical advanced oxidation processes for water treatment applications. Environ. Sci. Process. Impacts 2014, 16, 1182–1203. [Google Scholar] [CrossRef] [PubMed]
- Suffredini, H.B.; Machado, S.A.S.; Avaca, L.A. The water decomposition reactions on boron-doped diamond electrodes. J. Braz. Chem. Soc. 2004, 15, 16–21. [Google Scholar] [CrossRef]
- Perret, A.; Haenni, W.; Skinner, N.; Tang, X.M.; Gandini, D.; Comninellis, C.; Correa, B.; Foti, G. Electrochemical behavior of synthetic diamond thin film electrodes. Diam. Relat. Mater. 1999, 8, 820–823. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Yue, P.L. Anodic oxidation of dyes at novel Ti/B-diamond electrodes. Chem. Eng. Sci. 2003, 58, 995–1001. [Google Scholar] [CrossRef]
- Lai, C.L.; Lin, S.H. Treatment of chemical mechanical polishing wastewater by electrocoagulation: System performances and sludge settling characteristics. Chemosphere 2004, 54, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Iniesta, J.; Michaud, P.A.; Panizza, M.; Comninellis, C.H. Electrochemical oxidation of 3-methylpyridine at a boron-doped diamond electrode: Application to electroorganic synthesis and wastewater treatment. Electrochem. Commun. 2001, 3, 346–351. [Google Scholar] [CrossRef]
- Tenne, R.; Patel, K.; Hashimoto, K.; Fujishima, A. Efficient electrochemical reduction of nitrate to ammonia using conductive diamond film electrodes. J. Electroanal. Chem. 1993, 347, 409–415. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.H.; Lim, T.T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Scialdone, O.; Galia, A.; Randazzo, S. Oxidation of carboxylic acids in water at IrO2–Ta2O5 and boron doped diamond anodes. Chem. Eng. J. 2011, 174, 266–274. [Google Scholar] [CrossRef]
- Dong, H.; Yu, W.; Hoffman, M.R. Mixed Metal Oxide Electrodes and the Chlorine Evolution Reaction. J. Phys. Chem. C 2021, 125, 20745–20761. [Google Scholar] [CrossRef]
- Gandini, D.; Mahé, E.; Michaud, P.A.; Haenni, W.; Perret, A.; Comninellis, C. Oxidation of carboxylic acids at boron-doped diamond electrodes for wastewater treatment. J. Appl. Electrochem. 2000, 30, 1345–1350. [Google Scholar] [CrossRef]
- Cañizares, P.; García-Gómez, J.; Lobato, J.; Rodrigo, M.A. Electrochemical oxidation of aqueous carboxylic acid wastes using diamond thin-film electrodes. Ind. Eng. Chem. Res. 2003, 42, 956–962. [Google Scholar] [CrossRef]
- da Silva, S.W.; Navarro, E.M.O.; Rodrigues, M.A.S.; Bernardes, A.M.; Perez-Herranz, V. Using p-Si/BDD anode for the electrochemical oxidation of norfloxacin. J. Electroanal. Chem. 2019, 832, 112–120. [Google Scholar] [CrossRef]
- Marselli, B.; Garcia-Gomez, J.; Michaud, P.-A.; Rodrigo, M.A.; Comninellis, C. Electrogeneration of Hydroxyl Radicals on Boron-Doped Diamond Electrodes. J. Electrochem. Soc. 2003, 150, D79. [Google Scholar] [CrossRef]
- Heard, D.M.; Lennox, A.J.J. Electrode Materials in Modern Organic Electrochemistry. Angew. Chem. 2020, 59, 18866–18884. [Google Scholar] [CrossRef]
- Ben-Sasson, M.; Zidon, Y.; Calvo, R.; Adin, A. Enhanced removal of natural organic matter by hybrid process of electrocoagulation and dead-end microfiltration. Chem. Eng. J. 2013, 232, 338–345. [Google Scholar] [CrossRef]
- Bagga, A.; Chellam, S.; Clifford, D.A. Evaluation of iron chemical coagulation and electrocoagulation pretreatment for surface water microfiltration. J. Membr. Sci. 2008, 309, 82–93. [Google Scholar] [CrossRef]
- Gamage, N.P.; Chellam, S. Mechanisms of physically irreversible fouling during surface water microfiltration and mitigation by aluminum electroflotation pretreatment. Environ. Sci. Technol. 2014, 48, 1148–1157. [Google Scholar] [CrossRef]
- Chellam, S.; Sari, M.A. Aluminum electrocoagulation as pretreatment during microfiltration of surface water containing NOM: A review of fouling, NOM, DBP, and virus control. J. Hazard. Mater. 2016, 304, 490–501. [Google Scholar] [CrossRef]
- Ødegaard, H.; Østerhus, S.; Melin, E.; Eikebrokk, B. NOM removal technologies—Norwegian experiences. Drink. Water Eng. Sci. 2010, 3, 1–9. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef]
- Sillanpää, M.; Särkkä, H.; Vepsäläinen, M. NOM Removal by Electrochemical Methods. In Natural Organic Matter in Water: Characterization and Treatment Methods; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128017197. [Google Scholar]
- Soni, B.D.; Patel, U.D.; Agrawal, A.; Ruparelia, J.P. Application of BDD and DSA electrodes for the removal of RB 5 in batch and continuous operation. J. Water Process Eng. 2017, 17, 11–21. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, L.; Jiao, Y.; Wang, Q.; Tan, Q. Treatment of high-salinity reverse osmosis concentrate by electrochemical oxidation on BDD and DSA electrodes. Desalination 2011, 277, 201–206. [Google Scholar] [CrossRef]
- Zhou, M.; Särkkä, H.; Sillanpää, M. A comparative experimental study on methyl orange degradation by electrochemical oxidation on BDD and MMO electrodes. Sep. Purif. Technol. 2011, 78, 290–297. [Google Scholar] [CrossRef]
- Saha, P.; Bruning, H.; Wagner, T.V.; Rijnaarts, H.H.M. Removal of organic compounds from cooling tower blowdown by electrochemical oxidation: Role of electrodes and operational parameters. Chemosphere 2020, 259, 127491. [Google Scholar] [CrossRef] [PubMed]
- Lauzurique, Y.; Espinoza, L.C.; Huiliñir, C.; García, V.; Salazar, R. Anodic Oxidation of Industrial Winery Wastewater Using Different Anodes. Water 2022, 14, 95. [Google Scholar] [CrossRef]
- Can, O.T.; Gazigil, L.; Keyikoglu, R. Treatment of intermediate landfill leachate using different anode materials in electrooxidation process. Environ. Prog. Sustain. Energy 2022, 41, e13722. [Google Scholar] [CrossRef]
- Green, N.W.; McInnis, D.; Hertkorn, N.; Maurice, P.A.; Perdue, E.M. Suwannee River natural organic matter: Isolation of the 2R101N reference sample by reverse osmosis. Environ. Eng. Sci. 2015, 32, 38–44. [Google Scholar] [CrossRef]
- Rosenfeldt, E.J.; Linden, K.G. Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estradiol, and estradiol during UV photolysis and advanced oxidation processes. Environ. Sci. Technol. 2004, 38, 5476–5483. [Google Scholar] [CrossRef]
- Rosenfeldt, E.J.; Chen, P.J.; Kullman, S.; Linden, K.G. Destruction of estrogenic activity in water using UV advanced oxidation. Sci. Total Environ. 2007, 377, 105–113. [Google Scholar] [CrossRef]
- SpectraMax M Series Microplate Readers, The Ultimate Guide to Microplate Reader Solutions (eBook). Available online: https://www.moleculardevices.com/en/assets/ebook/br/ultimate-guide-to-microplate-reader-solutions (accessed on 26 August 2022).
- Manea, F.; Jakab, A.; Ardelean, M.; Pop, A.; Vlaicu, I. Boron-doped diamond electrode-based advanced treatment methods for drinking water. Environ. Eng. Manag. J. 2014, 13, 2167–2172. [Google Scholar] [CrossRef]
- Pétrier, C. The use of power ultrasound for water treatment. In Power Ultrasonics; Gallego-Juárez, J.A., Graff, K.F., Eds.; Woodhead Publishing: Oxford, UK, 2015; p. 953. [Google Scholar]
- Stefan, M.I. (Ed.) Chapter 2 UV/Hydrogen Peroxide Process. In Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017; pp. 50–52. [Google Scholar]
- Huang, K.L.; Liu, C.C.; Ma, C.Y.; Chen, T.T. Effects of operating parameters on electrochemical treatment of swine wastewater. Int. J. Electrochem. Sci. 2019, 14, 11325–11339. [Google Scholar] [CrossRef]
- Wiszniowski, J.; Robert, D.; Surmacz-Gorska, J.; Miksch, K.; Weber, J.-V. Photocatalytic mineralization of humic acids with TiO2: Effect of pH, sulfate and chloride anions. Int. J. Photoenergy 2003, 5, 69–74. [Google Scholar] [CrossRef]
- Piscopo, A.; Robert, D.; Weber, J.V. Influence of pH and chloride anion on the photocatalytic degradation of organic compounds: Part I. Effect on the benzamide and para-hydroxybenzoic acid in TiO2 aqueous solution. Appl. Catal. B Environ. 2001, 35, 117–124. [Google Scholar] [CrossRef]
- Abdullah, M.; Low, G.K.C.; Matthews, R.W. Effects of common inorganic anions on rates of photocatalytic oxidation of organic carbon over illuminated titanium dioxide. J. Phys. Chem. 1990, 94, 6820–6825. [Google Scholar] [CrossRef]
- da Silva, A.J.C.; dos Santos, E.V.; de Oliveira Morais, C.C.; Martínez-Huitle, C.A.; Castro, S.S.L. Electrochemical treatment of fresh, brine and saline produced water generated by petrochemical industry using Ti/IrO2-Ta2O5 and BDD in flow reactor. Chem. Eng. J. 2013, 233, 47–55. [Google Scholar] [CrossRef]
- Fattahi, A.; Arlos, M.J.; Bragg, L.M.; Liang, R.; Zhou, N.; Servos, M.R. Degradation of natural organic matter using Ag-P25 photocatalyst under continuous and periodic irradiation of 405 and 365 nm UV-LEDs. J. Environ. Chem. Eng. 2021, 9, 104844. [Google Scholar] [CrossRef]
- Clematis, D.; Klidi, N.; Barbucci, A.; Carpanese, M.P.; Delucchi, M.; Cerisola, G.; Panizza, M. Application of electro-fenton process for the treatment of methylene blue. Bulg. Chem. Commun. 2018, 50, 22–26. [Google Scholar]
- Santos, G.O.S.; Gonzaga, I.M.D.; Eguiluz, K.I.B.; Salazar-Banda, G.R.; Saez, C.; Rodrigo, M.A. Improving biodegradability of clopyralid wastes by photoelectrolysis: The role of the anode material. J. Electroanal. Chem. 2020, 864, 114084. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chang, J.E.; Wen, T.C. Indirect oxidation effect in electrochemical oxidation treatment of landfill leachate. Water Res. 1995, 29, 671–678. [Google Scholar] [CrossRef]
- Baker, J.R.; Milke, M.W.; Mihelcic, J.R. Relationship between chemical and theoretical oxygen demand for specific classes of organic chemicals. Water Res. 1999, 33, 327–334. [Google Scholar] [CrossRef]
- Stoddart, A.K.; Gagnon, G.A. Application of photoelectrochemical chemical oxygen demand to drinking water. J. Am. Water Works Assoc. 2014, 106, E383–E390. [Google Scholar] [CrossRef]
- He, W.; Chen, S.; Liu, X.; Chen, J. Water quality monitoring in a slightly-polluted inland water body through remote sensing—Case study of the Guanting Reservoir in Beijing, China. Front. Environ. Sci. Eng. China 2008, 2, 163–171. [Google Scholar] [CrossRef]
- Melin, E.S.; Ødegaard, H. Biofiltration of ozonated humic water in expanded clay aggregate filters. Water Sci. Technol. 1999, 40, 165–172. [Google Scholar] [CrossRef]
- Särkkä, H.; Vepsäläinen, M.; Pulliainen, M.; Sillanpää, M. Electrochemical inactivation of paper mill bacteria with mixed metal oxide electrode. J. Hazard. Mater. 2008, 156, 208–213. [Google Scholar] [CrossRef]
- Zhou, S.; Bu, L.; Yu, Y.; Zou, X.; Zhang, Y. A comparative study of microcystin-LR degradation by electrogenerated oxidants at BDD and MMO anodes. Chemosphere 2016, 165, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: US requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Jung, C.W.; Son, H.J. The relationship between disinfection by-products formation and characteristics of natural organic matter in raw water. Korean J. Chem. Eng. 2008, 25, 714–720. [Google Scholar] [CrossRef]
- Nkambule, T.I.; Kuvarega, A.T.; Krause, R.W.M.; Haarhoff, J.; Mamba, B.B. Synthesis and characterisation of Pd-modified N-doped TiO2 for photocatalytic degradation of natural organic matter (NOM) fractions. Environ. Sci. Pollut. Res. 2012, 19, 4120–4132. [Google Scholar] [CrossRef]
- Wong, H.; Mok, K.M.; Fan, X.J. Natural organic matter and formation of trihalomethanes in two water treatment processes. Desalination 2007, 210, 44–51. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Becker, W.C.; Wattier, K.L. Surrogate parameters for monitoring organic matter and THM precursors. J.-Am. Water Work. Assoc. 1985, 77, 122–132. [Google Scholar] [CrossRef]
- Dbira, S.; Bensalah, N.; Ahmad, M.I.; Bedoui, A. Electrochemical Oxidation/Disinfection of Urine Wastewaters with Different Anode Materials. Materials 2019, 12, 1254. [Google Scholar] [CrossRef]
- Moratalla, Á.; Lacasa, E.; Cañizares, P.; Rodrigo, M.A.; Sáez, C. Electro-Fenton-Based Technologies for Selectively Degrading Antibiotics in Aqueous Media. Catalysts 2022, 12, 602. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]


| Pollutant | Experimental Conditions | Average Efficiency | Refs. |
|---|---|---|---|
| Carboxylic Acids | i = 30 mA cm−2; T = 30 °C; 1 M H2SO4 | 70–90% | [16,20,23] |
| Polyacrylates | i = 1–30 mA cm−2; 1 M HClO4 | 100% | [23] |
| Industrial wastewaters | i = 7–36 mA cm−2; initial COD 1500–8000 mg L−1 | 85–100% | [25] |
| Carwash wastewater | i = 15–60 mA cm−2 | 40% | [26,27,28] |
| Wastewater from automotive industry | i = 30–50 mA cm−2; initial COD 2500 mg L−1 | >90% | [29] |
| Compound | % w/v |
|---|---|
| CaCl2 | 0.0226 |
| MgCl2·6H2O | 0.0836 |
| KNO3 | 0.0041 |
| CaSO4·2H2O | 0.0295 |
| NaOH | 0.0009 |
| NaHCO3 | 0.0126 |
| C6H11NO6 | 0.00258 |
| Suwannee River NOM | 0.0102 |
| Measurements | Units | Water Matrix 1 | Water Matrix 2 |
|---|---|---|---|
| pH | - | 6.5 | 8.5 |
| TOC | mg L−1 | 6.25 | 7.03 |
| UV254 | cm−1 | 0.16 | 0.18 |
| SUVA | L mg−1 m−1 | 2.3 | 2.9 |
| Oxygen Demand [mg L−1] | |
|---|---|
| Theoretical Calculation | 21.21 |
| PeCOD measurement | 18 |
| Electrodes | Current Density = 10 mA cm−2 | Current Density = 20 mA cm−2 | ||
|---|---|---|---|---|
| pH = 6.5 | pH = 8.5 | pH = 6.5 | pH = 8.5 | |
| BDD-BDD | 1.3 ± 0.7 | 1.01 ± 0.4 | 0.77 ± 0.07 | 0.73 ± 0.1 |
| BDD-SS | 1.74 ± 0.7 | 0.67 ± 0.4 | 0.55 ± 0.4 | 0.57 ± 0.2 |
| MMO-SS | 0.33 ± 0.3 | 0.67 ± 0.6 | 0.45 ± 0.1 | 0.59 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snowdon, M.R.; Rathod, S.; Fattahi, A.; Khan, A.; Bragg, L.M.; Liang, R.; Zhou, N.; Servos, M.R. Water Purification and Electrochemical Oxidation: Meeting Different Targets with BDD and MMO Anodes. Environments 2022, 9, 135. https://doi.org/10.3390/environments9110135
Snowdon MR, Rathod S, Fattahi A, Khan A, Bragg LM, Liang R, Zhou N, Servos MR. Water Purification and Electrochemical Oxidation: Meeting Different Targets with BDD and MMO Anodes. Environments. 2022; 9(11):135. https://doi.org/10.3390/environments9110135
Chicago/Turabian StyleSnowdon, Monika R., Shasvat Rathod, Azar Fattahi, Abrar Khan, Leslie M. Bragg, Robert Liang, Norman Zhou, and Mark R. Servos. 2022. "Water Purification and Electrochemical Oxidation: Meeting Different Targets with BDD and MMO Anodes" Environments 9, no. 11: 135. https://doi.org/10.3390/environments9110135
APA StyleSnowdon, M. R., Rathod, S., Fattahi, A., Khan, A., Bragg, L. M., Liang, R., Zhou, N., & Servos, M. R. (2022). Water Purification and Electrochemical Oxidation: Meeting Different Targets with BDD and MMO Anodes. Environments, 9(11), 135. https://doi.org/10.3390/environments9110135