1,2,3,4-Tetrahydropyrimidine Derivative for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
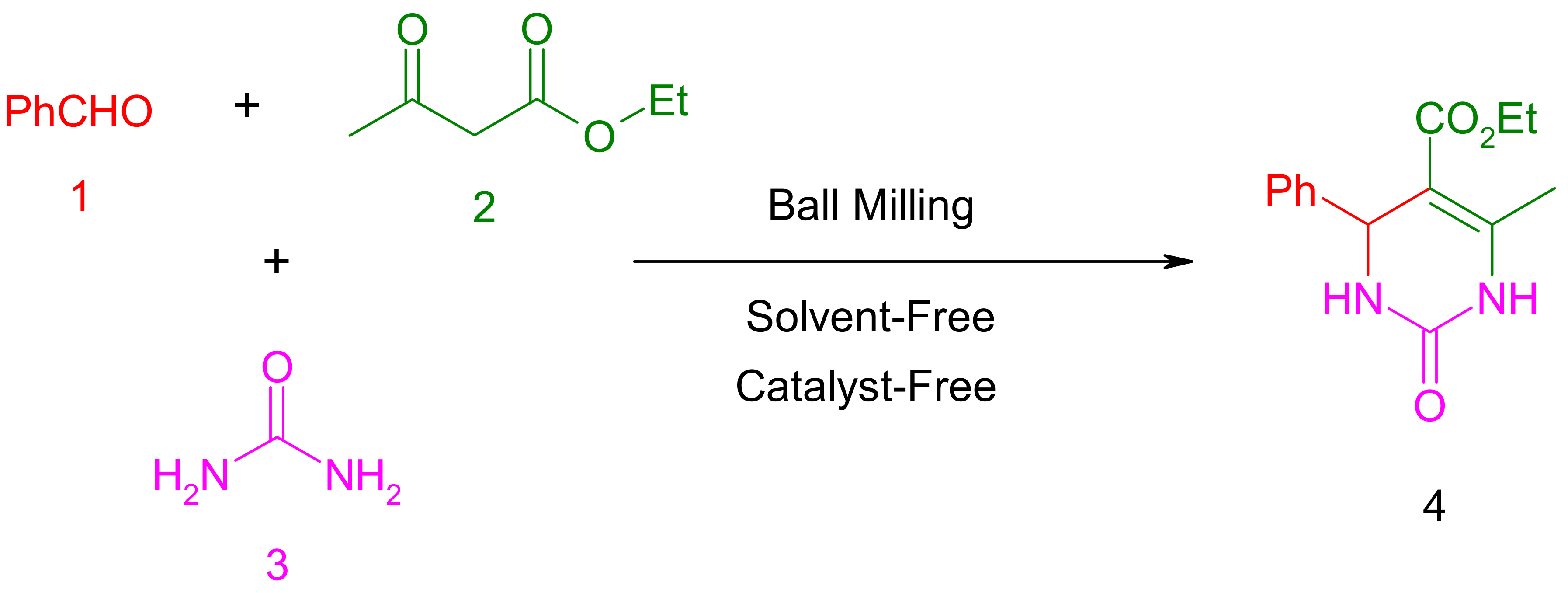
2.1. Synthesis of Ethyl 6-Methyl-2-oxo-4-Phenyl-1,2,3,4-Tetrahydropyrimidine-5-Carboxylate (4)
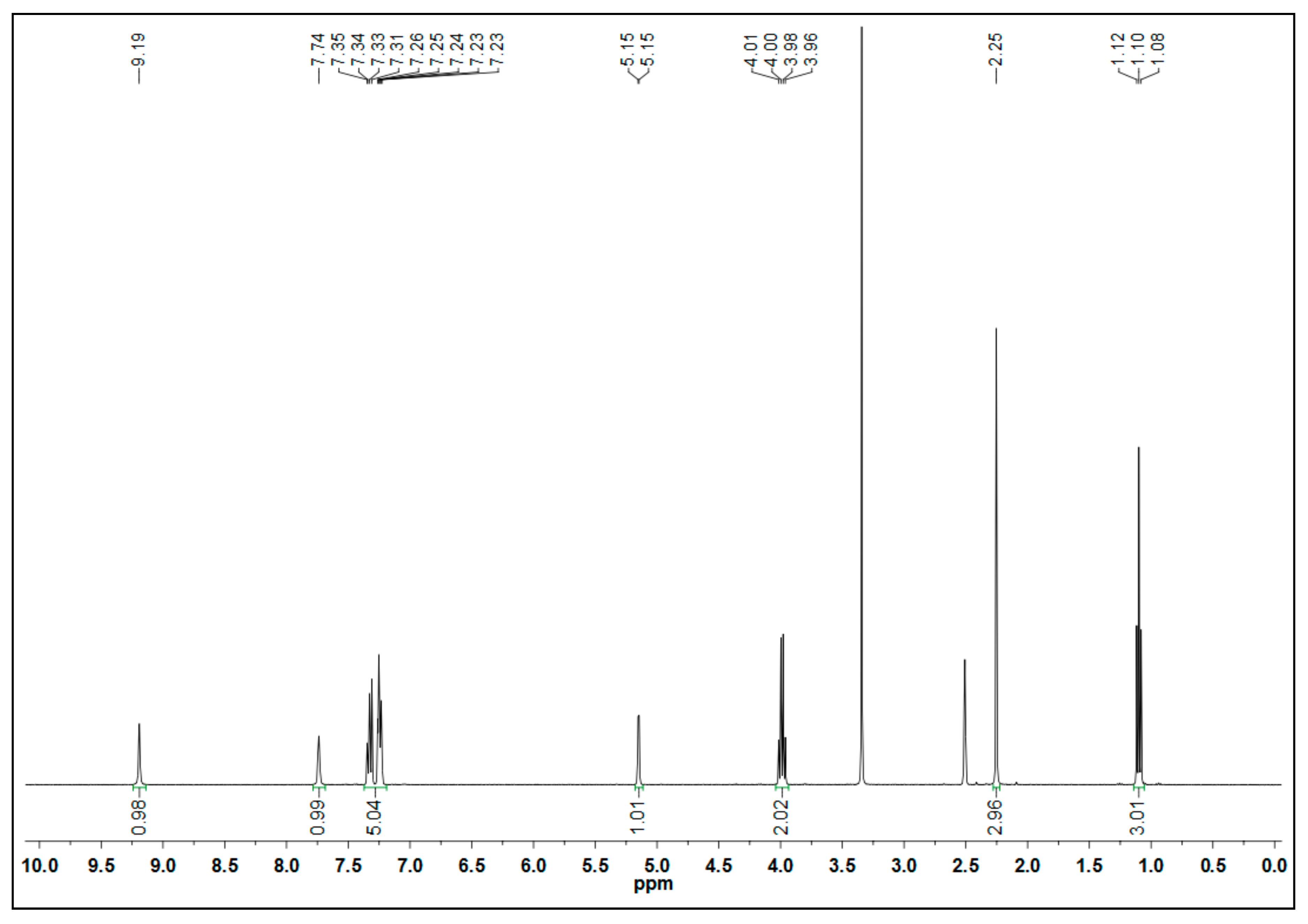
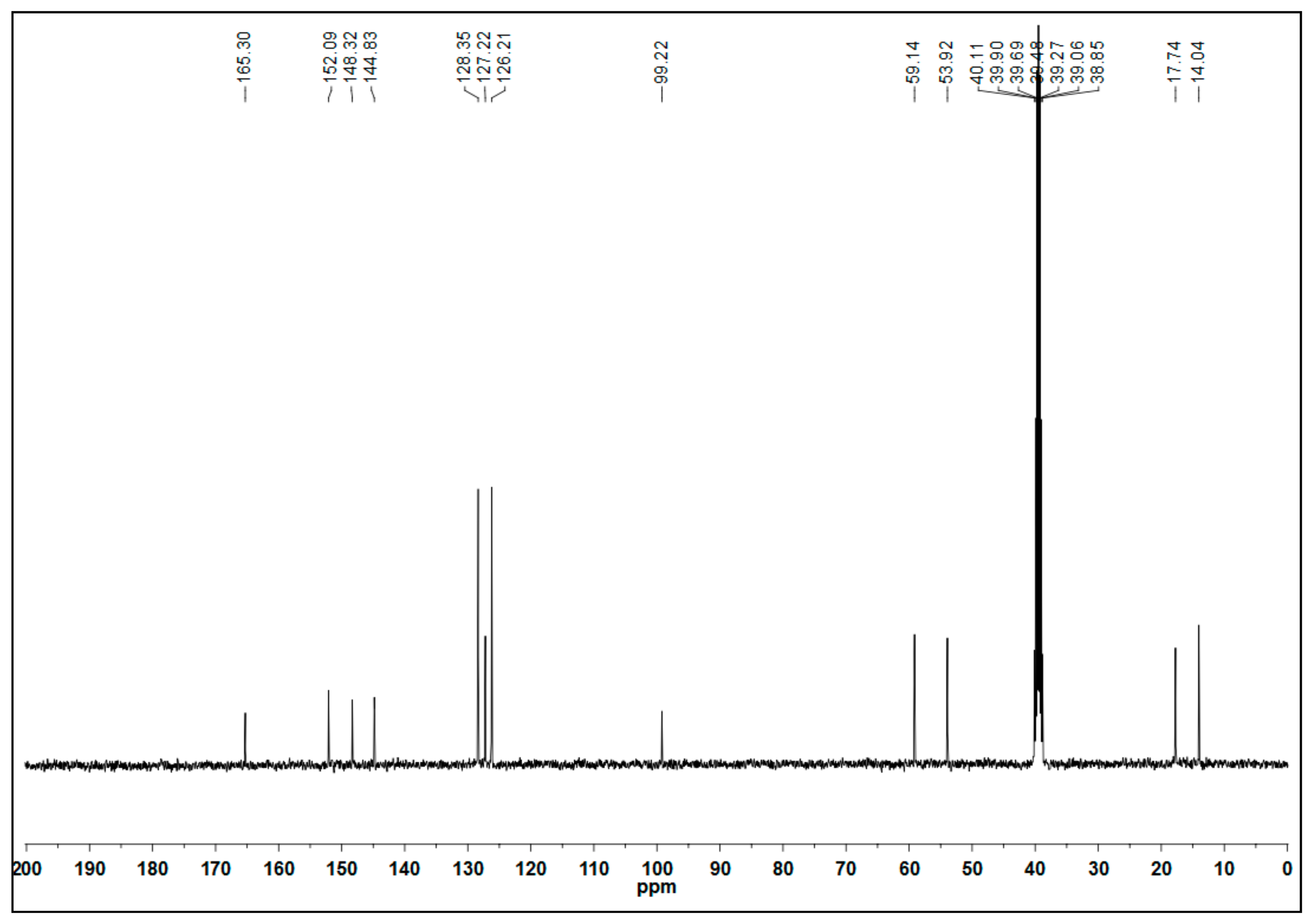
2.2. Characteristic Data of Ethyl 6-Methyl-2-oxo-4-Phenyl-1,2,3,4-Tetrahydropyrimidine-5-Carboxylate (4)
2.3. Adsorption Procedure
2.3.1. Preparation of Cd(II) Ion Solutions
2.3.2. Analysis of Cd(II) Ion Adsorption
3. Results and Discussion
3.1. Adsorption of Cd(II) Ions
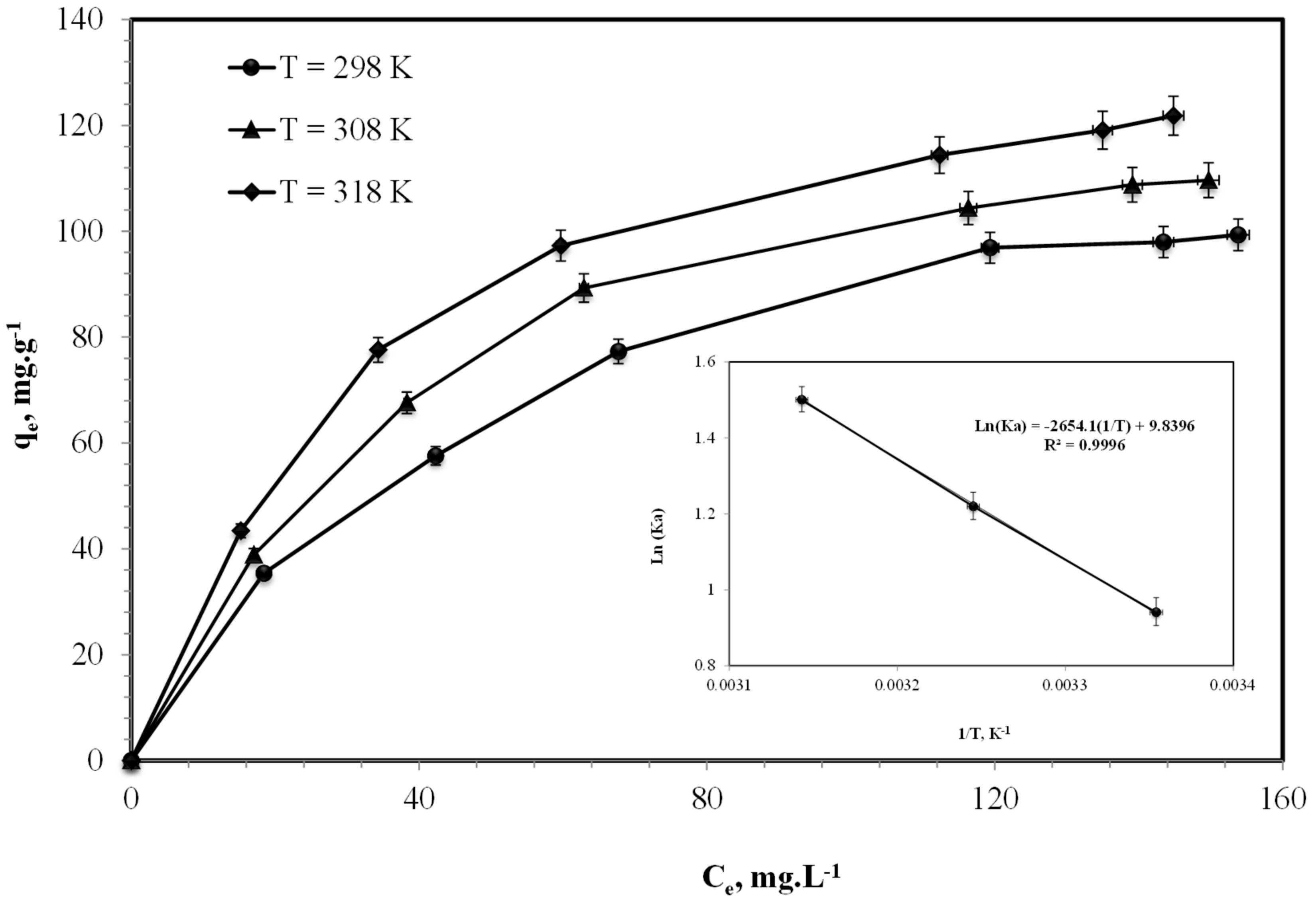
3.1.1. Effect of Initial Cd(II) Ion Concentrations
3.1.2. Effect of pH
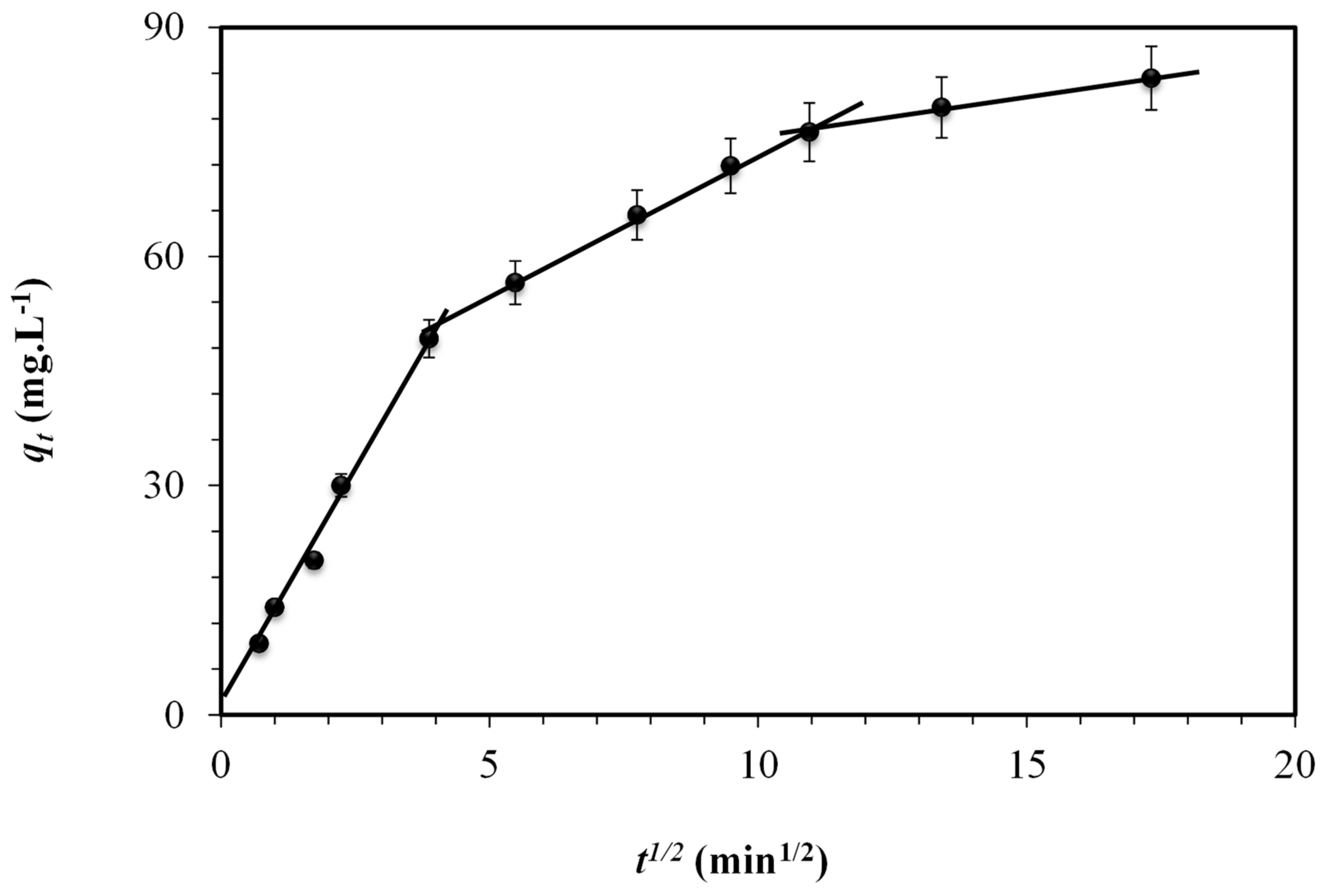
3.1.3. Kinetic Study
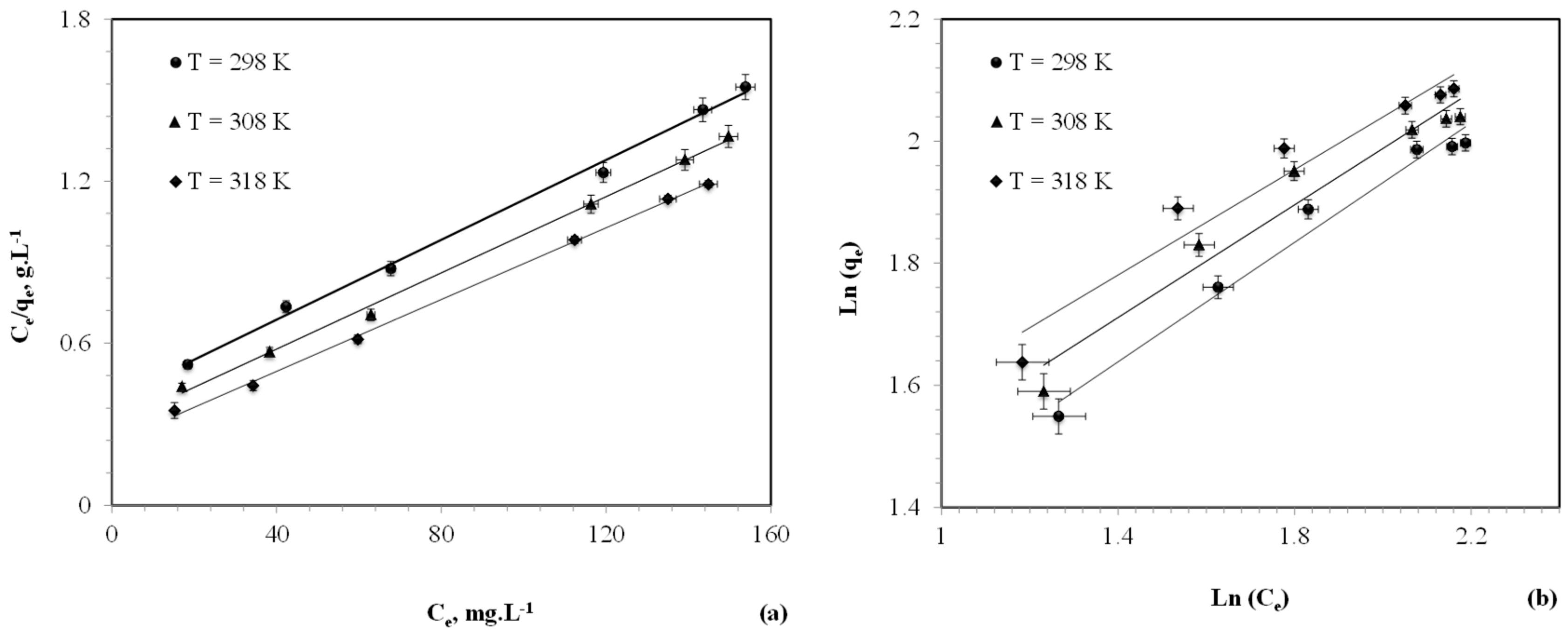
3.1.4. Adsorption Isotherms
3.1.5. Thermodynamic Study
3.2. Mechanism of Adsorption
3.3. Comparison of Cd(II) Adsorption Capacity with Other Previously Reported Adsorbents
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Bode-Aluko, C.A.; Pereao, O.; Ndayambje, G.; Petrik, L. Adsorption of toxic metals on modified polyacrylonitrile nanofibres: A review. Water Air Soil Poll. 2017, 228, 35. [Google Scholar] [CrossRef]
- Uzun, I.; Güzel, F. Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk. J. Chem. 2000, 24, 291–297. [Google Scholar]
- Gier, S.; Johns, W.D. Heavy metal-adsorption on micas and clay minerals studied by X–ray photoelectron spectroscopy. Appl. Clay Sci. 2000, 16, 289–299. [Google Scholar] [CrossRef]
- Koppelman, M.H.; Dillard, J.G. A study of the adsorption of Ni(II) and Cu(II) by clay minerals. Clays Clay Miner. 1977, 25, 457–462. [Google Scholar] [CrossRef]
- Biskup, B.; Subotic, B. Kinetic analysis of the exchange processes between sodium ions from zeolite A and cadmium, copper and nickel ions from solutions. Sep. Purif. Technol. 2004, 37, 17–31. [Google Scholar] [CrossRef]
- Cincotti, A.; Mameli, A.; Locci, A.M.; Orru, R.; Cao, G. Heavy metals uptake by Sardinian natural zeolites: Experiment and modeling. Ind. Eng. Chem. Res. 2006, 45, 1074–1084. [Google Scholar] [CrossRef]
- Araki, S.; Li, T.; Li, K.; Yamamoto, H. Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water. Sep. Purif. Technol. 2019, 221, 393–398. [Google Scholar] [CrossRef]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Dang, V.B.H.; Doan, H.D.; Dang-Vuc, T.; Lohi, A. Equilibrium and kinetics of biosorption of cadmium(II) and copper(II) ions by wheat straw. Bioresour. Technol. 2009, 100, 211–219. [Google Scholar] [CrossRef]
- Zewail, T.M.; El-Garf, S.A.M. Preparation of agriculture residue based adsorbents for heavy metal removal. Desalin. Water Treat. 2010, 22, 363–370. [Google Scholar] [CrossRef]
- Mo, J.; Yang, Q.; Zhang, N.; Zhang, W.; Zheng, Y.; Zhang, Z. A review on agro-industrial waste (AIW) derived adsorbents for water and wastewater treatment. J. Environ. Manag. 2018, 227, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, X.L.; Xu, W.H.; Huang, X.J.; Liu, J.H.; Xu, A.W. Stable organic-inorganic hybrid of polyaniline/α-zirconium phosphate for efficient removal of organic pollutants in water environment. Appl. Mater. Interfaces 2012, 4, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Gao, Y.; Li, Y. Preparation and chelation adsorption property of composite chelating material poly(amidoxime)/SiO2 towards heavy metal ions. Chem. Eng. J. 2010, 158, 542–549. [Google Scholar] [CrossRef]
- Zaitseva, N.; Zaitsev, V.; Walcarius, A. Chromium(VI) removal via reduction–sorption on bi-functional silica adsorbents. J. Hazard. Mater. 2013, 250–251, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Simsek, E.B.; Duranoglu, D.; Beker, U. Heavy metal adsorption by magnetic hybrid-sorbent: An experimental and theoretical approach. Sep. Sci. Technol. 2012, 47, 1334–1340. [Google Scholar] [CrossRef]
- Suchithra, P.S.; Vazhayal, L.; Mohamed, A.P.; Ananthakumar, S. Mesoporous organic-inorganic hybrid aerogels through ultrasonic assisted sol-gel intercalation of silica-PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200–202, 589–600. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.K.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan-silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Ge, P.; Li, F.; Zhang, B. Synthesis of modified mesoporous materials and comparative studies of removal of heavy metal from aqueous solutions. Pol. J. Environ. Stud. 2010, 19, 301–308. [Google Scholar]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination 2011, 266, 33–39. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Tighadouini, S.; Baquet, M. Solid-phase extraction of Hg(II), Zn(II) and Cd(II) from water using silica gel modified with bipyrazolic tripodal receptor. Ind. J. Chem. Technol. 2013, 20, 423–428. [Google Scholar]
- Radi, S.; Basbas, N.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F. New amine-modified silica: Synthesis, characterisation and its use in the Cu(II)-Removal from aqueous solutions. Prog. Nanotechnol. Nanomater. 2013, 2, 108–116. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, T.; Bacquet, M.; Degoutin, S.; Cazier, F. 1-(Pyridin-2-yl) Imine functionalized silica gel: Synthesis, Characterization and preliminary use in metal ion extraction. Sep. Sci. Technol. 2013, 48, 1349–1355. [Google Scholar] [CrossRef]
- Radi, S.; Toubi, Y.; Bacquet, M. Synthesis of pyridin-3-yl-functionalized silica as a chelating sorbent for solid-phase adsorption of Hg(II), Pb(II), Zn(II) and Cd(II) from water. Chem. Res. Intermed. 2013, 39, 3791–3802. [Google Scholar]
- Shami, R.B.; Shojaei, V.; Khoshdast, H. Efficient cadmium removal from aqueous solutions using a sample coal waste activated by rhamnolipid biosurfactant. J. Environ. Manag. 2019, 231, 1182–1192. [Google Scholar] [CrossRef]
- Dzhodzhyk, O.; Kolesnyk, I.; Konovalova, V.; Burban, A. Modified polyethersulfone membranes with photocatalytic properties. Chem. Chem. Technol. 2017, 11, 277–284. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: Factors affecting Hg(II) uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Ould M’hamed, M.; Alshammari, A.G.; Lemine, O.M. Green High-Yielding One-Pot Approach to Biginelli Reaction under Catalyst-Free and Solvent-Free Ball Milling Conditions. Appl. Sci. 2017, 6, 431. [Google Scholar] [CrossRef]
- Khezami, L.; Ould M’hamed, M.; Lemine, O.M.; Bououdina, M.; Bessadok-Jemai, A. Milled goethite nanocrystallinefor selective and fast uptake of cadmium ions from aqueous solution. Desalin. Water Treat. 2016, 57, 6531–6539. [Google Scholar] [CrossRef]
- Huang, C.P.; Smith, E.H. Removal of cadmium(II) from plating wastewater by activated carbon process. In Chemistry in Water Reuse; Cooper, W., Ed.; Ann Arbor Science: Ann Arbor, MI, USA, 1981; pp. 355–412. [Google Scholar]
- Gupta, V.K.; Sharma, S.; Yadau, I.S.; Dinesh, M. Utilisation of bagasses fly ash generated in the sugar industry for the removal of phenol and p-nitrophenol from waste water. J. Chem. Technol. Biot. 1998, 71, 180–186. [Google Scholar] [CrossRef]
- Benjamin, M.M. Effects of Competing Metals and Complexing Ligands on Trace Metal Adsorption at the Oxide/Solution Interface. PhD Thesis, Stanford University, Stanford, CA, USA, 1978. [Google Scholar]
- Haghesresht, F.; Lu, G. Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels. 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Hana, R.; Li, H.; Li, Y.; Zhang, J.; Xiao, H.; Shi, J. Biosorption of copper and lead ions by waste beer yeast. J. Hazard. Mater. 2006, B137, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Su, J.; Zhu, H.; Xiong, J.; Liu, X.; Li, D.; Chen, Y.; Li, Y. The removal of heavy metal ions from aqueous solutions by amine functionalized cellulose pretreated with microwave-H2O2. RSC Adv. 2017, 7, 34182. [Google Scholar] [CrossRef]
- Tobin, J.M.; Cooper, D.G.; Neufeld, R.J. Uptake of metal ions by Rhizopus arrhizus biomass. Appl. Environ. Microb. 1984, 47, 821–824. [Google Scholar]
- Reed, B.E.; Matsumoto, M.R. Modeling cadmium adsorption in single and binary adsorbent (powdered activated carbon) systems. J. Environ. Eng. 1993, 119, 332–348. [Google Scholar] [CrossRef]
- Mohapatra, M.; Anand, S. Cd(II) adsorption on synthetic goethite: Kinetics and thermodynamics aspects. Indian J. Environ. Prot. 2006, 26, 1057–1066. [Google Scholar]
- Mohapatra, M.; Mohapatra, L.; Singh, P.; Anand, S.; Mishra, B.K. A comparative study on Pb(II), Cd(II), Cu(II), Co(II) adsorption from single and binary aqueous solutions on additive assisted nano-structured goethite. Int. J. Eng. Sci. Technol. 2010, 2, 89–103. [Google Scholar] [CrossRef]
- Vinod, V.P.; Anirudhan, T.S. Adsorption Behaviour of Basic Dyes on the Humic Acid Immobilized Pillared Clay. Water Air Soil Pollut. 2003, 150, 193–217. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Ouazene, N. Mass-transfer processes in the adsorption of cationic dye by sawdust. Environ. Prog. Sustain. Energ. 2012, 31, 597–603. [Google Scholar] [CrossRef]
- Hameed, B.H.; Salman, J.M.; Ahmad, A.L. Adsorption isotherm and kinetic modeling of 2, 4-D pesticide on activated carbon derived from date stones. J. Hazard. Mater. 2009, 163, 121–126. [Google Scholar] [CrossRef] [PubMed]
- El-Sikaily, A.; El Nemr, A.; Khaled, A.; Abdelwehab, O. Removal of toxic chromium from wastewater using green alga Ulva lactuca and its activated carbon. J. Hazard. Mater. 2007, 148, 216–228. [Google Scholar] [CrossRef]
- Yazdani, M.; Tuutijärvi, T.; Bhatnagar, A.; Vahala, R. Adsorptive removal of arsenic (V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 2016, 214, 149–156. [Google Scholar] [CrossRef]
- Milosavljevic, N.B.; Ristic, M.; Peric-Grujic, A.A.; Filipovic, J.M.; Strbac, S.B.; Rakocevic, Z.L.; Krusic, M.T.K. Removal of Cu2+ ions using hydrogels of chitosan, itaconic and methacrylic acid: FTIR, SEM/EDX, AFM, kinetic and equilibrium study. Colloids and Surfaces A: Physicochem. Eng. Aspects 2011, 388, 59–69. [Google Scholar] [CrossRef]
- Radi, S.; Ramdani, A.; Lekchiri, Y.; Morcellet, M.; Crini, G.; Janus, L.; Bacquet, M. Immobilization of pyrazole compounds on silica gels and their preliminary use in metal ion extraction. New J. Chem. 2003, 27, 1224–1227. [Google Scholar] [CrossRef]
- Xie, F.; Lin, X.; Wu, X.; Xie, Z. Solid phase extraction of lead (II), copper (II), cadmium (II), and nickel (II) using gallic acid modified silica gel prior to determination by flame atomic adsorption spectrometry. Talanta 2008, 74, 836–843. [Google Scholar] [CrossRef]
- Shahbazi, A.; Younesi, H.; Badiei, A. Functionalized SBA-15 mesoporous silica by melamine-based dendrimer amines for adsorptive characteristics of Pb(II), Cu(II) and Cd(II) heavy metal ions in batch and fixed bed column. Chem. Eng. J. 2011, 168, 505–518. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, R.; Sun, C.; Ji, C.; Hou, C.; Wang, C.; Niu, Y.J. Adsorption for metal ions of chitosan coated cotton fiber. Appl. Polym. Sci. 2008, 110, 2321–2327. [Google Scholar]
- Meitei, M.D.; Prasad, M.N.V. Lead (II) and cadmium (II) biosorption on Spirodela polyrhiza (L.) Schleiden biomass. J. Environ. Chem. Eng. 2013, 1, 200–207. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules 2014, 19, 247–262. [Google Scholar] [CrossRef]
- Du, Z.; Zheng, T.; Wang, P.; Hao, L.; Wang, Y. Fast microwave-assisted preparation of a low-cost and recyclable carboxyl modified lignocellulose-biomass jute fiber for enhanced heavy metal removal from water. Bioresour. Technol. 2016, 201, 41–49. [Google Scholar]

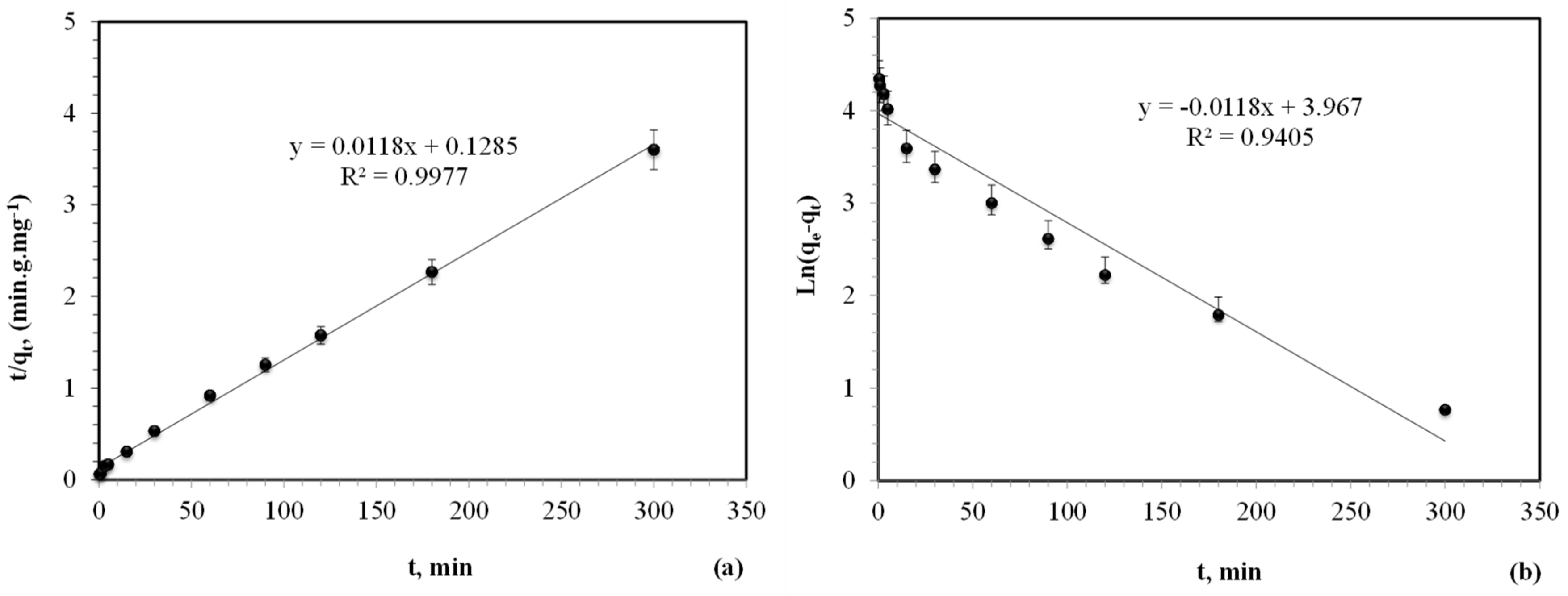
| t1/2 | First-Order | Second-Order | |||||
|---|---|---|---|---|---|---|---|
| (s) | qe (exp) a (mg g−1) | k1 (min−1) | qe(cal) b(mg g−1) | r2 | k2 × 104 (g mg−1 min−1) | qe(cal) b (mg g−1) | r2 |
| 665 | 77 | 0.0118 | 52.83 | 0.9405 | 10.6 | 84.75 | 0.9977 |
| Temperature (K) | Langmuir Constants | Freundlich Constants | |||||
|---|---|---|---|---|---|---|---|
| Q0 (mg g−1) | b (L mg−1) | r2 | RL | n | k | r2 | |
| 298 | 135.14 | 0.01896 | 0.9959 | 0.0815 | 2.044 | 8.98 | 0.9781 |
| 308 | 140.85 | 0.02404 | 0.9967 | 0.0679 | 2.164 | 11.58 | 0.9529 |
| 318 | 151.16 | 0.02959 | 0.9986 | 0.0521 | 2.316 | 14.99 | 0.9462 |
| Temperature (K) | Ka | ΔG0 (kJ mol−1) | ΔS0 (kJ mol−1 K−1) | ΔH0 (kJ mol−1) | r2 |
|---|---|---|---|---|---|
| 298 | 2.562 | –2.332 | 0.0818 | 22.07 | 0.9996 |
| 308 | 3.387 | –3.125 | 0.0818 | ||
| 318 | 4.485 | –3.970 | 0.0818 |
| Intraparticle Diffusion Equation Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|
| kdif1, mg/g min1/2 | C | r2 | kdif2, mg/g min1/2 | C | r2 | kdif2, mg/g min1/2 | C | r2 |
| 12.58 | 0.56 | 0.9928 | 3.83 | 35.15 | 0.9960 | 1.1 | 64.45 | 0.9934 |
| Adsorbent | qe (mg g−1) | Temp. (K) | Ref. |
|---|---|---|---|
| C,N-pyridylpyrazole | 4.8 | 298 | [47] |
| Gallic acid | 6.09 | 298 | [48] |
| 3-Aminopropytriethoxysilane | 14.1 | 323 | [49] |
| Chitosan/cotton fibers | 15.74 | 298 | [50] |
| Melamine-based NH2 dendrimer | 71.1 | 323 | [49] |
| Spirodela polyrhiza | 36 | 298 | [51] |
| Pyrazol-3-ylimine | 74.89 | 298 | [52] |
| CMJFMH | 88 | 298 | [53] |
| PEI/SA-MCCMV | 139.47 | 298 | [36] |
| Present Work | 135.14 | 298 | - |
| Present Work | 151.16 | 318 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M’hamed, M.O.; Khezami, L. 1,2,3,4-Tetrahydropyrimidine Derivative for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution. Environments 2019, 6, 68. https://doi.org/10.3390/environments6060068
M’hamed MO, Khezami L. 1,2,3,4-Tetrahydropyrimidine Derivative for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution. Environments. 2019; 6(6):68. https://doi.org/10.3390/environments6060068
Chicago/Turabian StyleM’hamed, Mohamed Ould, and Lotfi Khezami. 2019. "1,2,3,4-Tetrahydropyrimidine Derivative for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution" Environments 6, no. 6: 68. https://doi.org/10.3390/environments6060068
APA StyleM’hamed, M. O., & Khezami, L. (2019). 1,2,3,4-Tetrahydropyrimidine Derivative for Selective and Fast Uptake of Cadmium Ions from Aqueous Solution. Environments, 6(6), 68. https://doi.org/10.3390/environments6060068





