System to Evaluate Movement of Biological Contaminants in Soil
Abstract
1. Introduction
1.1. Review of Soil Column Design
1.2. Review of Soil Column Research
1.3. Gaps in Literature
2. Materials and Methods
2.1. Design of Flow-Reduction Cap
2.2. System Components
2.3. Construction of Soil Column
- Measure, mark, and cut the 3.81 cm (1.5 in) o.d. vinyl tubing with a power miter saw or hacksaw.
- Measure, mark, and cut the 0.635 cm (0.25 in) o.d. vinyl tubing.
- Print the flow-reduction cap. The flow-reduction cap can be printed with raised holes of differing sizes to increase or decrease flowrate into the soil column. The experiments done in this study used a flow-reduction cap with 0.635 cm (0.25 in) diameter raised holes. Autodesk® Inventor® files are available at https://grabcad.com/library/flow-reduction-cap-1 [20].
- Measure, mark, and cut the screen mesh.
- Measure and mark 6.35 cm (2.5 in) and 8.89 cm (3.5 in) concentric circles on a thin polycarbonate sheet. For this study, a clear polycarbonate sheet of 0.23622 cm (0.093 in) thickness was used. Cut the interior circle with a 6.35 cm (2.5 in) diameter hole saw. Cut the exterior circle with a 8.89 cm (3.5 in) diameter hole saw. If hole saws are not available, use a scoring tool then cut with a utility knife. File to clean the cuts.
- Measure and mark a point 1.5875 cm (0.625 in) from the bottom edge of the coupling spacer. Drill a 0.635 cm (0.25 in) hole with the power drill and 0.635 cm (0.25 in) drill bit through the marking.
- Measure and mark a point 3.96875 cm (1.5625 in) from the bottom edge of the coupling. Drill a 0.635 cm (0.25 in) hole through the marking.
- Place the coupling spacer inside of the coupling so that the holes line up.
- Push the overflow tube through the drilled holes in the coupling and coupling spacer.
- Super glue the outside of the end of the overflow tube and push it through the off-center hole in the flow-reduction cap, from the bottom (Figure 3). Match the surface of the top of the flow-reduction cap with the surface on the end of overflow tube. Water should be able to pool in the cap and easily drain through the overflow tube. Allow 24 h to dry.
- Draw a bead of silicone around the top of the coupling spacer with the silicone tube and caulk gun. Pull the flow-reduction cap towards the silicone bead with the overflow tube. Seal the flow-reduction cap to the coupling spacer and wipe away excess silicone. Allow 24 h to dry.
- Measure and mark a point 3.175 cm (1.25 in) from the top of the nozzle housing. Measure and mark another point 3.175 cm (1.25 in) from the top of the nozzle housing on the opposite side of the first marking. Drill a 1.27 cm (0.5 in) hole through both markings. From the top of the nozzle housing, use the hacksaw to create two cuts directly downwards towards the 2 and 10 o’clock positions on the 1.27 cm (0.5 in) drilled hole. The resulting slots should be large enough to force a 1.27 cm (0.5 in) o.d. PVC pipe past them and into the 1.27 cm (0.5 in) diameter drilled hole. The PVC piping should fit securely in the drilled hole. If the slot or hole is too small, use the file to widen it.
- Assemble the soil column (Figure 1b). Insert the discharge tube into the funnel coupling and tighten. Hold two layers of window mesh taut across the end of the soil column shell and insert it into the funnel coupling and tighten. Place two layers of window mesh on the other end of the soil column shell, place the sidewall ring on the window mesh, then insert it into the bottom of the coupling. Insert the nozzle housing into the top of the coupling. Insert the knockout test cap into the top of the nozzle housing. Figure 4 shows the finished soil column component.
2.4. Assembly of Soil Column Stand
- Measure, mark, and cut the 1.27 cm (0.5 in) o.d. PVC to the lengths and quantities shown for the upper leg, lower leg, and horizontal support with the PVC pipe cutter.
- Assemble the PVC and connectors (Figure 1c).
- Loosen or tighten two hose clamps to approximately 7.62 cm (3 in) diameters. Zip-tie a hose clamp to the top three horizontal supports. Zip-tie the second hose clamp to the lower two horizontal supports. Figure 5 shows the finished soil column stand.
2.5. Mist Nozzle and Piping
- Measure, mark, and cut the 1.27 cm (0.5 in) o.d. PVC to the lengths and quantities shown for the piping with the PVC pipe cutter.
- Assemble the PVC and connectors with PVC primer and PVC cement. Allow 24 h to dry.
- Assemble the ACF Greenhouses 1 gph mist nozzle. Wrap the threads with Teflon tape. Screw the mist nozzle into the ACF Greenhouses mist tee. Attach the garden hose to the ACF Greenhouses swivel hose connector. Figure 6 shows the finished soil column mist nozzle and piping.
2.6. Complete Soil Column System
- Place the bottom half of the soil column inside the stand by pushing the soil column shell, funnel coupling, and discharge tube between the stand’s lower legs.
- Slide the soil column shell upwards through the hose clamps and tighten them at a height that leaves enough space for a beaker under the discharge tube. Place a beaker under the discharge tube. Use a level to adjust the soil column to be vertical.
- Place the coupling on the soil column shell and tighten the hose clamp. Place the mist nozzle housing into the coupling and tighten the hose clamp.
- Attach the garden hose to the swivel hose connector and water source. Line the mist nozzle up with the center of the soil column. Push the 1.27 cm (0.5 in) piping into the slots and 1.27 cm (0.5 in) diameter drilled holes in the nozzle housing. If the slots or holes in the mist nozzle housing are too small, lightly file for a snug fit. Place the knockout test cap on the nozzle housing.
2.7. Soil Collection
2.8. Soil Packing
2.9. Testing Mister Nozzle Flowrates
2.10. Flow-Reduction Cap Flowrate
2.11. Infiltration Time
3. Results
3.1. Design
3.2. Flowrate through Mist Nozzle and Flow-Reduction Cap
3.3. Infiltration Time
4. Discussion
4.1. Infiltration Time
4.2. Design Justification
4.2.1. Soil Column
4.2.2. Soil Column Stand
4.2.3. Mist Nozzle and Piping
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howell, T.A. Lysimetry. In Encyclopedia of Soils in the Environment; USDA Agricultural Research Service: Bushland, TX, USA, 2005; pp. 379–386. [Google Scholar]
- Lewis, J.; Sjöstrom, J. Optimizing the experimental design of soil columns in saturated and unsaturated transport experiments. J. Contam. Hydrol. 2010, 115, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Butters, G.L.; Bandaranayake, W. Demonstrations in solute transport using dyes: I. Procedures and results. J. Nat. Resour. Life Sci. Educ. 1993, 22, 121–125. [Google Scholar]
- Schlossberg, M.J.; Karnok, K.J. Use of transparent columns for demonstrating water movement in golf green root zones. J. Nat. Resour. Life Sci. Educ. 2002, 31, 1–4. [Google Scholar]
- Ngo, V.V.; Michel, J.; Gujisaite, V.; Latifi, A.; Simonnot, M. Parameters describing nonequilibrium transport of polycyclic aromatic hydrocarbons through contaminated soil columns: Estimability analysis, correlation, and optimization. J. Contam. Hydrol. 2014, 158, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.K.; Shelton, D.R.; Pachepsky, Y.A. Transport and retention of manure-borne coliforms in soil. Vadose Zone J. 2005, 4, 828–837. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Medina, J.; González, A.; Diez, M.C.; Cartes, P.; Monreal, C.; Navia, R. Evaluation of biodegradable polymers as encapsulating agents for the development of a urea controlled-release fertilizer using biochar as support material. Sci. Total Environ. 2014, 505, 446–453. [Google Scholar] [CrossRef]
- Laird, D.A.; Fleming, P.; Davis, D.D.; Horton, R.; Wang, B.; Karlen, D.L. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma 2010, 158, 443–449. [Google Scholar] [CrossRef]
- Schäfer, A.; Ustohal, P.; Harms, H.; Stauffer, F.; Dracos, T.; Zehnder, A.J.B. Transport of bacteria in unsaturated porous media. J. Contam. Hydrol. 1998, 33, 149–169. [Google Scholar] [CrossRef]
- Corwin, D.L. Evaluation of a simple lysimeter-design modification to minimize sidewall flow. J. Contam. Hydrol. 2000, 42, 35–49. [Google Scholar] [CrossRef]
- Lewis, J.; Sjöstrom, J. Optimizing the experimental design of unsaturated soil columns. In Proceedings of the 19th World Congress of Soil Science: Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 1–6. [Google Scholar]
- Bergström, L. Leaching of agrochemicals in field lysimeters—A method to test mobility of chemicals in soil. In Pesticide/Soil Interactions; INRA: Paris, France, 2000; pp. 279–285. [Google Scholar]
- Feyereisen, G.W.; Folmar, G.J. Development of a laboratory-scale lysimeter system to simultaneously study runoff and leaching dynamics. Trans. ASABE 2009, 52, 1585–1591. [Google Scholar] [CrossRef]
- Chrysikopoulos, C.V.; Syngouna, V.I. Effect of gravity on colloid transport through water-saturated columns packed with glass beads: Modeling and experiments. Environ. Sci. Technol. 2014, 48, 6805–6813. [Google Scholar] [CrossRef]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2005, 144, 31–43. [Google Scholar] [CrossRef]
- Schijven, J.F.; Hassanizadeh, S.M. Removal of viruses by soil passage: Overview of modeling, processes, and parameters. Crit. Rev. Environ. Sci. Technol. 2000, 30, 49–127. [Google Scholar] [CrossRef]
- Camobreco, V.J.; Richards, B.K.; Steenhuis, T.S.; Peverly, J.H.; McBride, M.B. Movement of heavy metals through undisturbed and homogenized soil columns. Soil Sci. 1996, 161, 740–750. [Google Scholar] [CrossRef]
- Wehrhan, A.; Kasteel, R.; Simunek, J.; Groeneweg, J.; Vereecken, H. Transport of sulfadiazine in soil columns—Experiments and modelling approaches. J. Contam. Hydrol. 2006, 89, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Rabølle, M.; Spliid, N.H. Sorption and mobility of metronidazole, olaquindox, oxytetracycline and tylosin in soil. Chemosphere 2000, 40, 715–722. [Google Scholar] [CrossRef]
- GrabCAD Community. Available online: https://grabcad.com/library/flow-reduction-cap-1 (accessed on 7 March 2019).
- ACF Greenhouses. Available online: http://www.littlegreenhouse.com/accessory/mist.shtml (accessed on 7 March 2019).
- Oliviera, I.B.; Demond, A.H.; Salehzadeh, A. Packing of sands for the production of homogeneous porous media. Soil Sci. Soc. Am. J. 1996, 60, 49–53. [Google Scholar] [CrossRef]
- Cornell University Cooperative Extension of Suffolk County. General Soil Information and Specs. Available online: https://s3.amazonaws.com/assets.cce.cornell.edu/attachments/3341/general_soil_info_and_specs.pdf?1413396358 (accessed on 30 January 2019).
- Precipitation Frequency Data Server—NOAA. Available online: https://hdsc.nws.noaa.gov/hdsc/pfds/ (accessed on 30 January 2019).
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Ball, J. Soil and Water Relationships. Available online: http://kbsgk12project.kbs.msu.edu/wp-content/uploads/2011/09/Soil-and-Water-Relationships.pdf (accessed on 30 January 2019).
- Sentenac, P.; Lynch, R.J.; Bolton, M.D. Measurement of a side-wall boundary effect in soil columns using fiber-optics sensing. Int. J. Phys. Model. Geotech. 2001, 1, 35–41. [Google Scholar] [CrossRef]
- Weihermüller, L.; Siemens, J.; Deurer, M.; Knoblauch, S.; Rupp, H.; Göttlein, A.; Pütz, T. In situ soil water extraction: A review. J. Environ. Qual. 2007, 36, 1735–1748. [Google Scholar] [CrossRef]
- Rais, D.; Nowack, B.; Schulin, R.; Luster, J. Sorption of trace metals by standard and micro suction cups in the absence and presence of dissolved organic carbon. J. Environ. Qual. 2006, 35, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, W.W.; Sletten, R.S.; Brandstetter, A.; Wieshammer, G.; Stingeder, G. Adsorption of trace metals by tension lysimeters: Nylon membrane vs. porous ceramic cup. J. Environ. Qual. 1997, 26, 1430–1434. [Google Scholar] [CrossRef]
- Sheppard, S.C. Toxicity testing using microcosms. In Soil Ecotoxicology; CRC Press: Boca Raton, FL, USA, 1997; pp. 345–373. [Google Scholar]
- Davidson, P.C. Characterization of Pathogen Transport in Overland Flow. Ph.D. Thesis, Department of Agricultural and Biological Engineering, University of Illinois at Urbana-Illinois, Urbana-Champaign, IL, USA, 2010. [Google Scholar]
- Davidson, P.C.; Kuhlenschmidt, T.B.; Bhattarai, R.; Kalita, P.K.; Kuhlenschmidt, M.K. Effects of soil type and cover condition on Cryptosporidium parvum transport in overland flow. Water Air Soil Pollut. 2014, 225, 1882–1893. [Google Scholar] [CrossRef]
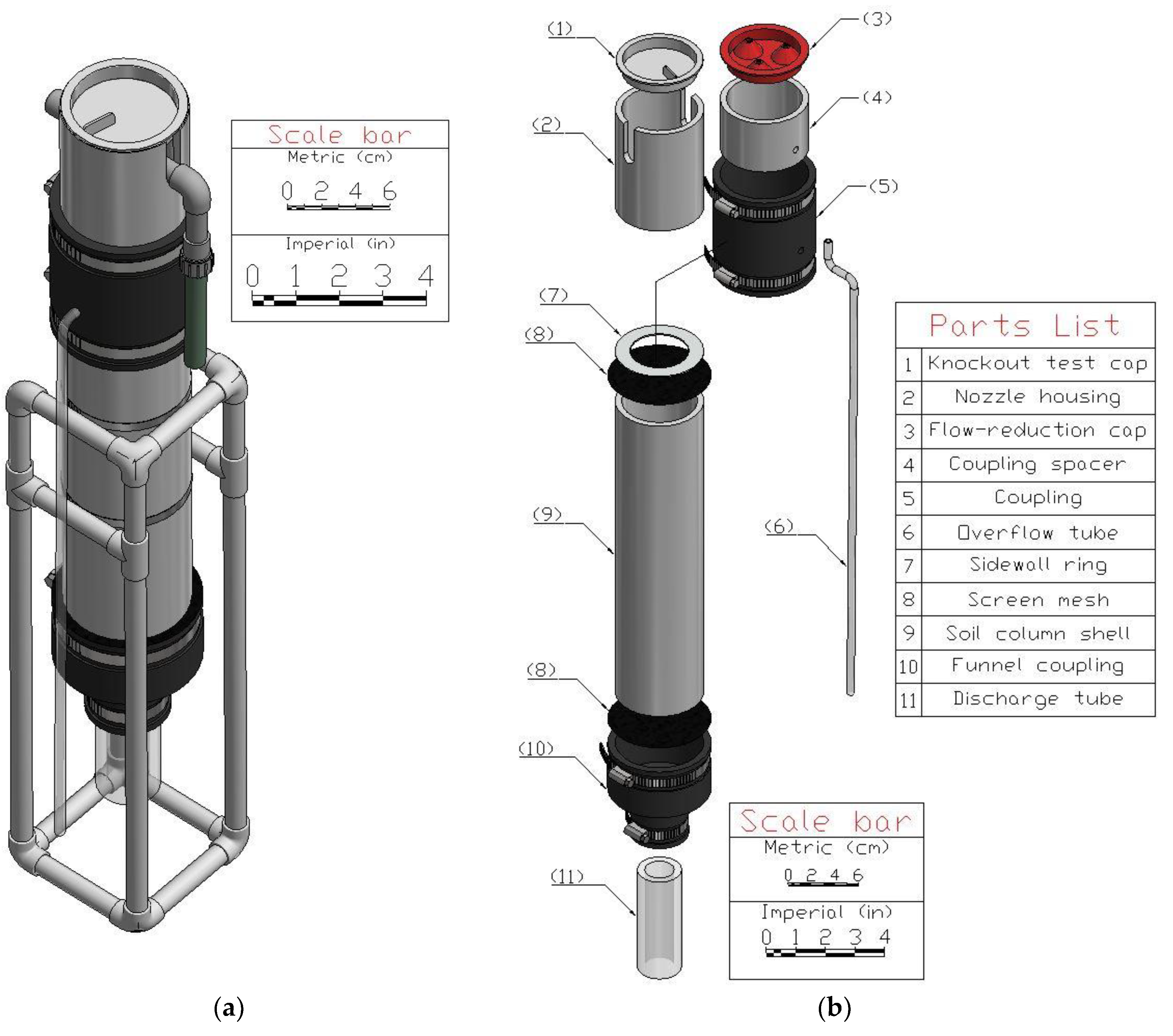






| Part Name | Material | Sizing Specifications | Quantity | |
|---|---|---|---|---|
| Soil column shell | PVC | i.d.: 7.62 cm (3 in) | Length: 33.02 cm (13 in) | 1 |
| Coupling spacer | i.d.: 7.62 cm (3 in) | Length: 5.08 cm (2 in) | 1 | |
| Nozzle housing | i.d.: 7.62 cm (3 in) | Length: 7.62 cm (3 in) | 1 | |
| Knockout test cap | o.d.: 7.62 cm (3 in) | 1 | ||
| Overflow tube | Vinyl | o.d.: 0.635 cm (0.25 in) | Length: 147.32 cm (58 in) | 1 |
| Discharge tube | o.d.: 3.81 cm (1.5 in) | Length: 15.24 cm (6 in) | 1 | |
| Flow-reduction cap | 3D-printed ABS | See Figure 2 | 1 | |
| Screen mesh | Plastic | 18 × 16 [a] | Diameter: 10.16 cm (4 in) | 4 |
| Sidewall ring | Polycarbonate | i.d.: 6.35 cm (2.5 in) | o.d.: 8.89 cm (3.5 in) | 1 |
| Funnel coupling | Rubber | i.d.: 3.81 cm (1.5 in) to 7.62 cm (3 in) | 1 | |
| Coupling | i.d.: 7.62 cm (3 in) | 1 | ||
| Part Name | Material | Sizing Specifications | Quantity | |
|---|---|---|---|---|
| Lower leg | PVC | o.d.: 1.27 cm (0.5 in) | Length: 31.115 cm (12.25 in) | 4 |
| Upper leg | Length: 7.62 cm (3 in) | 4 | ||
| Horizontal support | Length: 8.89 cm (3.5 in) | 9 | ||
| Tee connector | i.d.:1.27 cm (0.5 in) | 4 | ||
| 90° elbow connector | 2 | |||
| 3-way connector | 6 | |||
| Hose clamp | Stainless steel | Diameter: 7.62 cm (3 in) | 2 | |
| Zip ties | Plastic | Length: 15.24 cm (6 in) | 6 | |
| Part Name | Material | Sizing Specifications | Quantity | |
|---|---|---|---|---|
| Piping | PVC | i.d.: 1.27 cm (0.5 in) | Length: 6.35 cm (2.5 in) | 3 |
| 90° elbow connector | PVC | i.d.: 1.27 cm (0.5 in) | 1 | |
| Tube end cap | PVC | i.d.: 1.27 cm (0.5 in) | 1 | |
| ACF Greenhouses [a] swivel hose connector | PVC | 1.905 cm (0.75 in) f screw to 1.27 cm (0.5 in) f slip | 1 | |
| ACF Greenhouses misting tee | PVC | i.d.: 1.27 cm (0.5 in) | 1 | |
| ACF Greenhouses poly mist nozzle | Plastic | 1 gph | 1 | |
| Hose clamp | Stainless steel | Diameter: 10.16 cm (4 in) | 1 | |
| Garden hose | Rubber | 1.905 cm (0.75 in) | 1 | |
| Nozzle | Trial | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Ave. | |
| 1 | 69.5 | 69.9 | 70.0 | 70.1 | 69.0 | 70.0 | 70.2 | 69.9 | 69.0 | 69.1 | 69.7 |
| 2 | 75.0 | 75.0 | 75.0 | 74.7 | 75.0 | 74.3 | 74.8 | 75.3 | 74.4 | 74.8 | 74.8 |
| 3 | 67.7 | 68.4 | 68.9 | 69.0 | 68.5 | 67.9 | 67.0 | 68.9 | 68.1 | 68.0 | 68.2 |
| 4 | 72.3 | 73.2 | 73.5 | 73.6 | 73.0 | 72.2 | 73.0 | 73.9 | 72.9 | 72.1 | 73.0 |
| Flow-Reduction Cap Size (in) [a] | Trial 1 | Trial 2 | Trial 3 | Ave |
|---|---|---|---|---|
| 0.15 | 1.5 | 1.55 | 1.6 | 1.55 |
| 0.20 | 2.35 | 2.2 | 2.1 | 2.22 |
| 0.25 | 3.2 | 3.1 | 2.9 | 3.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, M.R.; Onstad, N.H.; Green, M.L.; Witola, W.H.; Davidson, P.C. System to Evaluate Movement of Biological Contaminants in Soil. Environments 2019, 6, 33. https://doi.org/10.3390/environments6030033
Miller MR, Onstad NH, Green ML, Witola WH, Davidson PC. System to Evaluate Movement of Biological Contaminants in Soil. Environments. 2019; 6(3):33. https://doi.org/10.3390/environments6030033
Chicago/Turabian StyleMiller, Matthew R., Nora H. Onstad, Michelle L. Green, William H. Witola, and Paul C. Davidson. 2019. "System to Evaluate Movement of Biological Contaminants in Soil" Environments 6, no. 3: 33. https://doi.org/10.3390/environments6030033
APA StyleMiller, M. R., Onstad, N. H., Green, M. L., Witola, W. H., & Davidson, P. C. (2019). System to Evaluate Movement of Biological Contaminants in Soil. Environments, 6(3), 33. https://doi.org/10.3390/environments6030033






