Abstract
Olive leaves (OLL) represent a major waste generated during the production of olive oil, but there is a great potential for their valorization, because they provide important content in polyphenolic phytochemicals, which possess several bioactivities. In spite of the high number of studies dealing with polyphenol recovery from olive leaves, green processes involving environmentally benign solvents are scarce. In this study, a novel renewable natural low-transition temperature mixture (LTTM), composed of glycerol and sodium-potassium tartrate, was tested for its efficient ability to extract polyphenolic substances from OLL. The extraction process was optimised by using response surface methodology and the maximum yield in total polyphenols was 26.75 ± 3.22 mg caffeic acid equivalents per g dry weight, achieved with 50% (v/v) aqueous LTTM, liquid-to-solid ratio of 45 mL g−1 and at 73 °C. The LTTM was proven to be equally effective with 60% aqueous methanol, but it displayed inferior antioxidant properties. Liquid chromatography-diode array-mass spectrometry analyses revealed no significant qualitative differences between the LTTM and the aqueous methanolic extract.
1. Introduction
It is nowadays regarded central to the emerging bioeconomy that the residual biomass originating from the agricultural and food sector should not be simply treated as waste, but rather as a bioresource with high potential for the production of precious chemicals and fuels. Agro-industrial activity accounts for the generation of a large volume of wastes, including processing residues, such as leaves, branches, peels, roots, stems and seeds. This biomass is usually undervalorized, in spite its richness in a spectrum of high value-added substances. A prominent class of phytochemicals of abundant occurrence in agri-food wastes is polyphenols, encompassing an outstanding diversity of structures [1]. Numerous of these compounds possess biological properties of particular interest, including anti-inflammatory and chemopreventive activities [2] and therefore they are considered to have a significant prospect as bioactive agents in food, pharmaceutical and cosmetics industry.
In this context, Mediterranean countries are facing challenges to deal with problems associated with agri-food wastes, typical to their major crops, such olives. Olive industry wastes that have not been properly treated are a major ecological issue for olive-producing countries, and therefore approaches aiming at valorising olive wastes, primarily those allowing a sustainable recovery of valuable natural components, are gaining acceptance. Olive leaves, a regular olive-processing residue, contain high amounts of polyphenols with pharmacological potency [3]; hence, various extraction methods have been developed for their effective recovery. However, green technologies pertaining to such a process are particularly limited. The use of environmentally friendly extraction techniques may represent innovative opportunities to face such challenge in a sustainable manner.
In the framework of deploying eco-friendly procedures for purposes of solid-liquid extraction of precious chemicals, a new generation of solvents, termed low-transition temperature mixtures (LTTMs) or deep eutectic solvents (DES), appears as a very promising path. These novel materials are composed of non-toxic, natural and renewable substances and have attracting properties not encountered with conventional volatile solvents, such as low vapour pressure, absence of flammability and low cost [4]. Moreover, several LTTMs have been proven to be far more effective extraction means of recovering polyphenols [5,6]. On this ground, this study was carried out to optimise extraction of polyphenols from OLL, assisted by ultrasounds and the variables taken into consideration for the optimisation included LTTM concentration, liquid-to-solid ratio and temperature. Extractions with water and aqueous ethanol were also performed for comparison, because water and ethanol are the most common bio-solvents employed for polyphenol recovery. The extracts obtained were evaluated for antioxidant activity and the principal phenolics were tentatively identified by liquid chromatography-diode array-mass spectrometry.
2. Materials and Methods
2.1. Chemicals
Solvents used for liquid chromatography-mass spectrometry were HPLC grade. Εthanol (99.8%) was from Acros Organics (Geel, Belgium). Anhydrous sodium carbonate was from Carlo Erba Reactifs (Val de Reuil, France). Glycerol (99%) was from Sigma-Aldrich (St. Lois, MO, USA). Aluminium chloride hexahydrate, sodium acetate trihydrate, and sodium-potassium tartrate tetrahydrate (SPT) were from Penta (Prague, Czeck Republic). 2,4,6-Tripyridyl-s-triazine (TPTZ, 99%) was from Fluka (Steinheim, Germany). 2,2-Diphenyl-1-picrylhydrazyl radical (DPPH) was from Aldrich (Steinheim, Germany).
2.2. LTTM Synthesis
Amount of 138.14 g glycerol (1.5 mol) was mixed with 60.45 g SPT (0.214 mol) and 13.5 g distilled water (0.75 mol) in glass vial to give a molar ratio of 7:1:2. The mixture was heated at 60–70 °C for approximately 60 min, under continuous stirring at 700 rpm and the perfectly transparent, moderately viscous, pale brown liquid formed was left to cool down to ambient temperature. Appropriate amounts of this LTTM were then used to make up aqueous solutions with concentrations 50–80% (w/v). These solutions were used for the extractions, as dictated by the experimental design (Table 1).

Table 1.
Experimental values and coded levels of the independent variables used for the 23 full-factorial design.
2.3. Olive Leaves (OLL)
Analytical information regarding olive variety and method of collection of OLL has been reported elsewhere [7]. The OLL collected were pooled and dried, and the dried material was sieved to give a powder with approximate average particle diameter of 0.3 mm. The material was placed in screw-cap plastic tubes and stored in a dry and dark chamber.
2.4. Extraction Procedure
Appropriate amount of OLL was transferred in a 15 mL screw-cap tube and 10 mL of solvent was added. The mixture was extracted for 60 min in a temperature-controlled sonication bath (Elma P70, Singen, Germany) operated in pulse mode. Sonication was carried out at a frequency of 37 kHz, ultrasonic power of 140 W and acoustic energy density of 35 W L−1.
2.5. Sample Preparation and Determinations
Following extraction, an aliquot of 1 mL of each sample was placed in a 1.5 mL Eppendorf tube and centrifuged in a table centrifugator (Hermle, Wehingen, Germany) for 10 min, at 10,000× g. A suitable volume of the clear supernatant was diluted 1:20 with methanol prior to determinations. Total polyphenol yield (YTP) was determined with the Folin-Ciocalteu methodology [8] and results were expressed as mg caffeic acid equivalents (CAE) per g of dry weight. Total flavonoid yield (YTFn) was assayed with AlCl3 reagent, as previously reported [9] and given as mg rutin equivalents (RtE) per g of dry weight. The antioxidant activity was estimated by determining the antiradical activity (AAR) and the ferric-reducing power (PR) of the extracts. Both tests were carried out using published protocols [8].
2.6. Experimental Design
A 23-full factorial design was used, as described previously [10], with YTP as the response. The three independent variables considered were CLTTM (X1, varying between 50 and 80%, w/v), RL/S (X2, varying between 15 and 45 mL g−1) and T (X3, varying between 50 and 80 °C). Each variable was coded at three levels, −1, 0 and 1 (Table 1), according to the following equation:
where xi and Xi are the dimensionless and the actual value of the independent variable i, X0 the actual value of the independent variable i at the central point, and ΔXi the step change of Xi corresponding to a unit variation of the dimensionless value. Data from the experimental design were subjected to regression analysis using least square regression methodology to obtain the parameters of the mathematical models. Analysis of variance (ANOVA) was used to assess the significance of the individual terms and the model. Data for each design point were recorded and 3D plots were obtained using the fitted model.
2.7. Qualitative Liquid Chromatography-Diode Array-Mass Spectrometry (LC-DAD-MS)
A previously described methodology [11] was employed to tentatively characterise the principal polyphenolic metabolites, with some modifications. Briefly, the equipment used was a Finnigan MAT Spectra System P4000 pump, coupled with a UV6000LP diode array detector and a Finnigan AQA mass spectrometer. A Fortis RP-18 column, 150 × 2.1 mm, 3 µm, was used, at 40 °C. Analyses were performed with electrospray ionization (ESI) in both positive and negative ion mode, with acquisition set at 50 eV, capillary voltage 4 kV, source voltage 25 V, detector voltage 650 V and probe temperature 350 °C. Eluent (A) and eluent (B) were 1% acetic acid and methanol, respectively. The flow rate was 0.2 mL min−1, and the elution programme used was as follows: 0–2 min, 10% B; 2–40 min, 100% B; 45 min, 100% B.
2.8. Statistics
All extractions were carried out twice and all determinations in triplicate. Values reported are averages. Response surface design and associated statistics, as well as value distribution were performed with JMP™ 10, at least at a 95% significance level (p < 0.05).
3. Results and Discussion
3.1. LTTM Synthesis
In a previous study, an LTTM composed of glycerol, SPT and water at a molar ratio of 5:1:4 was tested for its efficiency in extracting polyphenolic antioxidants from various agri-food wastes, including OLL [5]. The YTP from OLL achieved with this particular solvent was lower compared to those obtained with other glycerol-based LTTMs, yet for the extraction of materials such as onion solid wastes, the solvent performed satisfactorily. Given the nature of the solvent, which is composed of inexpensive and fully food-compatible materials (glycerol and SPT), it was deemed that it would merit a profounder investigation as a food-grade solvent with a prospect in processes pertaining to the extraction of natural antioxidants.
In this framework, improvements were first accomplished with the aim of tailoring its composition. Considering the polarity of OLL polyphenols (sparingly water-soluble), the percentage of glycerol was increased whereas that of water was minimised, maintaining the appropriate molar ratio that would provide LTTM stability. Thus, following preliminary experimentation, the molar proportion that yielded a stable LTTM was found to be glycerol:SPT:water 7:1:3. Lower glycerol ratio did not result in sufficient HBD:HBA interaction, so SPT was not fully melted, even after prolonged heating (>2 h). On the other hand, water ratio < 3 gave LTTM that tended to crystalize upon cooling down to ambient temperature. This was presumably because the HBD groups, as well as spatial arrangement and number could affect LTTM stability. Water molecules could contribute in this regard, facilitating hydrogen bond formation and enhancing their stability [12]. The LTTM synthesised as described above was stable for several weeks at room temperature and it has not been previously reported.
3.2. Process Optimisation
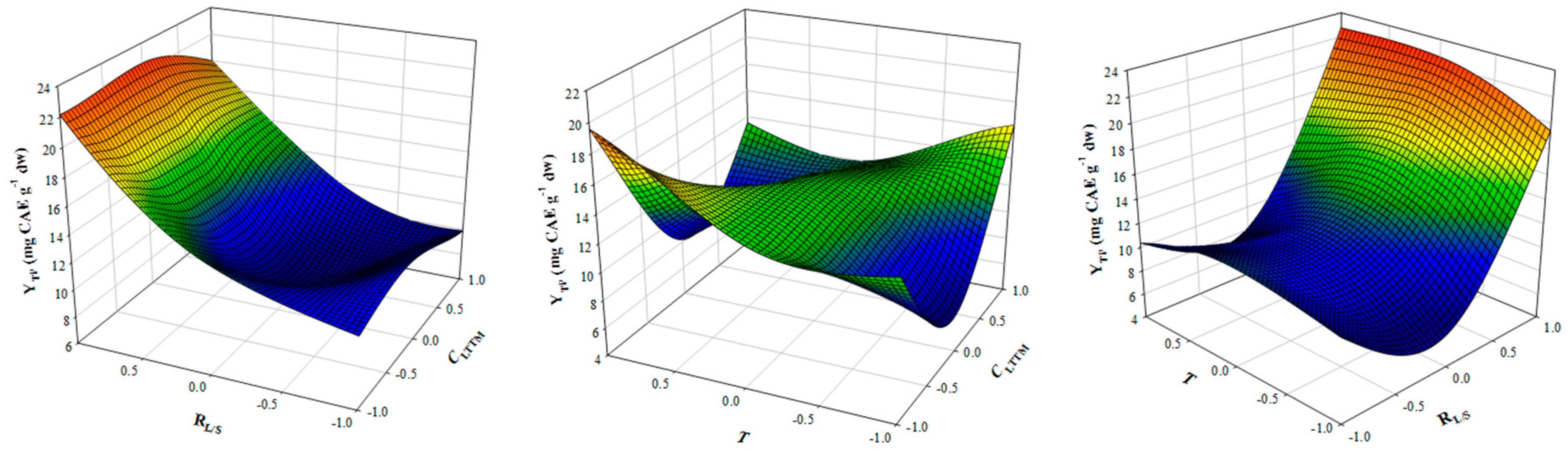
The next step in optimising polyphenol extraction from OLL was the regulation of critical conditions that may affect extraction yield, including the concentration of the LTTM in an aqueous solution (CLTTM), the liquid-to-solid ratio (RL/S) and temperature (T). To do so, a 23-full factorial design was deployed, with two central points (points # 15 and 16, Table 2). This enabled the recording of the response (YTP) upon simultaneous variation of all three independent variables (CLTTM, RL/S and T) (Figure 1). Assessment of term contribution by performing ANOVA showed that T (X3), CLTTM·RL/S (X1·X2), RL/S·T (X2·X3) and () were non-significant (p < 0.05). Hence by omitting the non-significant terms, the following model was obtained:

Table 2.
Measured and predicted values of YTP, determined for individual design points of the experimental design.
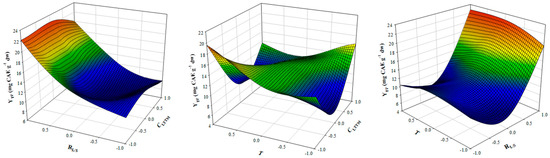
Figure 1.
3D plot displaying the effect of simultaneous variation of CLTTM, RL/S and T on YTP. OLL Extractions were carried out under continuous stirring at 600 rpm, for 60 min.
With the use of the desirability function, the recommended settings to achieve the highest YTP were estimated to be CLTTM = 50% (w/v), RL/S = 45 mL g−1 and T = 73 °C. Under these conditions, the maximum predicted YTP was 26.75 ± 3.22 mg CAE g−1 dw.
The optimal YTP determined matched exactly the one previously obtained (27.68 ± 1.66 mg CAE g−1 dw) [5], demonstrating that the changes brought about to the composition of the solvent, did not compromise extraction yield. Instead, critical evaluation of the optimal conditions found through the process optimisation revealed that:
- CLTTM was reduced from 80% (w/v) to 50% (w/v); this fact illustrated that with significantly lower amount of solvent, the same yield can be achieved.
- RL/S was reduced from 100 to 45 mL g−1, which demonstrated that a lower volume of solvent would suffice to obtain the same yield, resulting in even less solvent requirements.
- T was reduced from 80 to 73 °C, hence making the whole process less energy-demanding and therefore more cost-effective and environmentally friendly.
- The resident time was 60 min instead of 90 min, and this was another improvement with regard to the points mentioned in 3.
With respect to CLTTM, it has been suggested that the amount of water required depends on the polarity of the solute (polyphenols), with flavonoid glycosides, such as rutin, being more soluble in LTTMs with a higher water proportion, compared with its aglycone quercetin [12]. On such a ground, it has been supported that the extraction of more polar compounds may require LTTMs with higher water analogy [13]. Additionally, as many LTTMs are rather quite viscous, appropriate viscosity tuning may be achieved through water incorporation, to facilitate extraction efficiency [14]. On the other hand, water content as high as 63.8% (v/v) has been used in glycerol-based LTTMs for the extraction of polyphenolic antioxidants [15]. However, water proportion exceeding 50% may involve danger of LTTM disintegration and loss of their peculiar properties [16].
Regarding RL/S, the optimum level of 45 mL g−1 found was higher than those used in other cases of polyphenol extraction with various LTTMs, which ranged between 10 and 20 mL g−1 [17,18,19]. Yet, RL/S of 36.2 mL g−1 has also been reported [15]. It should be stressed that optima RL/S determined for polyphenol extraction with conventional solvents may reach up to 100–120 mL g−1 [20,21], but such high values have never been predicted for polyphenol extraction with LTTMs. As pointed out earlier, a minimum RL/S is always required for sufficient mixing, which would allow for attaining appropriate diffusivity and hence increased extraction yield. If RL/S is low, then the various equilibria established may hinder mass transfer [22]. It appears that adequate mass transfer can be achieved in LTTMs at lower RL/S, thus turning extraction of polyphenols into a less solvent-demanding process.
Likewise, in the limited number of available studies dealing with polyphenol extraction with LTTMs, the effect of T, which plays central role in an extraction process, appears to be stronger at relatively moderate levels, varying from 60 to 80 °C [17,18,19], whereas higher T have not been tested. The optimal value determined in this study was 73 °C, which fell within such a range. Recently, it was shown that yield of polyphenol extraction from red grape pomace dropped sharply by switching T from 60 to 70 °C, to recover partly at 80 °C [23]. The effect of T was studied more thoroughly in another recent examination, where an apparent anti-Arrhenius behaviour was revealed [15]. To explain this phenomenon, it was supported that increasing T beyond a certain limit, there might be decomposition of the LTTM, which would lead to weakening of their capacity to solubilise the solute. It would appear that as the temperature rises, the energy provided to the system is sufficient to initiate disintegration of the LTTM through thermally-induced hydrogen bond rupture.
3.3. Assessment of Model Validity and Extraction Efficiency
To ascertain the validity and hence the applicability of the model built via the response surface methodology, extraction was performed under optimal conditions and afforded 30.09 ± 2.71 mg CAE g−1 dw. This value fell within the limits of statistical error, as predicted for the maximum value determined by the model (26.75 ± 3.22 mg CAE g−1 dw). Additionally, to set the efficiency of the LTTM on a comparative basis with other eco-friendly conventional solvents, extractions were carried out under the optimised conditions, and the extracts were analysed for YTP, YTFn, AAR and PR. The solvents used were 60% (v/v) ethanol [7], 9% (w/v) glycerol [24] and water, while 60% methanol served as control solvent.
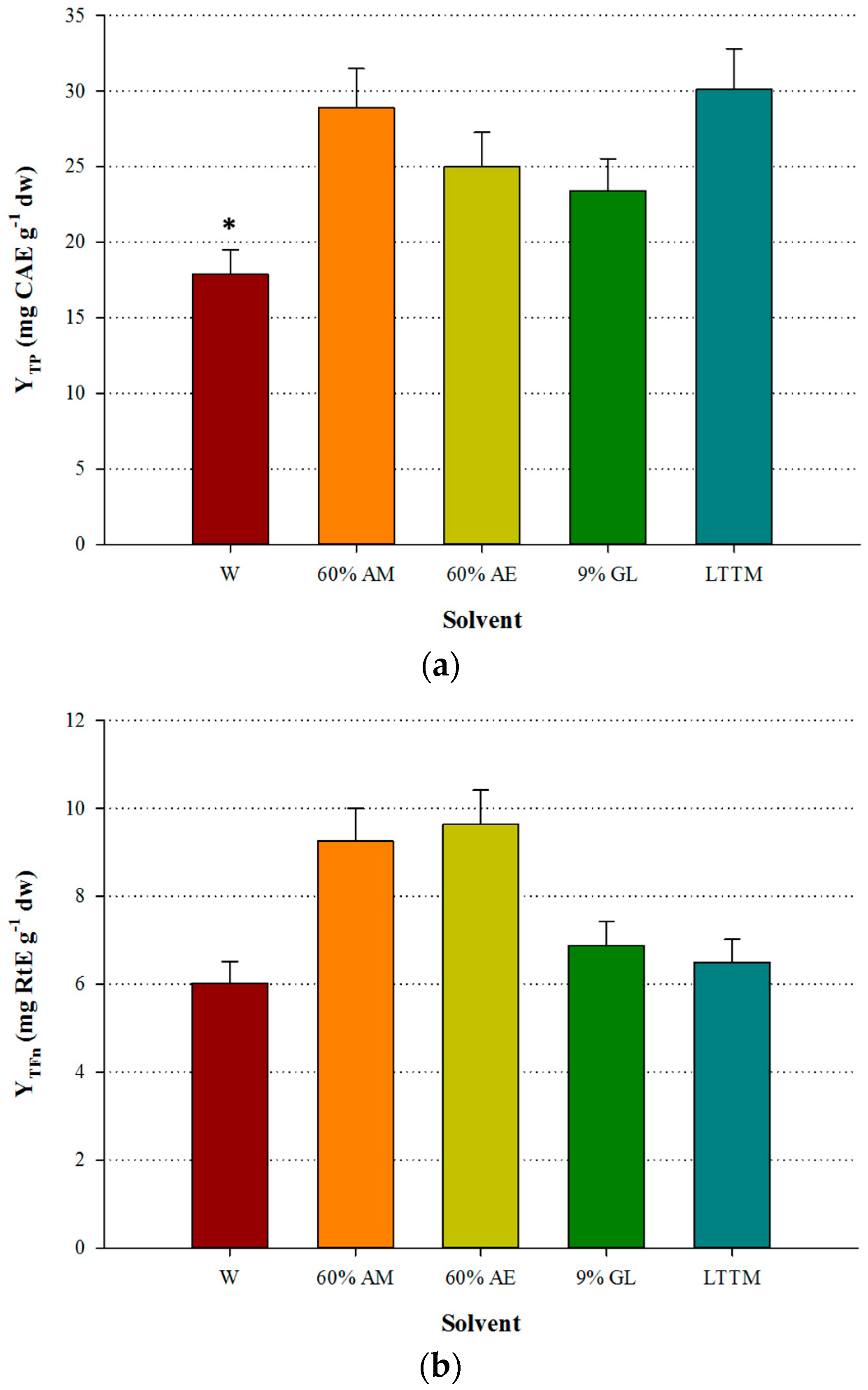
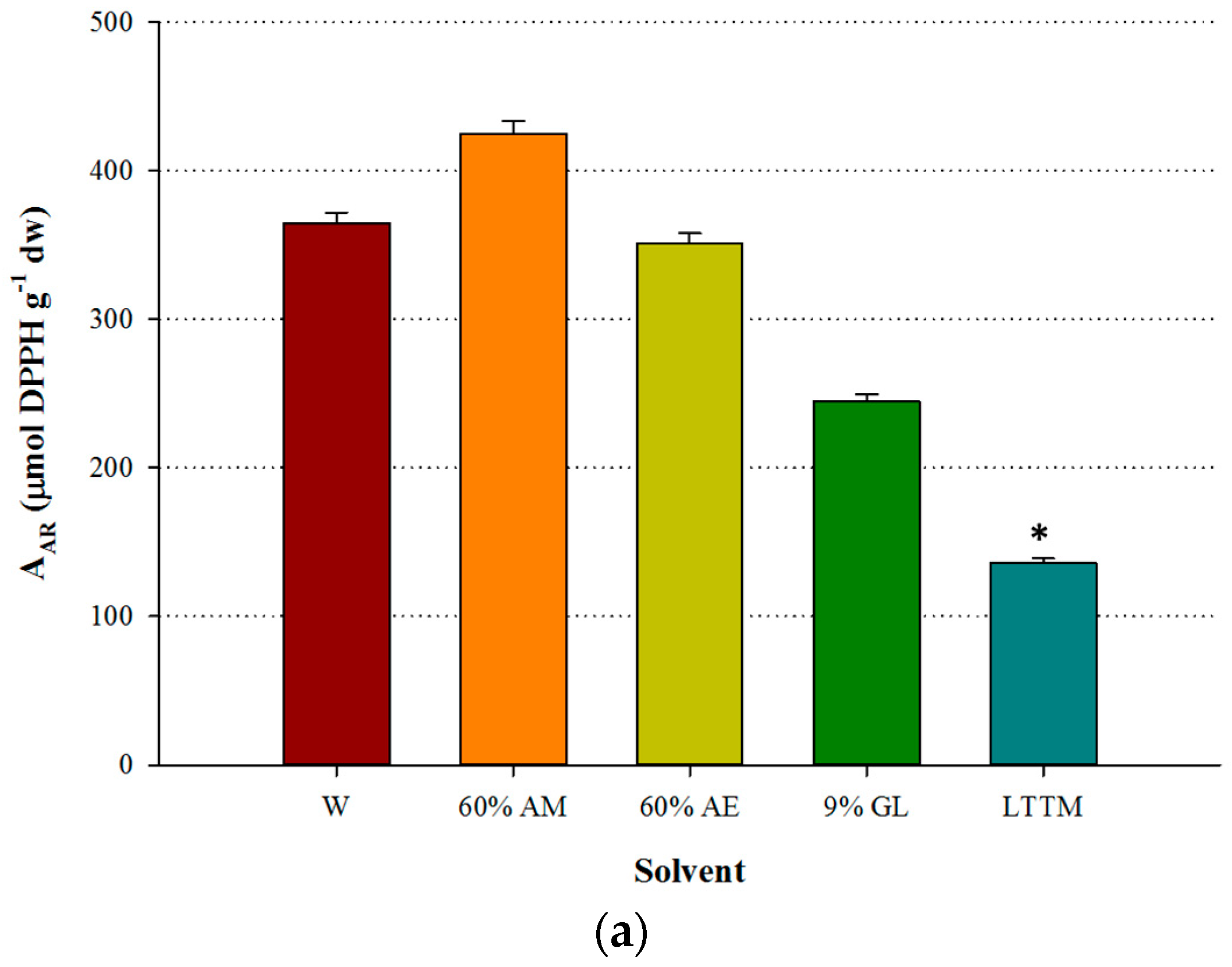
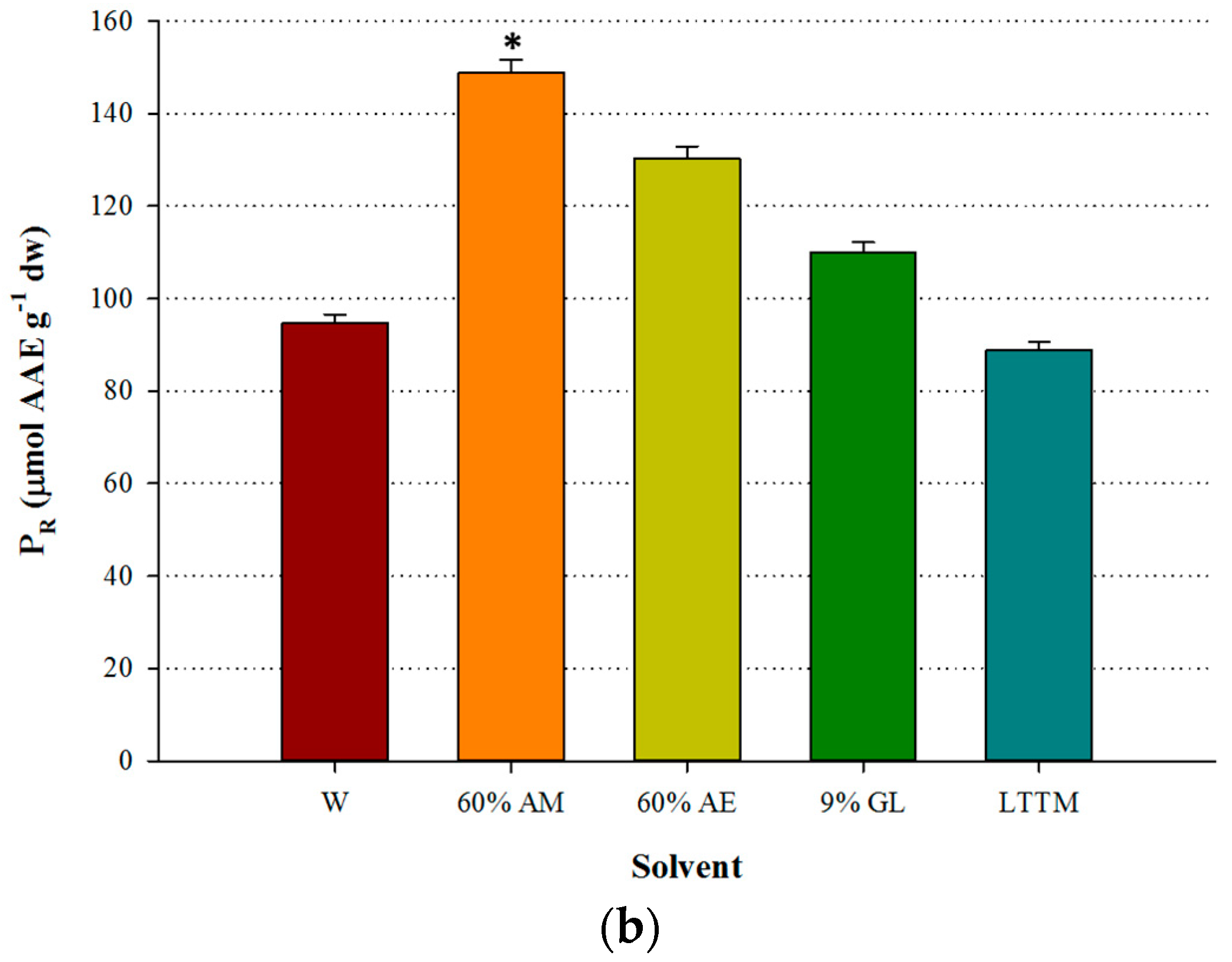
The LTTM was proven to be equally efficient with aqueous methanol, giving by 4% higher YTP (p > 0.05). However, it was significantly more efficient than water, as the difference in YTP was by 40.6% higher (p < 0.05) (Figure 2a). Aqueous ethanol provided higher YTFn compared with the LTTM, which gave YTFn values comparable to those of aqueous glycerol and water (Figure 2b), yet in this case no statistical differences were detected (p > 0.05). On the contrary, the extract obtained with the LTTM displayed significantly lower AAR and PR (p < 0.05), compared with the aqueous methanolic extract (Figure 3). This finding contrasted a general trend observed in studies on LTTM extracts, where increased polyphenol levels were associated with proportional antioxidant activity [5,9,23], but such a phenomenon has been previously seen in OLL extracts, with correlation between YTP and AAR being low and statistically non-significant [7]. In concurrence with this, a more recent study showed that high YTP did not fully coincide with high AAR in OLL extracts [25].
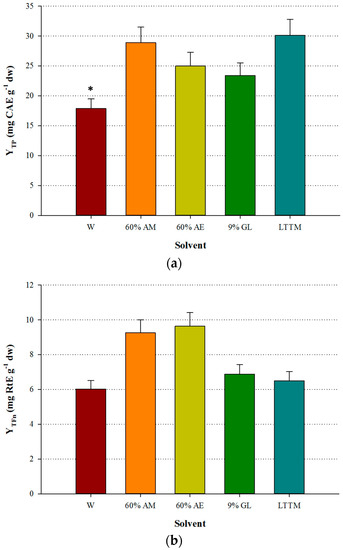
Figure 2.
Comparative diagram for YTP (a) and YTFn (b), obtained using 50% (v/v) LTTM, 60% aqueous methanol (AM), 60% aqueous ethanol (AE), 9% (w/v) aqueous glycerol and distilled water (W). Extractions were carried out at 73 °C, at RL/S = 45 mL g−1, under continuous stirring at 600 rpm, for 60 min. Asterisk (*) denotes statistically different value (p < 0.05).
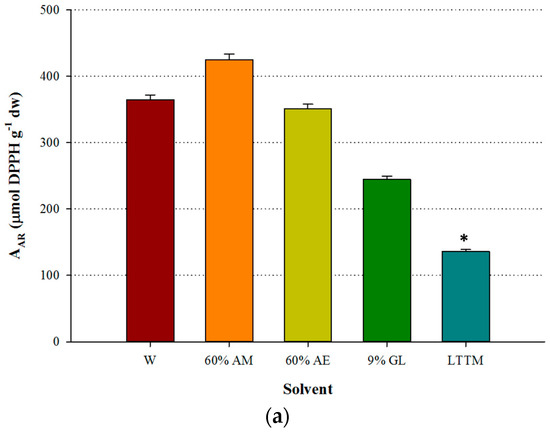
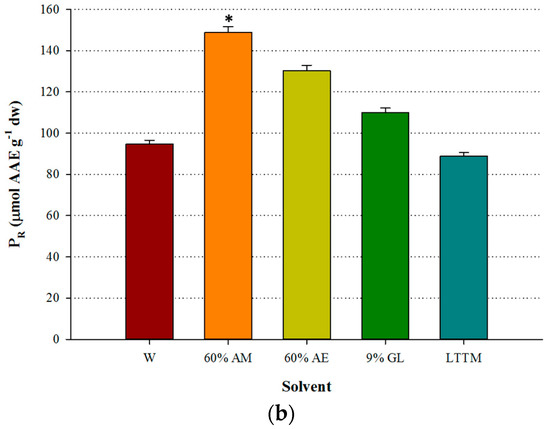
Figure 3.
Comparative diagram for AAR (a) and PR (b), obtained using 50% (v/v) LTTM, 60% aqueous methanol (AM), 60% aqueous ethanol (AE), 9% (w/v) aqueous glycerol and distilled water (W). Extractions were carried out at 73 °C, at RL/S = 45 mL g−1, under continuous stirring at 600 rpm, for 60 min. Asterisk (*) denotes statistically different value (p < 0.05).
The antioxidant activity measured in OLL extracts has been suggested to be the outcome of interactions amongst the various polyphenolic substances [26], a claim further supported by studies that assessed the antiradical behaviour of mixtures of pure antioxidants and extracts [27]. The discrepancy observed regarding the weak antioxidant effects of the LTTM extract, in spite its high polyphenol concentration, may be attributed to differences in the amount of flavonoids and other phenolics, major representative of which is oleuropein. This might be manifested because it was found that the AAR exhibited stronger correlation with the flavonoid content, rather than total polyphenols [28]. On such a ground and taking into account that the LTTM extracts had lower total flavonoid concentration, it might be reasonable that they also had lower levels of both AAR and PR. Similar findings have been previously reported [29]. In any case, it could be speculated that the differences in the antioxidant potency seen between the LTTM and the aqueous methanolic extract, might reflect differences in the relative amounts of the polyphenolic antioxidants, as well as phenomena of synergism and antagonism [27]. The latter aspect is strongly supported by the fact that OLL may behave as a more powerful antioxidant than its individual polyphenols [30].
3.4. Tentative Characterisation of Extract Composition
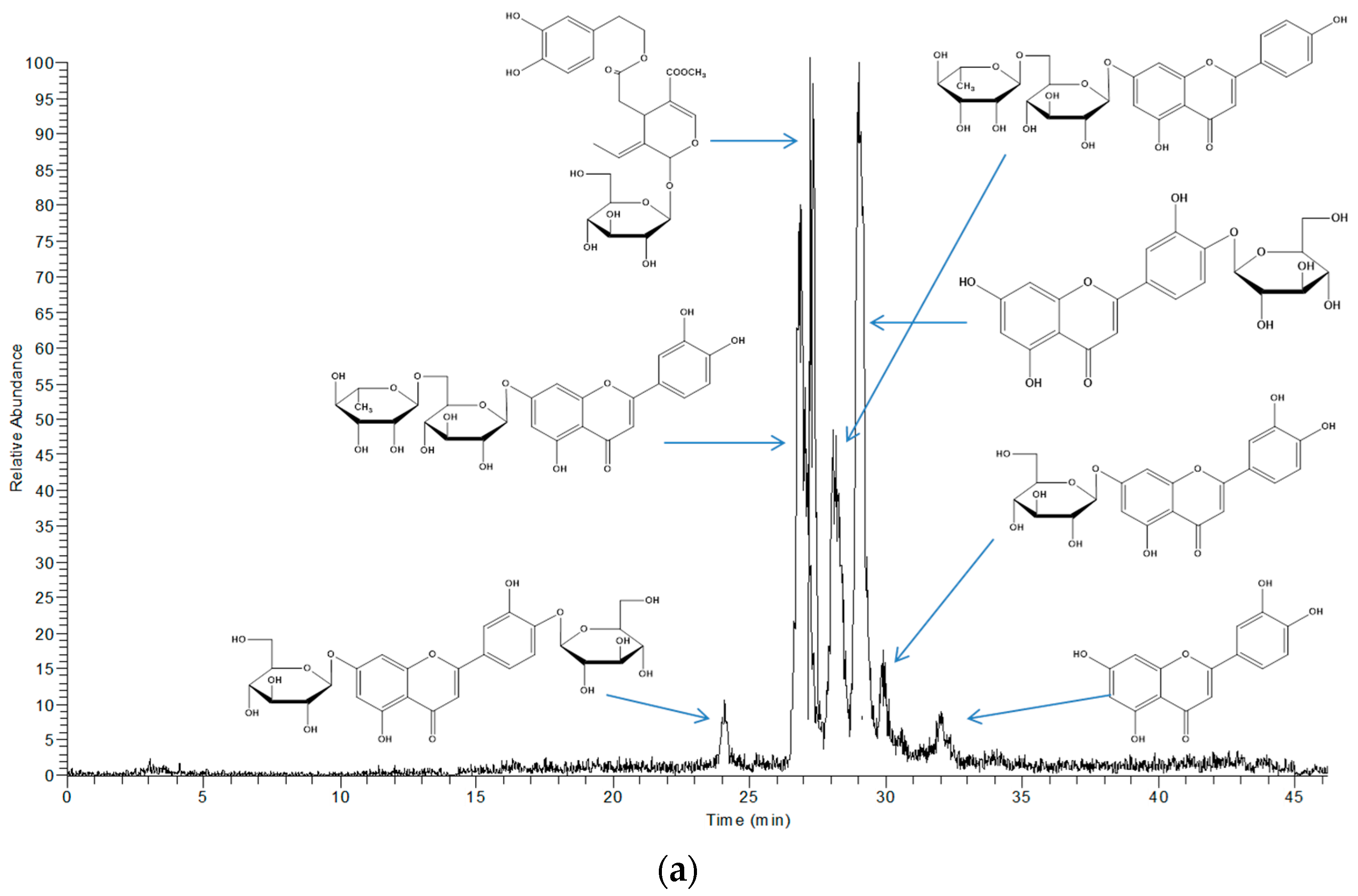
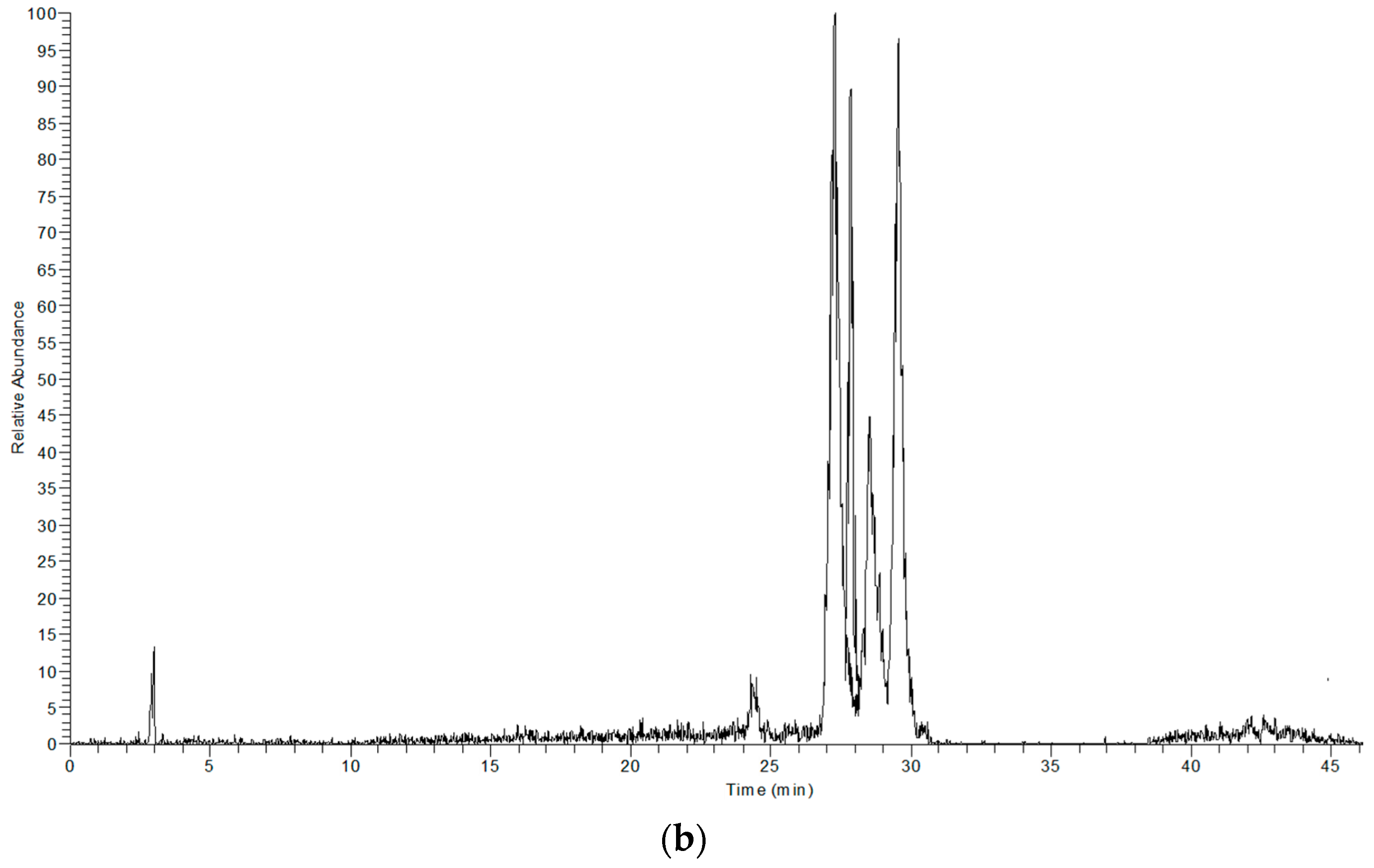
The extracts obtained with LTTM and 60% (v/v) methanol were analysed by LC-DAD-MS to identify possible qualitative differences in their polyphenolic profile, which could be indication of selectivity. Based on mass spectral and UV-vis characteristics, the principal phytochemicals tentatively identified were luteolin and four glycosides thereof, along with an apigenin glycoside and oleuropein (Table 3). These constituents are regularly detected in OLL and represent major polyphenolic metabolites [31]. In Figure 4, it is given the total ion current of the 60% (v/v) methanolic extract (upper chromatogram) and the LTTM extract (lower chromatogram). As can be seen, there were no major differences with respect to the main peaks detected, with the exception of luteolin aglycone, which appeared only in the aqueous methanolic extract. This was probably due to lower polarity of the aglycone, which would endow it with higher solubility in aqueous methanol, rather than the more polar LTTM. On the other hand, its presence might be an indication of luteolin glycoside hydrolysis in aqueous methanol.

Table 3.
UV-vis and mass spectrometric data of the principal polyphenols detected in the OLL extracts, obtained with 50% (v/v) LTTM, at 73 °C.
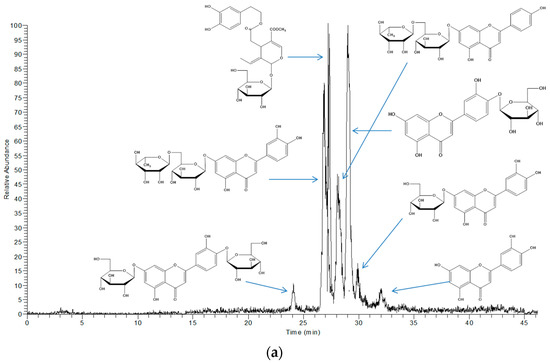
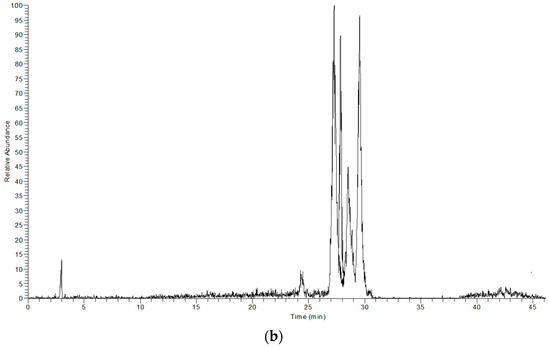
Figure 4.
Total ion chromatograms showing the principal polyphenols detected in 60% (v/v) aqueous methanol (a) and 50% (v/v) aqueous LTTM (b).
4. Conclusions
The newly launched LTTM tested in this study was demonstrated to be equally efficient with 60% aqueous methanol, in extracting polyphenolic compounds from OLL. On the other hand, it was evidenced that it extracted relatively lower amounts of flavonoids, a fact that might negatively impact the antioxidant properties of the extracts obtained, as indicated by comparison with the aqueous methanolic extract. However, the chromatographic profiling of both extracts did not show significant qualitative differences in the polyphenolic composition, and therefore the differences in the antioxidant potency might arise from differences in the relative amounts of the polyphenolic antioxidants occurring in the extracts. Given the exceptionally benign nature of the LTTM used, which is composed of non-toxic and edible substances, it would be recommended that its potency as an effective means of recovering natural antioxidants merits profounder investigation.
Acknowledgments
The Department of Food Science & Nutrition (University of the Aegean) and the Food Quality & Chemistry of Natural Products Programme are thanked for providing all the means necessary to carry out this study.
Author Contributions
Marianna Dedousi, Valentina Mamoudaki, laboratory experiments; Spyros Grigorakis, liquid chromatography-mass spectrometry; Dimitris P. Makris, experimental design, data handling, statistics, manuscript editing.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AAR | antiradical activity (μmol DPPH g−1) |
| PR | reducing power (μmol AAE g−1) |
| RL/S | liquid-to-solid ratio (mL g−1) |
| T | temperature (°C) |
| YTFn | yield in total flavonoids (mg RtE g−1) |
| YTP | yield in total polyphenols (mg GAE g−1) |
Abbreviations
| AAE | ascorbic acid equivalents |
| CAE | caffeic acid equivalents |
| DPPH | 2,2-diphenyl-picrylhydrazyl radical |
| dw | dry weight |
| HBA | hydrogen bond acceptor |
| HBD | hydrogen bond donor |
| LTTM | low-transition temperature mixture |
| RtE | rutin (quercetin 3-O-rutinoside) equivalents |
| SPT | sodium-potassium tartrate |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
References
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Mojzer, B.E.; Knez, H.M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Prenzler, P.D.; Omar, S.H.; Ismael, R.; Servili, M.; Esposto, S.; Taticchi, A.; Selvaggini, R.; Urbani, S. Pharmacology of olive biophenols. Adv. Mol. Toxicol. 2012, 6, 195–242. [Google Scholar]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Ang. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel glycerol-based natural eutectic mixtures and their efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from agri-food waste biomass. Waste Biomass Valoriz. 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Kottaras, P.; Koulianos, M.; Makris, D.P. Low-Transition temperature mixtures (LTTMs) made of bioorganic molecules: Enhanced extraction of antioxidant phenolics from industrial cereal solid wastes. Recycling 2017, 2, 3. [Google Scholar] [CrossRef]
- Mylonaki, S.; Kiassos, E.; Makris, D.P.; Kefalas, P. Optimisation of the extraction of olive (Olea europaea) leaf phenolics using water/ethanol-based solvent systems and response surface methodology. Anal. Bioanal. Chem. 2008, 392, 977. [Google Scholar] [CrossRef] [PubMed]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a green ultrasound-assisted extraction process for potato peel (Solanum tuberosum) polyphenols using bio-solvents and response surface methodology. Biomass Convers. Biorefinery 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Manousaki, A.; Jancheva, M.; Grigorakis, S.; Makris, D.P. Extraction of antioxidant phenolics from Agri-food waste biomass using a newly designed glycerol-based natural low-transition temperature mixture: A comparison with conventional eco-friendly solvents. Recycling 2016, 1, 194. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T.; Kefalas, P. Factorial design optimisation of grape (Vitis vinifera) seed polyphenol extraction. Eur. Food Res. Technol. 2009, 229, 731–742. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K.; Kefalas, P. Characterisation of certain major polyphenolic antioxidants in grape (Vitis vinifera cv. Roditis) stems by liquid chromatography-mass spectrometry. Eur. Food Res. Technol. 2008, 226, 1075–1079. [Google Scholar]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as a new extraction media for phenolic metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.; Tian, M.; Row, K.H. Evaluation of alcohol-based deep eutectic solvent in extraction and determination of flavonoids with response surface methodology optimization. J. Chromatogr. A 2013, 1285, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Jancheva, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimised extraction of antioxidant polyphenols from Satureja thymbra using newly designed glycerol-based natural low-transition temperature mixtures (LTTMs). J. Appl. Res. Med. Aromat. Plants 2016. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Peng, X.; Yao, X.-H.; Wei, Z.-F.; Luo, M.; Wang, W.; Zhao, C.-J.; Fu, Y.-J.; Zu, Y.-G. Deep eutectic solvent-based microwave-assisted extraction of genistin, genistein and apigenin from pigeon pea roots. Sep. Purif. Technol. 2015, 150, 63–72. [Google Scholar] [CrossRef]
- Wei, Z.; Qi, X.; Li, T.; Luo, M.; Wang, W.; Zu, Y.; Fu, Y. Application of natural deep eutectic solvents for extraction and determination of phenolics in Cajanus cajan leaves by ultra performance liquid chromatography. Sep. Purif. Technol. 2015, 149, 237–244. [Google Scholar] [CrossRef]
- Yao, X.-H.; Zhang, D.-Y.; Duan, M.-H.; Cui, Q.; Xu, W.-J.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Fu, Y.-J. Preparation and determination of phenolic compounds from Pyrola incarnata Fisch. with a green polyols based-deep eutectic solvent. Sep. Purif. Technol. 2015, 149, 116–123. [Google Scholar] [CrossRef]
- Blidi, S.; Bikaki, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. A comparative evaluation of bio-solvents for the efficient extraction of polyphenolic phytochemicals: Apple waste peels as a case study. Waste Biomass Valoriz. 2015, 6, 1125–1133. [Google Scholar] [CrossRef]
- Vetal, M.D.; Lade, V.G.; Rathod, V.K. Extraction of ursolic acid from Ocimum sanctum by ultrasound: Process intensification and kinetic studies. Chem. Eng. Proc. Process. Intensif. 2013, 69, 24–30. [Google Scholar] [CrossRef]
- Rakotondramasy-Rabesiaka, L.; Havet, J.-L.; Porte, C.; Fauduet, H. Estimation of effective diffusion and transfer rate during the protopine extraction process from Fumaria officinalis L. Sep. Purif. Technol. 2010, 76, 126–131. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of Natural Sodium Acetate-Based Low-Transition Temperature Mixtures (LTTMs) for Enhanced Extraction of Antioxidants and Pigments from Red Vinification Solid Wastes. Environ. Proc. 2017, 1–13. [Google Scholar] [CrossRef]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimisation and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Sep. Purif. Technol. 2014, 128, 89–95. [Google Scholar] [CrossRef]
- Mourtzinos, I.; Anastasopoulou, E.; Petrou, A.; Grigorakis, S.; Makris, D.; Biliaderis, C.G. Optimization of a green extraction method for the recovery of polyphenols from olive leaf using cyclodextrins and glycerin as co-solvents. J. Food Sci. Technol. 2016, 53, 3939–3947. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef] [PubMed]
- Karvela, E.; Makris, D.P.; Karathanos, V.T. Implementation of response surface methodology to assess the antiradical behaviour in mixtures of ascorbic acid and α-tocopherol with grape (Vitis vinifera) stem extracts. Food Chem. 2012, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Abaza, L.; Youssef, N.B.; Manai, H.; Haddada, F.M.; Methenni, K.; Zarrouk, M. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas. Aceites. 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y.; Lee, J.; Lee, H.-B.; Son, J.-Y.; Park, C.-S.; Shetty, K.; Kim, Y.-C. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour. Technol. 2009, 100, 6107–6113. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Japón-Luján, R.; Capote, F.P.; Marinas, A.; de Castro, M.D.L. Liquid chromatography/triple quadrupole tandem mass spectrometry with multiple reaction monitoring for optimal selection of transitions to evaluate nutraceuticals from olive-tree materials. Rapid Com. Mass Spectrom. 2008, 22, 855–864. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).