Optimization of a Green Extraction/Inclusion Complex Formation Process to Recover Antioxidant Polyphenols from Oak Acorn Husks (Quercus Robur) Using Aqueous 2-Hydroxypropyl-β-Cyclodextrin/Glycerol Mixtures
Abstract
:1. Introduction
2. Method and Materials
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extraction Procedure
2.4. Determination of Total Polyphenol Yield (YTP)
2.5. Determination of the Antiradical Activity (AAR)
2.6. Determination of the Reducing Power (PR)
2.7. Experimental Design
| Independent Variables | Code Units | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| CCD (%, w/v) | X1 | 1 | 7 | 13 |
| Cgl (%, w/v) | X2 | 0 | 30 | 60 |
| T (°C) | X3 | 40 | 60 | 80 |
2.8. Statistical Analysis
3. Results and Discussion
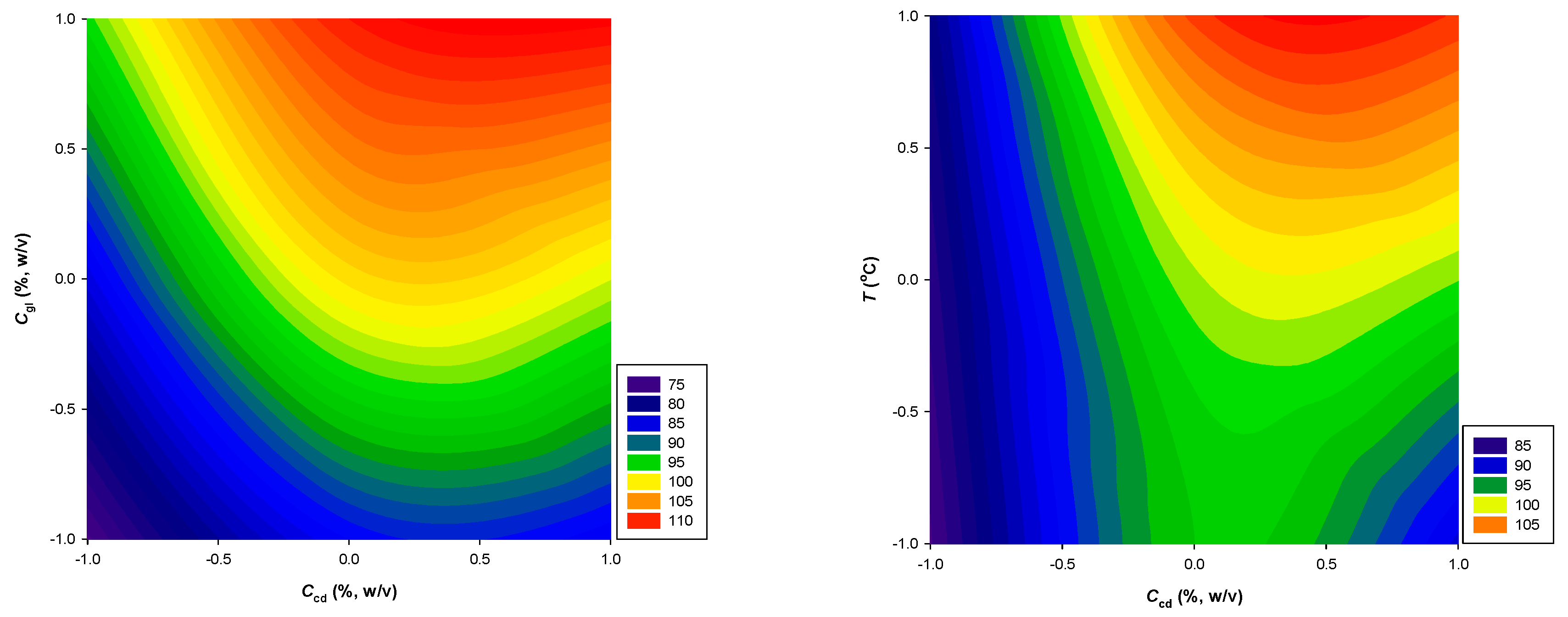
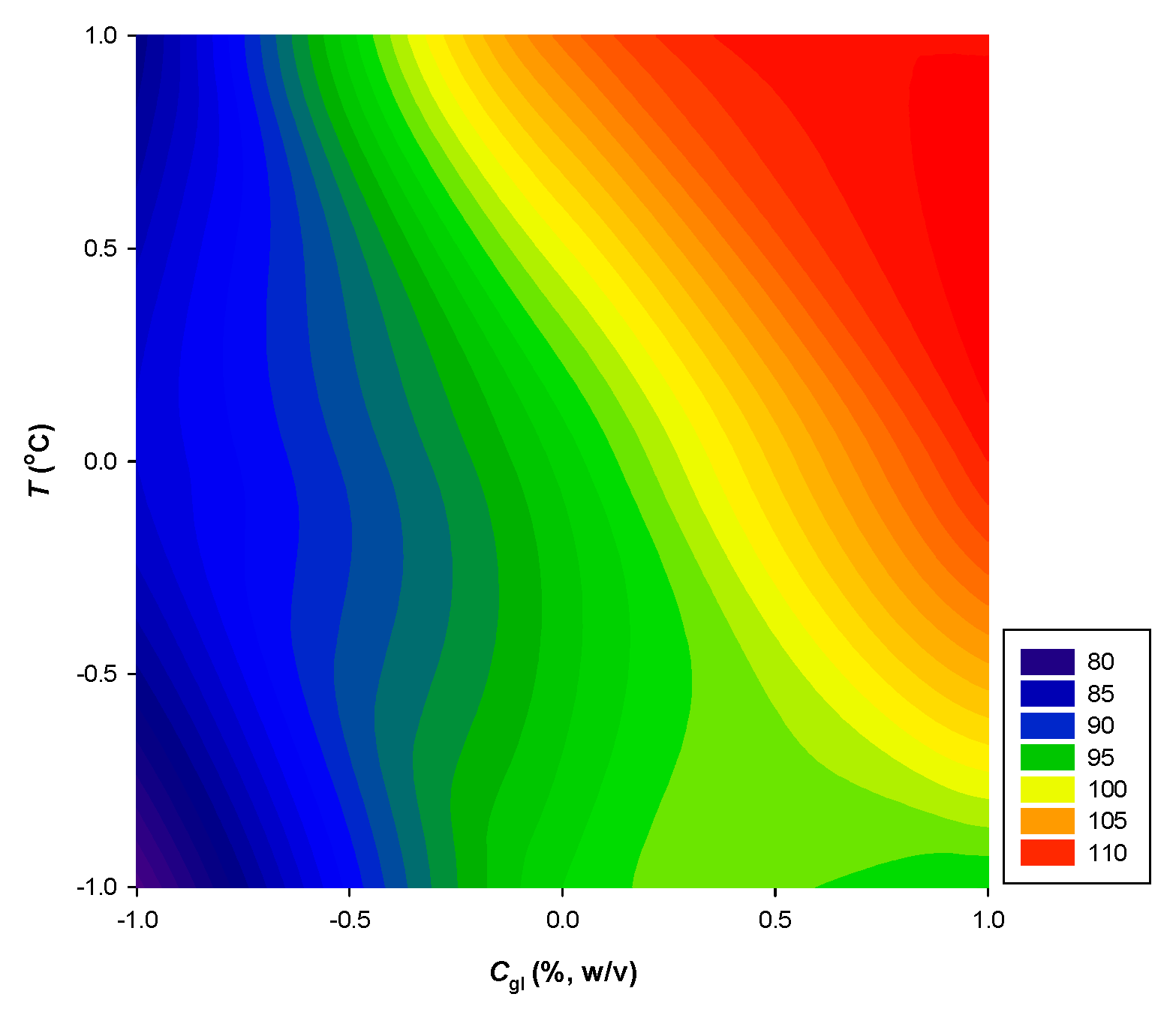
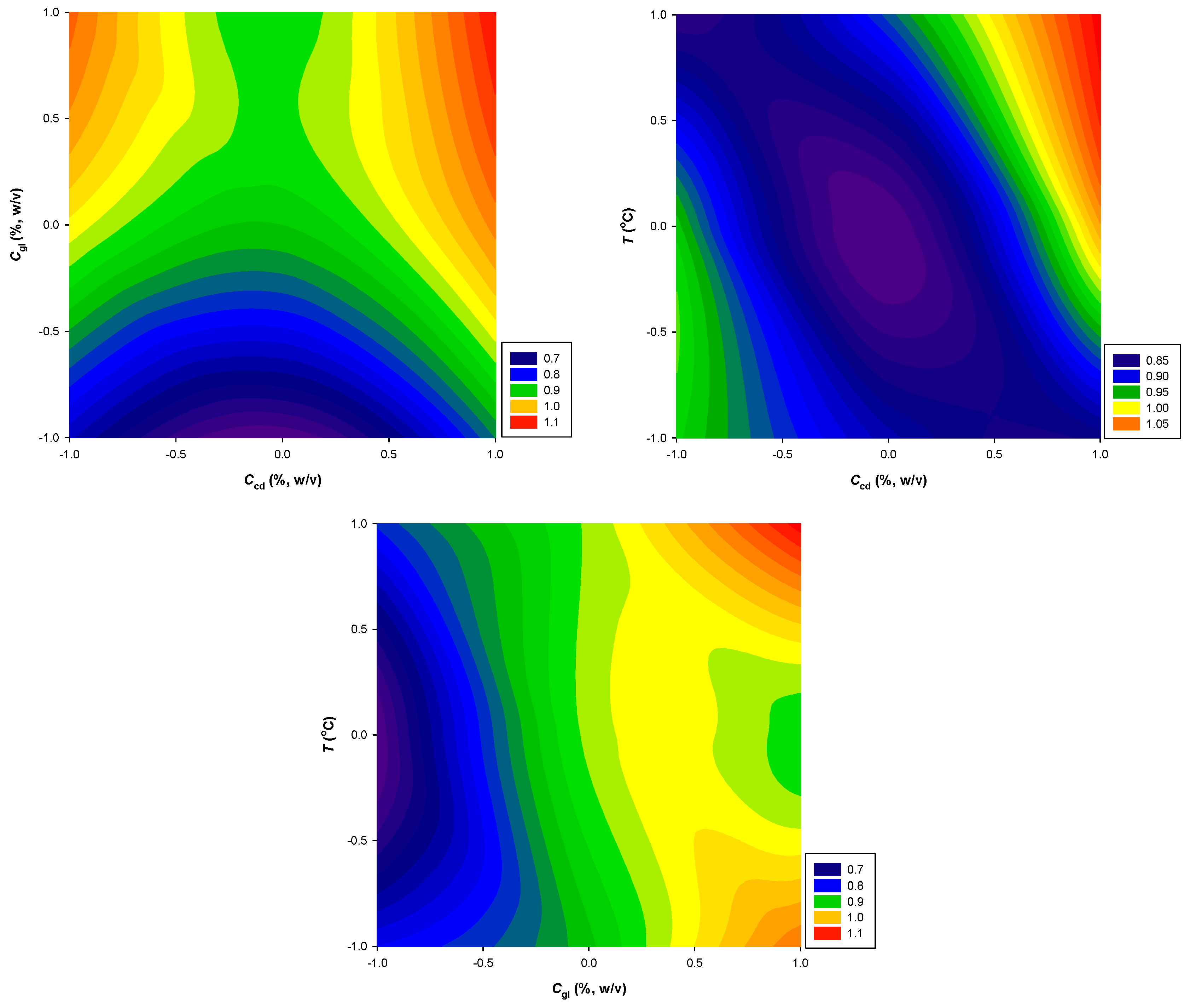
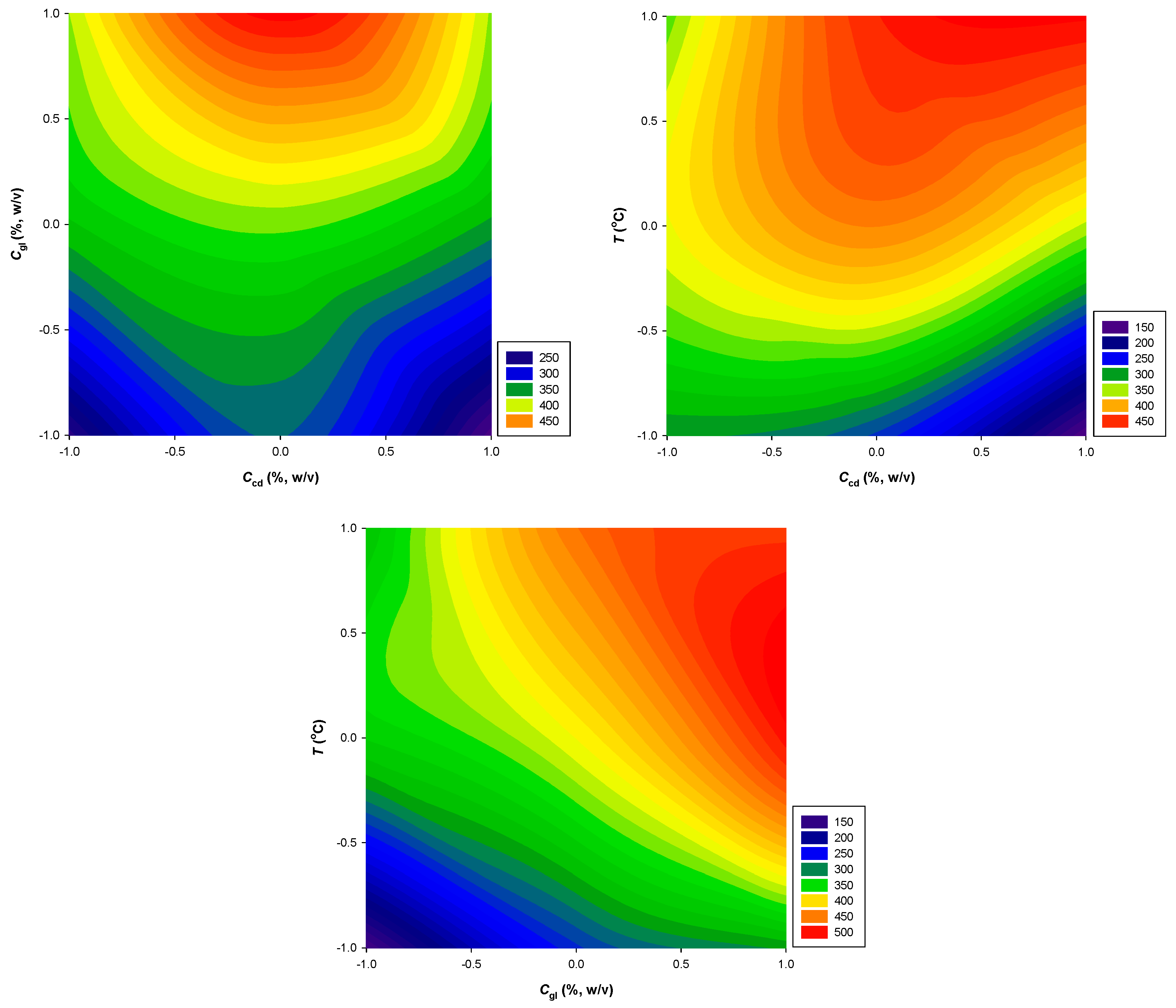
| Response Variables | 2nd Order Polynomial Equations | R 2 | p |
|---|---|---|---|
| YTP (mg GAE g-1·dw) | 99.47 + 6.82X1 + 12.00X2 | 0.95 | 0.0308 |
| AAR (μmol TRE g-1·dw) | 860.4 + 0.14X2 + 0.09X1X3 + 0.13 − 0.1 | 0.96 | 0.0166 |
| PR (μmol AAE g-1·dw) | 408.1 + 81.8X2 + 69.7X1X3 | 0.94 | 0.0418 |
| Design Point | Independent Variables | Responses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| YTP (mg GAE g−1·dw) | AAR (μmol TRE g−1·dw) | PR (μmol AAE g−1·dw) | |||||||
| X1 | X2 | X3 | Measured | Predicted | Measured | Predicted | Measured | Predicted | |
| 1 | −1 | −1 | −1 | 72.73 | 74.21 | 862.8 | 822.9 | 237.5 | 209.4 |
| 2 | −1 | −1 | 1 | 72.73 | 73.21 | 672.8 | 665.4 | 237.5 | 261.2 |
| 3 | −1 | 1 | −1 | 90.91 | 91.41 | 1063.6 | 1101.3 | 377.6 | 373.0 |
| 4 | −1 | 1 | 1 | 100.00 | 99.50 | 986.7 | 995.3 | 457.7 | 406.2 |
| 5 | 1 | −1 | −1 | 77.27 | 78.80 | 746.6 | 736.9 | 482.0 | 491.7 |
| 6 | 1 | −1 | 1 | 90.91 | 91.40 | 981.7 | 942.9 | 377.6 | 379.9 |
| 7 | 1 | 1 | −1 | 100.00 | 100.50 | 966.0 | 971.8 | 257.5 | 231.4 |
| 8 | 1 | 1 | 1 | 122.73 | 122.19 | 1190.9 | 1229.3 | 517.8 | 543.5 |
| 9 | −1 | 0 | 0 | 86.36 | 84.37 | 952.3 | 954.0 | 297.6 | 358.1 |
| 10 | 1 | 0 | 0 | 100.09 | 98.01 | 1023.4 | 1028.0 | 397.7 | 346.7 |
| 11 | 0 | −1 | 0 | 90.45 | 85.98 | 526.4 | 622.8 | 377.6 | 330.7 |
| 12 | 0 | 1 | 0 | 107.00 | 109.98 | 994.6 | 905.2 | 437.7 | 494.3 |
| 13 | 0 | 0 | −1 | 100.00 | 95.98 | 866.5 | 874.1 | 257.5 | 267.1 |
| 14 | 0 | 0 | 1 | 95.45 | 106.33 | 901.2 | 924.1 | 397.7 | 449.0 |
| 15 | 0 | 0 | 0 | 100.00 | 99.47 | 931.3 | 860.4 | 437.7 | 408.1 |
| 16 | 0 | 0 | 0 | 90.91 | 99.47 | 803.9 | 860.4 | 397.7 | 408.1 |
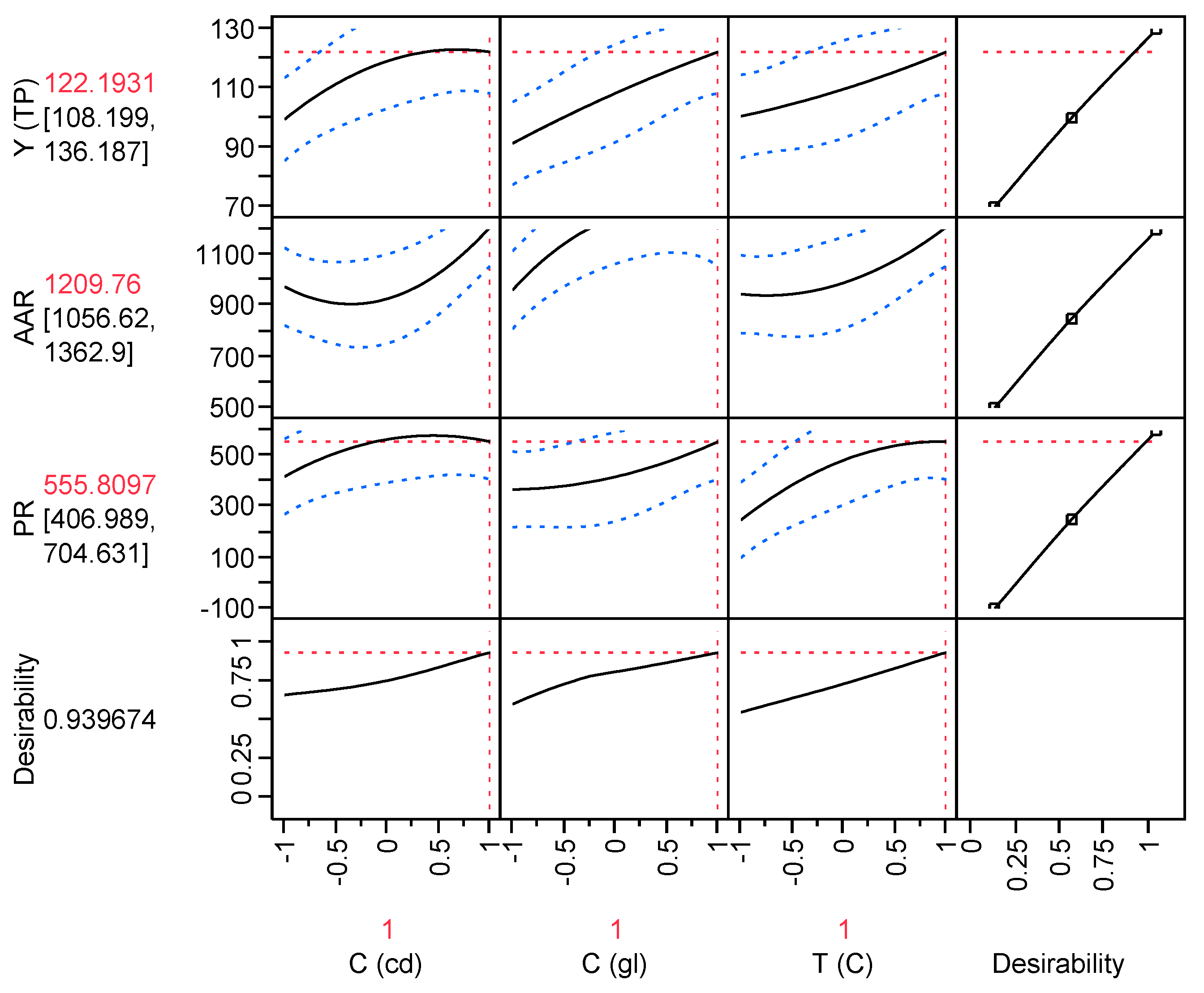
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAR | antiradical activity (μmol TRE g−1·dw) |
| CCD | hydroxypropyl-β-cyclodextrin concentration (%, w/v) |
| Cgl | glycerol concentration (%, w/v) |
| CTP | total polyphenol concentration (mg GAE L−1) |
| PR | reducing power (μmol AAE g−1·dw) |
| T | temperature (°C) |
| YTP | yield in total polyphenols (mg GAE g−1·dw) |
| AAE | ascorbic acid equivalents |
| CD | hydroxypropyl-β-cyclodextrin |
| DPPH | 2,2-diphenyl-picrylhydrazyl |
| GAE | gallic acid equivalents |
| TRE | trolox equivalents |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
References
- Arancon, R.A.D.; Lin, C.S.K.; Chan, K.M.; Kwan, T.H.; Luque, R. Advances on waste valorization: New horizons for a more sustainable society. Energy Sci. Eng. 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrasonics Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Tech. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Pinho, E.; Grootveld, M.; Soares, G.; Henriques, M. Cyclodextrins as encapsulation agents for plant bioactive compounds. Carbohydrate Polym. 2014, 101, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Kuang, S.S.; Oliveira, J.C.; Crean, A.M. Microencapsulation as a tool for incorporating bioactive ingredients into food. Crit. Rev. Food Sci. Nutr. 2010, 50, 951–968. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food Bioproc. Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Blidi, S.; Bikaki, M.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. A Comparative Evaluation of Bio-solvents for the Efficient Extraction of Polyphenolic Phytochemicals: Apple Waste Peels as a Case Study. Waste Biomass Valor. 2015, 6, 1125–1133. [Google Scholar] [CrossRef]
- Shehata, E.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Extraction optimization using water/glycerol for the efficient recovery of polyphenolic antioxidants from two Artemisia species. Separ. Purif. Technol. 2015, 149, 462–469. [Google Scholar] [CrossRef]
- Ofcarcik, R.; Burns, E. Chemical and physical properties of selected acorns. J. Food Sci. 1971, 36, 576–578. [Google Scholar] [CrossRef]
- Tejerina, D.; García-Torres, S.; de Vaca, M.C.; Vázquez, F.; Cava, R. Acorns (Quercus rotundifolia Lam.) and grass as natural sources of antioxidants and fatty acids in the “montanera” feeding of Iberian pig: Intra-and inter-annual variations. Food Chem. 2011, 124, 997–1004. [Google Scholar] [CrossRef]
- Çoruh, N.; Nebigil, C.; Özgökce, F. Rapid and comprehensive separation for the phenolic constituents of Quercus brantii acorns by RP-HPLC-DAD. J. Liquid Chrom. Related Technol. 2014, 37, 907–915. [Google Scholar] [CrossRef]
- Rakić, S.; Petrović, S.; Kukić, J.; Jadranin, M.; Tešević, V.; Povrenović, D.; Šiler-Marinković, S. Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 2007, 104, 830–834. [Google Scholar] [CrossRef]
- Kosseva, M.; Webb, C. Food Industry Wastes: Assessment and Recuperation of Commodities; Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Popović, B.M.; Štajner, D.; Ždero, R.; Orlović, S.; Galić, Z. Antioxidant characterization of oak extracts combining spectrophotometric assays and chemometrics. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Heo, S.-I.; Jung, M.-J.; Wang, M.-H. Antioxidant properties of water extract from acorn. J. Applied Biol. Chem. 2007, 50, 70–73. [Google Scholar]
- Igueld, S.B.; Abidi, H.; Trabelsi-Ayadi, M.; Chérif, J.K. Study of physicochemicals characteristics and antioxidant capacity of cork oak acorns (Quercus suber L.) grown in three regions in Tunisia. J. Applied Pharmaceut. Sci. 2015, 5, 26–32. [Google Scholar] [CrossRef]
- Karimi, A.; Moradi, M.-T. Total Phenolic Compounds and In Vitro Antioxidant Potential of Crude Methanol Extract and the Correspond Fractions of Quercus Brantii L. Acorn. J. Herb. Med. Pharmacol. 2015, 4, 35–39. [Google Scholar]
- Karakashov, B.; Grigorakis, S.; Loupassaki, S.; Makris, D.P. Optimization of polyphenol extraction from Hypericum perforatum (St. John's Wort) using aqueous glycerol and response surface methodology. J. Applied Res. Med. Arom. Plants 2015, 2, 1–8. [Google Scholar]
- Bassil, D.; Makris, D.P.; Kefalas, P. Oxidation of caffeic acid in the presence of L-cysteine: Isolation of 2-S-cysteinylcaffeic acid and evaluation of its antioxidant properties. Food Res. Inter. 2005, 38, 395–402. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Kalogeropoulos, N.; Karathanos, V.T. Deployment of response surface methodology to optimise recovery of grape (Vitis vinifera) stem polyphenols. Talanta 2009, 79, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kiassos, E.; Mylonaki, S.; Makris, D.P.; Kefalas, P. Implementation of response surface methodology to optimise extraction of onion (Allium cepa) solid waste phenolics. Innov. Food Sci. Emerg. Technol. 2009, 10, 246–252. [Google Scholar] [CrossRef]
- Apostolakis, A.; Grigorakis, S.; Makris, D.P. Optimization and comparative kinetics study of polyphenol extraction from olive leaves (Olea europaea) using heated water/glycerol mixtures. Separ. Purif. Technol. 2014, 128, 89–95. [Google Scholar]
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J. R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2 K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equil. 2014, 382, 279–285. [Google Scholar]
- Katsampa, P.; Valsamedou, E.; Grigorakis, S.; Makris, D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box—Behnken experimental design and kinetics. Ind. Crops Prod. 2015, 77, 535–543. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Laura, A.; Torres-Rivas, F.; Rodrigo-Garcia, J.; González-Aguilar, G.A. Complexation of apple antioxidants: Chlorogenic acid, quercetin and rutin by β-cyclodextrin (β-CD). J. Inclus. Phenom. Macrocyclic Chem. 2005, 53, 121–129. [Google Scholar] [CrossRef]
- Calabrò, M.; Tommasini, S.; Donato, P.; Raneri, D.; Stancanelli, R.; Ficarra, P.; Ficarra, R.; Costa, C.; Catania, S.; Rustichelli, C. Effects of α- and β-cyclodextrin complexation on the physico-chemical properties and antioxidant activity of some 3-hydroxyflavones. J. Pharmaceut. Biomed. Anal. 2004, 35, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Jullian, C.; Miranda, S.; Zapata-Torres, G.; Mendizábal, F.; Olea-Azar, C. Studies of inclusion complexes of natural and modified cyclodextrin with (+)catechin by NMR and molecular modeling. Bioorg. Med. Chem. 2007, 15, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Tommasini, S.; Raneri, D.; Ficarra, R.; Calabrò, M.L.; Stancanelli, R.; Ficarra, P. Improvement in solubility and dissolution rate of flavonoids by complexation with β-cyclodextrin. J. Pharmaceut. Biomed. Anal. 2004, 35, 379–387. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Bilia, A.R.; Di Bari, L.; Mazzi, G.; Vincieri, F.F. Studies on the interactions between some flavonols and cyclodextrins. Bioorg. Med. Chem. Letters 2007, 17, 5744–5748. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Lucas-Abellán, C.; Fortea, M.; Gabaldón, J.; Núñez-Delicado, E. Complexation of resveratrol by native and modified cyclodextrins: determination of complexation constant by enzymatic, solubility and fluorimetric assays. Food Chem. 2008, 111, 262–267. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Karvela, E.; Makris, D.P.; Karathanos, V.T. Implementation of response surface methodology to assess the antiradical behaviour in mixtures of ascorbic acid and α-tocopherol with grape (Vitis vinifera) stem extracts. Food Chem. 2012, 132, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha Ferreira, F.; Valentim, I.B.; Ramones, E.L.C.; Trevisan, M.T.S.; Olea-Azar, C.; Perez-Cruz, F.; de Abreu, F.C.; Goulart, M.O.F. Antioxidant activity of the mangiferin inclusion complex with β-cyclodextrin. LWT-Food Sci. Technol. 2013, 51, 129–134. [Google Scholar] [CrossRef]
- Stražišar, M.; Andrenšek, S.; Šmidovnik, A. Effect of β-cyclodextrin on antioxidant activity of coumaric acids. Food Chemistry 2008, 110, 636–642. [Google Scholar] [CrossRef]
- Budryn, G.; Nebesny, E.; Pałecz, B.; Rachwał-Rosiak, D.; Hodurek, P.; Miśkiewicz, K.; Oracz, J.; Żyżelewicz, D. Inclusion complexes of β-cyclodextrin with chlorogenic acids (CHAs) from crude and purified aqueous extracts of green Robusta coffee beans (Coffea canephora L.). Food Res. Inter. 2014, 61, 202–213. [Google Scholar] [CrossRef]
- Amyrgialaki, E.; Makris, D.P.; Mauromoustakos, A.; Kefalas, P. Optimization of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind. Crops Prod. 2014, 59, 216–222. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyriakidou, K.; Mourtzinos, I.; Biliaderis, C.G.; Makris, D.P. Optimization of a Green Extraction/Inclusion Complex Formation Process to Recover Antioxidant Polyphenols from Oak Acorn Husks (Quercus Robur) Using Aqueous 2-Hydroxypropyl-β-Cyclodextrin/Glycerol Mixtures. Environments 2016, 3, 3. https://doi.org/10.3390/environments3010003
Kyriakidou K, Mourtzinos I, Biliaderis CG, Makris DP. Optimization of a Green Extraction/Inclusion Complex Formation Process to Recover Antioxidant Polyphenols from Oak Acorn Husks (Quercus Robur) Using Aqueous 2-Hydroxypropyl-β-Cyclodextrin/Glycerol Mixtures. Environments. 2016; 3(1):3. https://doi.org/10.3390/environments3010003
Chicago/Turabian StyleKyriakidou, Katerina, Ioannis Mourtzinos, Costas G. Biliaderis, and Dimitris P. Makris. 2016. "Optimization of a Green Extraction/Inclusion Complex Formation Process to Recover Antioxidant Polyphenols from Oak Acorn Husks (Quercus Robur) Using Aqueous 2-Hydroxypropyl-β-Cyclodextrin/Glycerol Mixtures" Environments 3, no. 1: 3. https://doi.org/10.3390/environments3010003
APA StyleKyriakidou, K., Mourtzinos, I., Biliaderis, C. G., & Makris, D. P. (2016). Optimization of a Green Extraction/Inclusion Complex Formation Process to Recover Antioxidant Polyphenols from Oak Acorn Husks (Quercus Robur) Using Aqueous 2-Hydroxypropyl-β-Cyclodextrin/Glycerol Mixtures. Environments, 3(1), 3. https://doi.org/10.3390/environments3010003








