Preliminary Study of Cellulose and Polycaprolactone-Based Materials for Enhancing Bacteriological and Physicochemical Quality of Contaminated Water
Abstract
1. Introduction
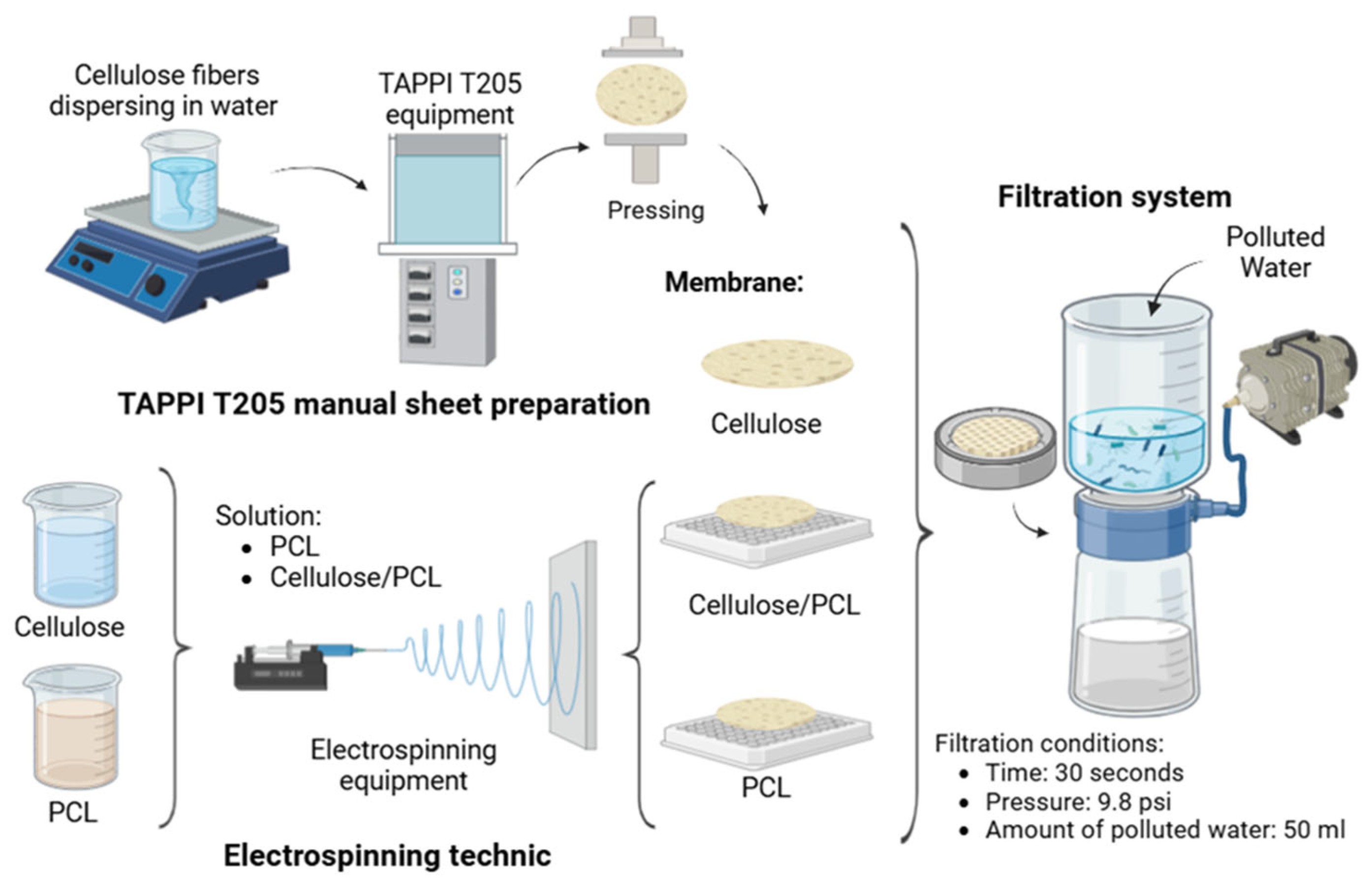
2. Materials and Methods
2.1. Preparation and Characterization of the Cellulose-Based Membranes
2.2. Filtration Tests with Cellulose-Based and Polycaprolactone Membranes
2.3. Water Quality Parameters
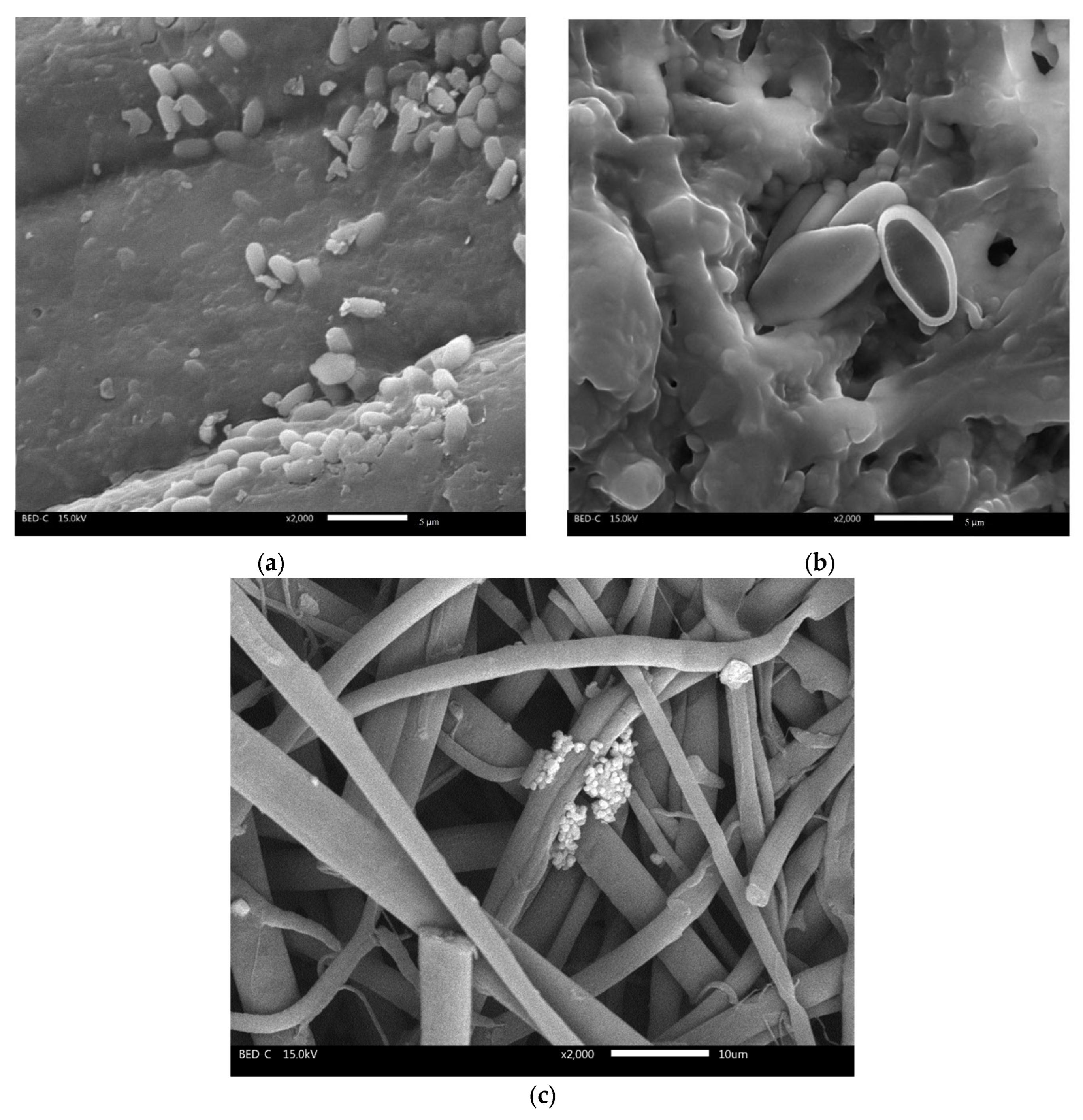
3. Results and Discussion
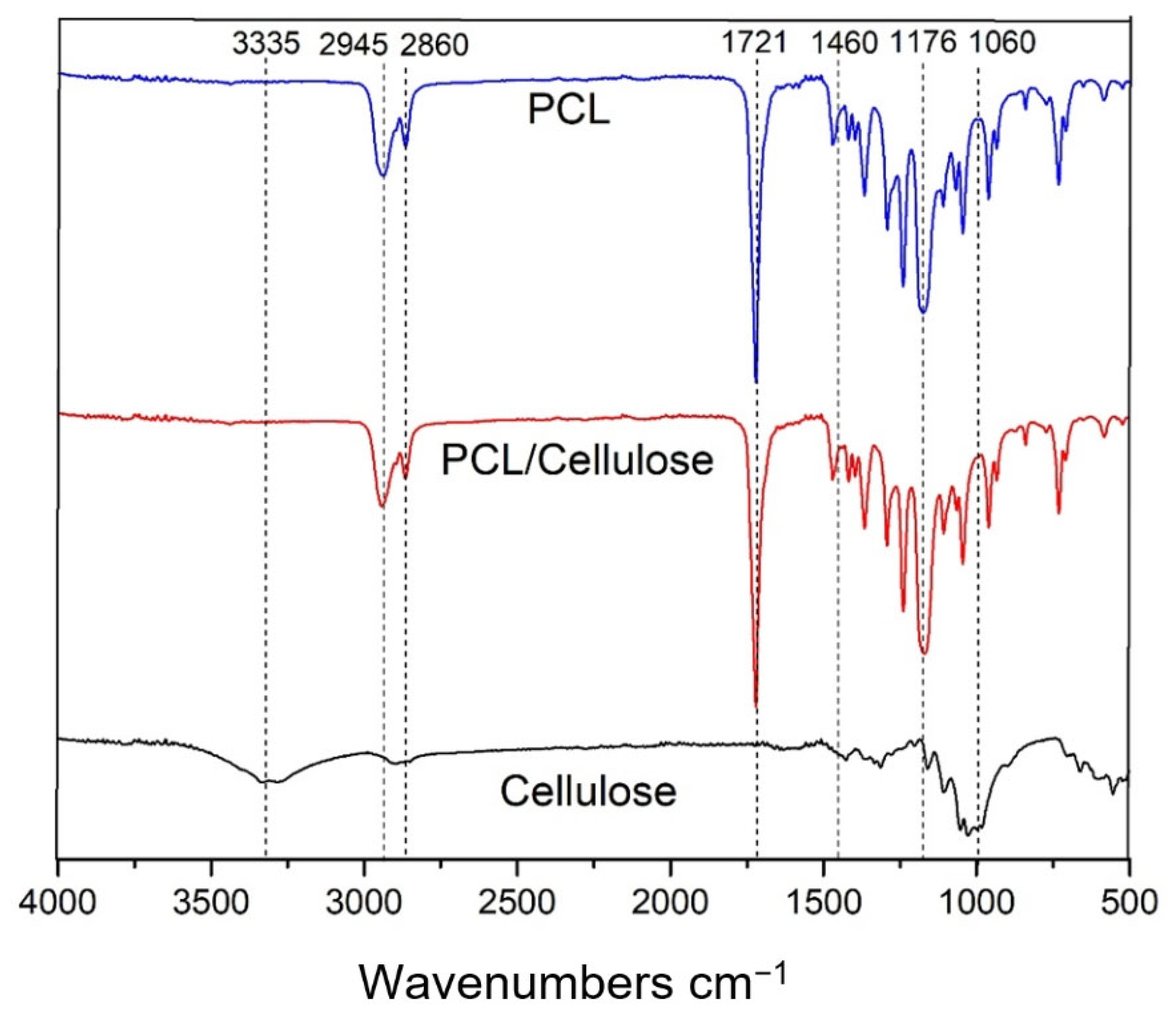
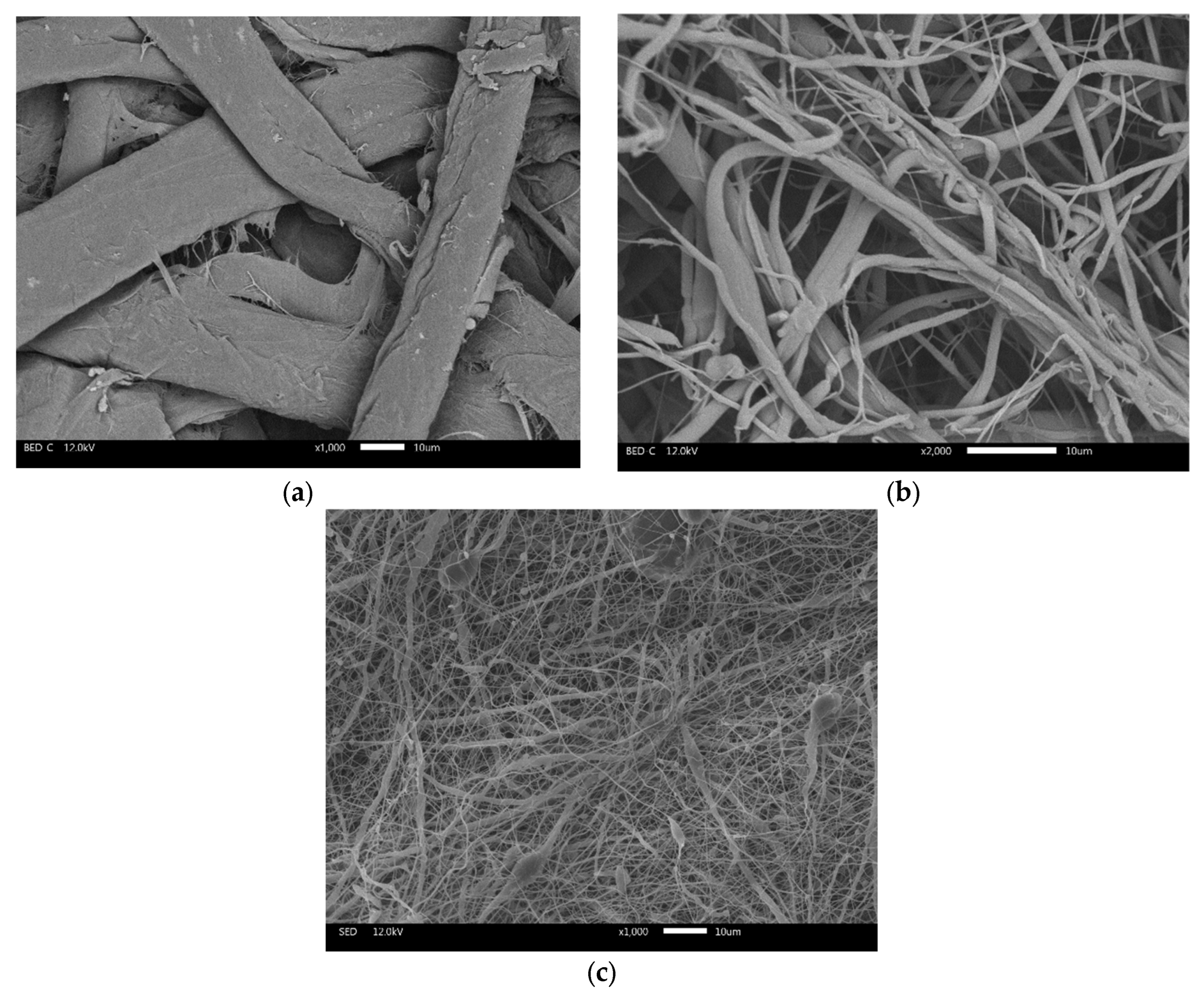
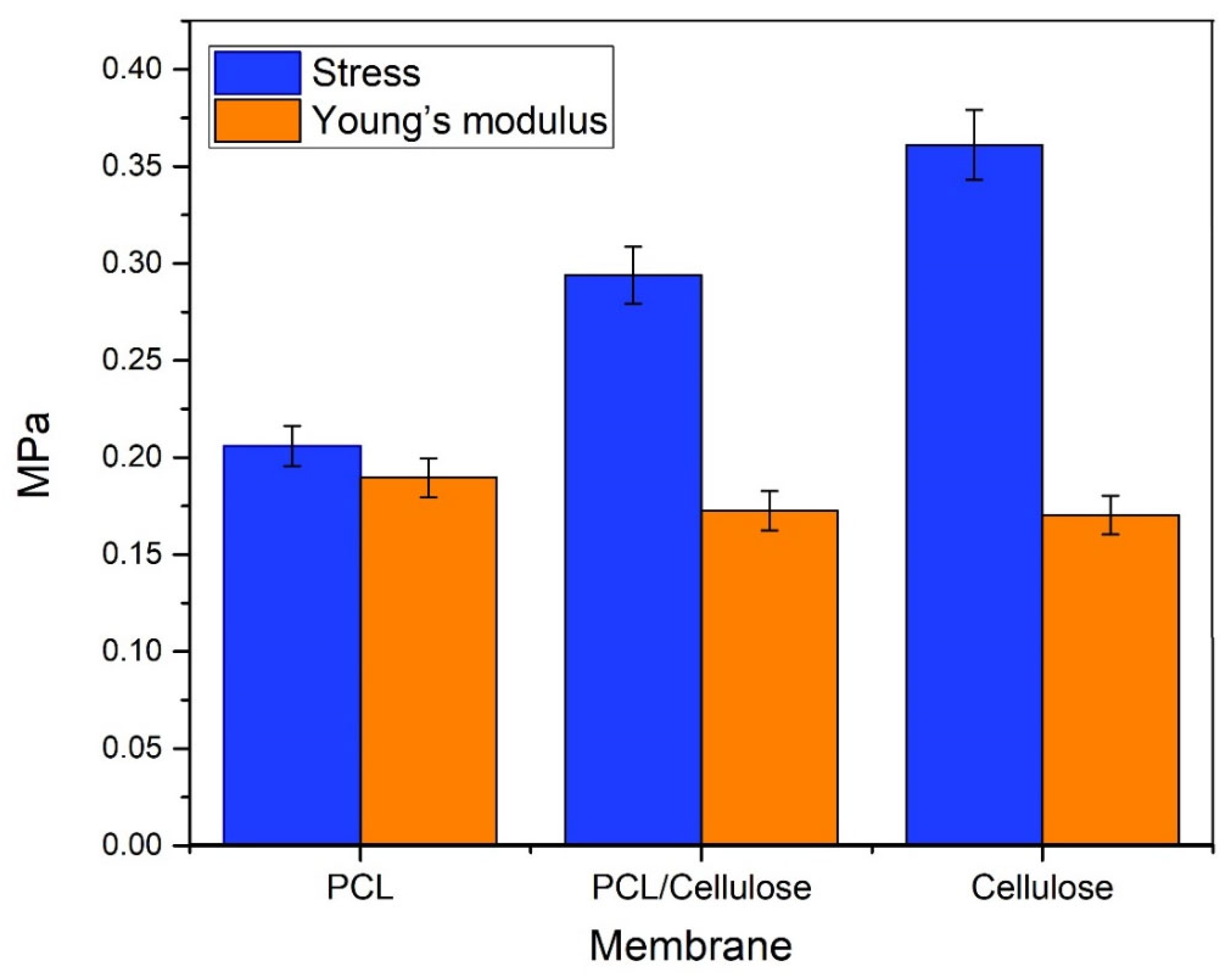
3.1. Characteristics of Cellulose-Based Membranes
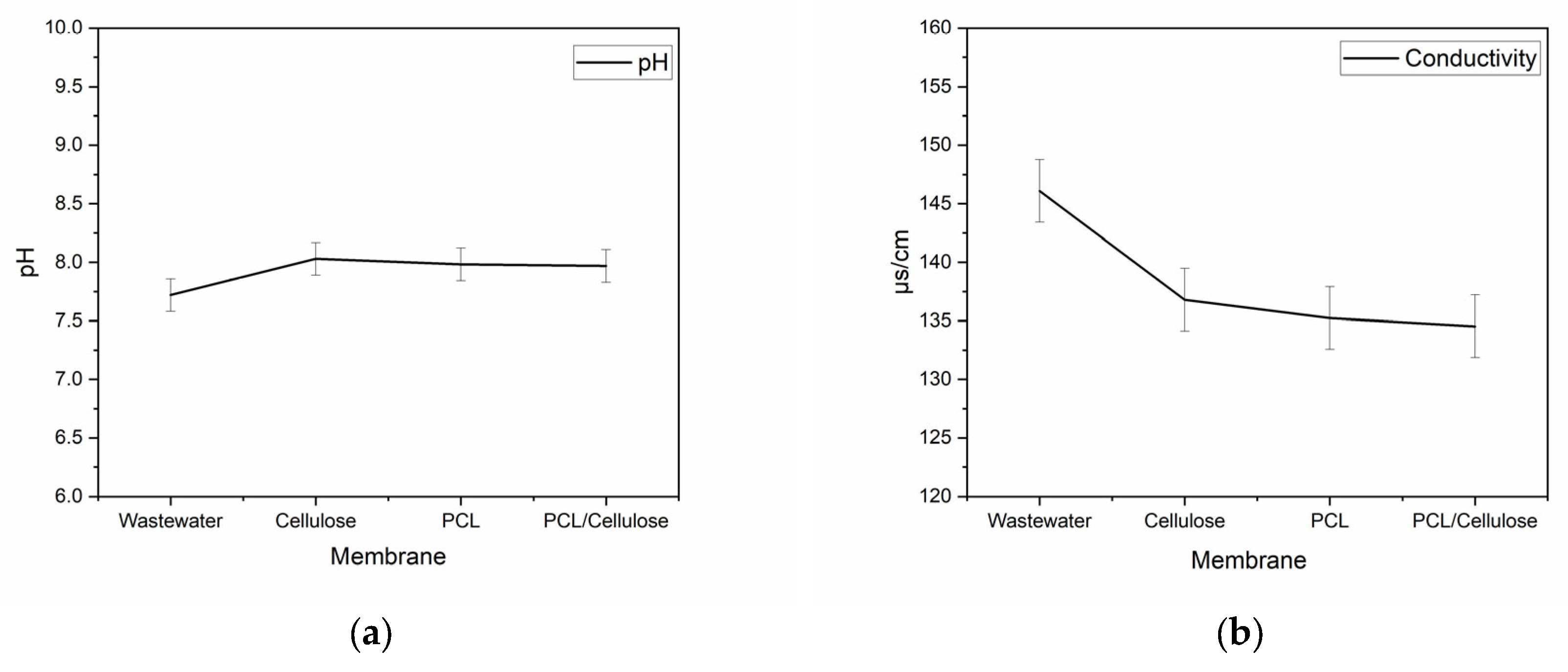
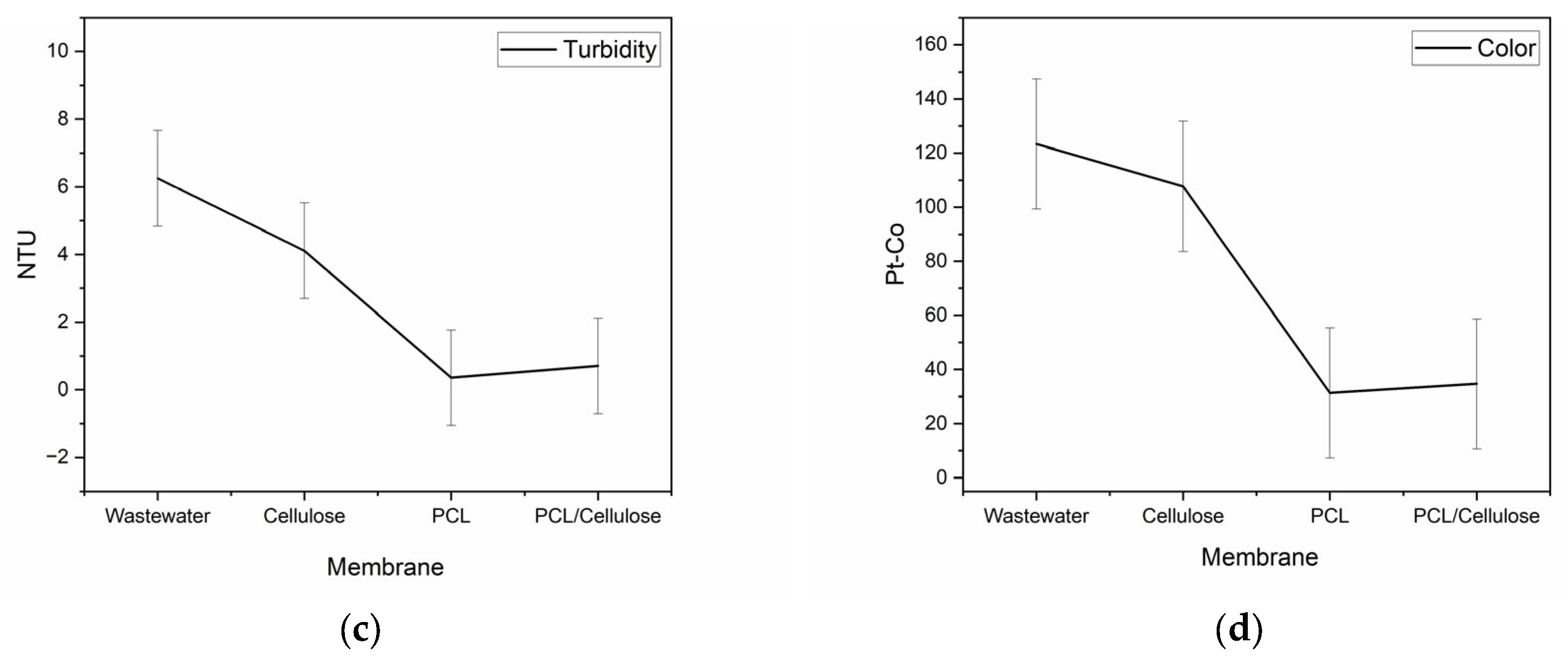
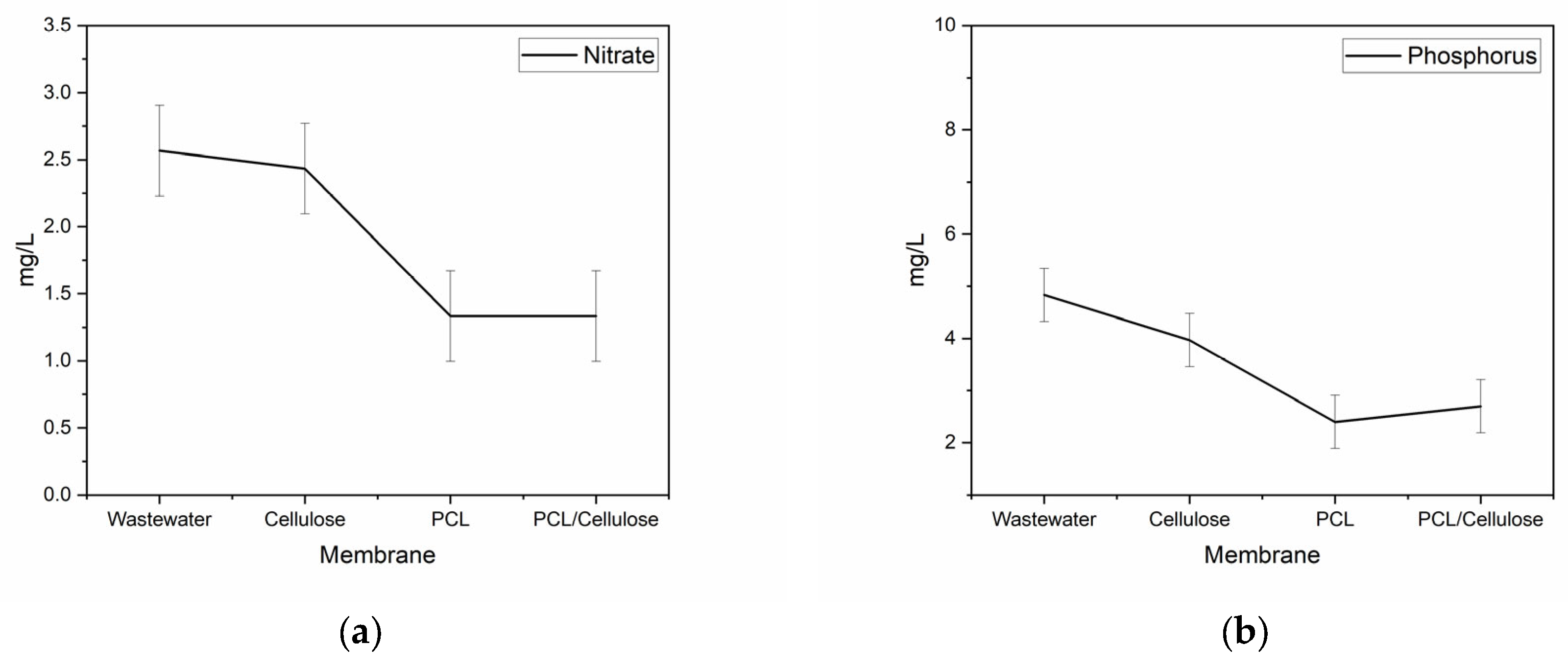
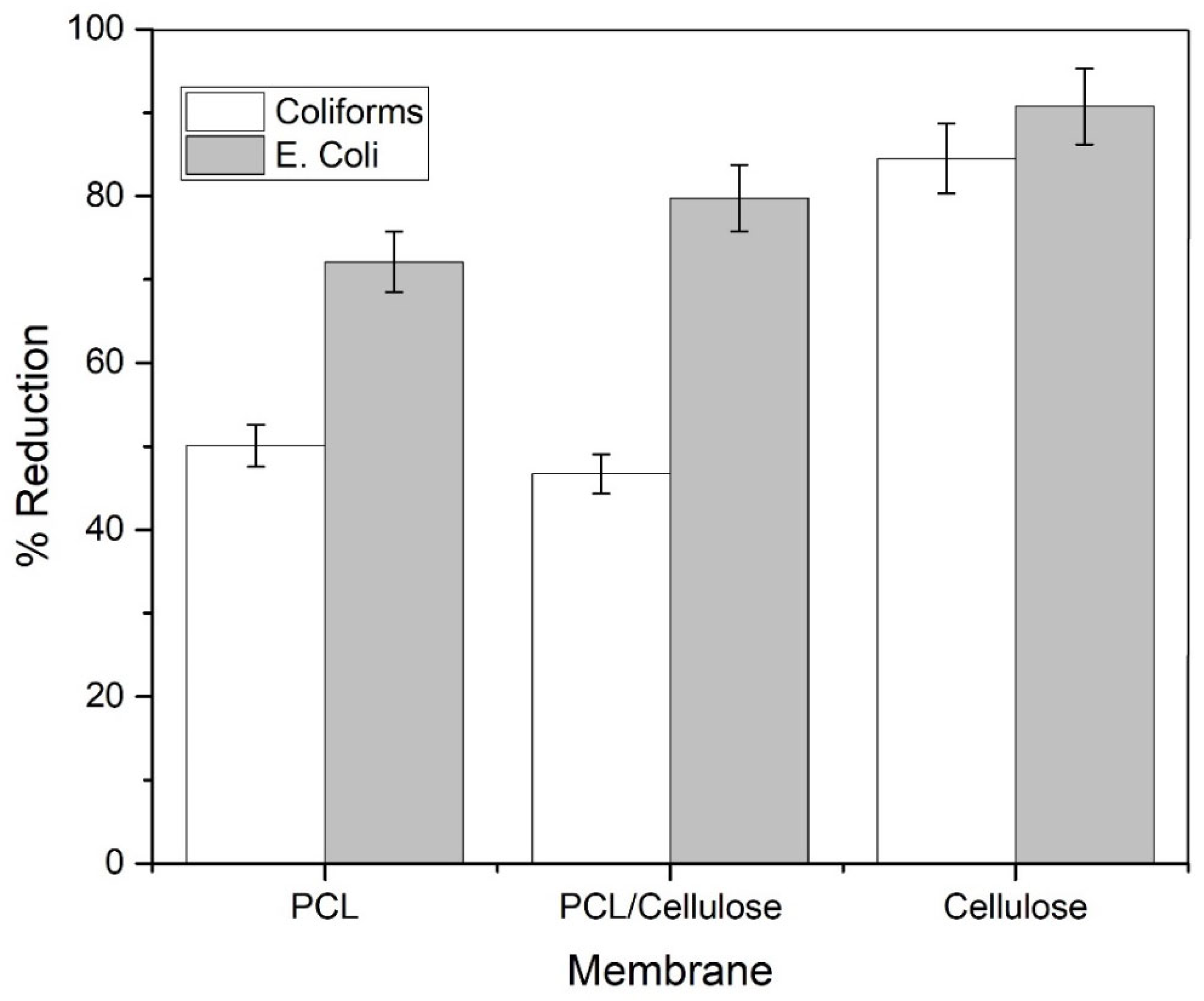
3.2. Improvement of Water Quality When Using the Three Types of Membranes in a Filtration System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uprety, S.; Ngo, I.; Maggos, M.; Dangol, B.; Sherchan, S.P.; Shisler, J.L.; Amarasiri, M.; Sano, D.; Nguyen, T.H. Multiple pathogen contamination of water, hands, and fomites in rural Nepal and the effect of WaSH interventions. Int. J. Hyg. Environ. Health 2024, 257, 114341. [Google Scholar] [CrossRef]
- Boyd, C. Water Quality: An Introduction; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Clarke, R.; Peyton, D.; Healy, M.G.; Fenton, O.; Cummins, E. A quantitative microbial risk assessment model for total coliforms and E. coli in surface runoff following application of biosolids to grassland. Environ. Pollut. 2017, 224, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.L.; Pokhrel, L.R.; Williams, A.; Iverson, G. Understanding factors influencing total coliform and E. coli sampling outcomes in new private water wells in North Carolina, USA. Groundw. Sustain. Dev. 2022, 17, 100759. [Google Scholar] [CrossRef]
- Xu, G.; Wang, T.; Wei, Y.; Zhang, Y.; Chen, J. Fecal coliform distribution and health risk assessment in surface water in an urban-intensive catchment. J. Hydrol. 2022, 604, 127204. [Google Scholar] [CrossRef]
- Pérez Guillemette, T.; Albrechtsen, H.-J. Microbial indicators for water quality in recirculating shower technology. Sci. Total Environ. 2025, 965, 178610. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Hu, Y.; Abidi, N. Cellulose Dissolution in Ionic Liquid under Mild Conditions: Effect of Hydrolysis and Temperature. Fibers 2021, 9, 5. [Google Scholar] [CrossRef]
- Tanpichai, S.; Boonmahitthisud, A.; Soykeabkaew, N.; Ongthip, L. Review of the recent developments in all-cellulose nanocomposites: Properties and applications. Carbohydr. Polym. 2022, 286, 119192. [Google Scholar] [CrossRef]
- Debnath, B.; Haldar, D.; Purkait, M.K. A critical review on the techniques used for the synthesis and applications of crystalline cellulose derived from agricultural wastes and forest residues. Carbohydr. Polym. 2021, 273, 118537. [Google Scholar] [CrossRef]
- Tehrani, A.D.; Tahriri, F.; Najafabadi, A.K.; Arefizadeh, K. Preparation of new green poly (amino amide) based on cellulose nanoparticles for adsorption of Congo red and its adaptive neuro-fuzzy modeling. Int. J. Biol. Macromol. 2024, 281, 136287. [Google Scholar] [CrossRef]
- Aziz, T.; Farid, A.; Haq, F.; Kiran, M.; Ullah, A.; Zhang, K.; Li, C.; Ghazanfar, S.; Sun, H.; Ullah, R.; et al. A Review on the Modification of Cellulose and Its Applications. Polymers 2022, 14, 3206. [Google Scholar] [CrossRef]
- Recio-Colmenares, C.L.; Flores-Gómez, J.; Morales Rivera, J.P.; Palacios Hinestroza, H.; Sulbarán-Rangel, B. Green Materials for Water and Wastewater Treatment: Mechanisms and Artificial Intelligence. Processes 2025, 13, 566. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Nanocellulose as a novel nanostructured adsorbent for environmental remediation: A review. Cellulose 2017, 24, 1171–1197. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic Nanocellulose-Based Membranes for Organic Dye Removal. Materials 2019, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Batmaz, R.; Mohammed, N.; Zaman, M.; Minhas, G.; Berry, R.M.; Tam, K.C. Cellulose nanocrystals as promising adsorbents for the removal of cationic dyes. Cellulose 2014, 21, 1655–1665. [Google Scholar] [CrossRef]
- Jin, L.; Li, W.; Xu, Q.; Sun, Q. Amino-functionalized nanocrystalline cellulose as an adsorbent for anionic dyes. Cellulose 2015, 22, 2443–2456. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, C.; Mathew, A.P. Mechanically robust high flux graphene oxide-nanocellulose membranes for dye removal from water. J. Hazard. Mater. 2019, 371, 484–493. [Google Scholar] [CrossRef]
- Anulaya, S.V.; Subash, A.; Gholap, V.; Kandasubramanian, B. Electrospinning of cellulose acetate for methylene blue dye removal. Hybrid Adv. 2024, 6, 100205. [Google Scholar] [CrossRef]
- Karim, Z.; Khan, M.J.; Hussain, A.; Ahmed, F.; Khan, Z.H. Impact of functionalized and structurally tuned cellulosic composite membranes on removal of metal ions, dye, drug, and proteins. Colloids Surf. A: Physicochem. Eng. Asp. 2024, 692, 134031. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, B.; Yin, X.; Ma, H.; Hsiao, B.S. Highly permeable nanofibrous composite microfiltration membranes for removal of nanoparticles and heavy metal ions. Sep. Purif. Technol. 2020, 233, 115976. [Google Scholar] [CrossRef]
- Palacios, H.; Urena-Saborio, H.; Zurita, F.; Guerrero de León, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef]
- Sulbarán-Rangel, B. Nanocellulose-Based Materials in the Removal of Contaminants from Water. Polym. Sci. Peer Rev. J. 2022, 3, 1–4. [Google Scholar] [CrossRef]
- Wang, J.; Abbas, S.; Li, L.; Walker, C.; Ni, Y.; Cai, Z. Cellulose Membranes: Synthesis and Applications for Water and Gas Separation and Purification. Membranes 2024, 14, 148. [Google Scholar] [CrossRef]
- Ma, H.; Burger, C.; Hsiao, B.S.; Chu, B. Ultrafine polysaccharide nanofibrous membranes for water purification. Biomacromolecules 2011, 12, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Chen, C.; Law, J.L.M.; Houghton, M.; Chen, L. Fabrication of flexible self-standing all-cellulose nanofibrous composite membranes for virus removal. Carbohydr. Polym. 2016, 143, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Alam, K.S.; Fatema-Tuj-Johora, M.; Khan, G.M.A. Fundamental aspects and developments in cellulose-based membrane technologies for virus retention: A review. J. Environ. Chem. Eng. 2021, 9, 106401. [Google Scholar] [CrossRef]
- Baghdad, K.; Hasnaoui, A.M. Zeolite–cellulose composite membranes: Synthesis and applications in metals and bacteria removal. J. Environ. Chem. Eng. 2020, 8, 104047. [Google Scholar] [CrossRef]
- Ottenhall, A.; Henschen, J.; Illergård, J.; Ek, M. Cellulose-based water purification using paper filters modified with polyelectrolyte multilayers to remove bacteria from water through electrostatic interactions. Environ. Sci. Water Res. Technol. 2018, 4, 2070–2079. [Google Scholar] [CrossRef]
- Jha, H.; Dubey, B.K. Challenges and Opportunities in Enabling Circular Economy for Sustainable Wastewater Treatment. In Biological and Hybrid Wastewater Treatment Technology: Recent Developments in India; Ghangrekar, M.M., Yadav, S., Yadava, R.N., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2024; pp. 483–507. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, Y.; Pang, J. Electrospun konjac glucomannan-gelatin/polycaprolactone bilayer nanofibrous films with improved hydrophobicity and mechanical properties for food packaging. Food Biosci. 2024, 61, 104544. [Google Scholar] [CrossRef]
- Palacios, H.; Hernandez, J.; Esquivel, M.; Toriz, G.; Rojas, O.J.; Sulbaran-Rangel, B. Isolation and Characterization of Nanofibrillar Cellulose from Agave tequilana Weber Bagasse. Adv. Mater. Sci. Eng. 2019, 2019, 7. [Google Scholar] [CrossRef]
- Hernández, J.; Romero, V.H.; Escalante, A.; Toriz, G.; Rojas, O.; Sulbarán-Rangel, B. Agave tequilana bagasse as source of cellulose nanocrystals via organosolv treatment. BioResources 2018, 13, 3603–3614. [Google Scholar] [CrossRef]
- TAPPI. Forming Handsheets for Physical Tests of Pulp; TAPPI T 205 sp-06; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2006. [Google Scholar]
- Toure, A.; Wenbiao, D.; Keita, Z.; Dembele, A. Investigation of the water quality of daily used surface-sources for drinking and irrigation by the population of Segou in the center of Mali. J. Water Health 2019, 17, 338–349. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; APHA Press: Washington, DC, USA, 2005. [Google Scholar]
- Correa, E.; Moncada, M.E.; Zapata, V.H. Electrical characterization of an ionic conductivity polymer electrolyte based on polycaprolactone and silver nitrate for medical applications. Mater. Lett. 2017, 205, 155–157. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, Q.; Lei, M.; He, J.; Liu, G. The use of solvent-soaking treatment to enhance the anisotropic mechanical properties of electrospun nanofiber membranes for water filtration. RSC Adv. 2016, 6, 66807–66813. [Google Scholar] [CrossRef]
- Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Recent Advances in Biopolymeric Membranes towards the Removal of Emerging Organic Pollutants from Water. Membranes 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Materials for Water Remediation: Adsorption, Catalysis, and Antifouling. Front. Chem. Eng. 2021, 3, 790314. [Google Scholar] [CrossRef]
- Sanchis-Perucho, P.; Aguado, D.; Ferrer, J.; Seco, A.; Robles, Á. A comprehensive review of the direct membrane filtration of municipal wastewater. Environ. Technol. Innov. 2024, 35, 103732. [Google Scholar] [CrossRef]
- Callister, W.J.; Rethwisch, D. Fundamentals of Materials Science and Engineering: An Integrated Approach, 6th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2021; p. 976. [Google Scholar]
- Huang, L.; Manickam, S.S.; McCutcheon, J.R. Increasing strength of electrospun nanofiber membranes for water filtration using solvent vapor. J. Membr. Sci. 2013, 436, 213–220. [Google Scholar] [CrossRef]
- Hami, S.S.; Affandi, N.D.; Indrie, L.; Tripa, S.; Harun, A.M.; Ahmad, M.R. Enhancing Mechanical Properties and Flux of Nanofibre Membranes for Water Filtration. Polymers 2023, 15, 3281. [Google Scholar] [CrossRef]
- Tejeda, A.; Montoya, A.; Sulbarán-Rangel, B.; Zurita, F. Possible Pollution of Surface Water Bodies with Tequila Vinasses. Water 2023, 15, 3773. [Google Scholar] [CrossRef]
- Kubínová, T.; Kyncl, M. Comparison of standard methods for determining the color of water in several countries. IOP Conf. Ser. Earth Environ. Sci. 2021, 900, 012018. [Google Scholar] [CrossRef]
- Hasan, M.K.; Shahriar, A.; Jim, K.U. Water pollution in Bangladesh and its impact on public health. Heliyon 2019, 5, e02145. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Drinking-Water Quality: Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Sperling, M. Wastewater Characteristics, Treatment and Disposal. In Biological Wastewater Treatment Series; IWA Publishing: London, UK, 2007; p. 304. [Google Scholar]
- Zheng, S.; Yang, S.; Ouyang, Z.; Zhang, Y. Robust and highly hydrophilic ultrafiltration membrane with multi-branched cellulose nanocrystals for permeability-selectivity anti-trade-off property. Appl. Surf. Sci. 2023, 614, 156157. [Google Scholar] [CrossRef]
- Li, Q.; Liang, W.; Lv, L.; Fang, Z.; Xu, D.; Liao, J.; Liu, Y. Preparation of PCL/lecithin/bacteriocin CAMT6 antimicrobial and antioxidant nanofiber films using emulsion electrospinning: Characteristics and application in chilled salmon preservation. Food Res. Int. 2024, 175, 113747. [Google Scholar] [CrossRef] [PubMed]
- Trikannad, S.A.; van Halem, D.; Foppen, J.W.; van der Hoek, J.P. The contribution of deeper layers in slow sand filters to pathogens removal. Water Res. 2023, 237, 119994. [Google Scholar] [CrossRef]
- Hong, H.R.; Kim, J. Nanoroughness-Mediated Bacterial Adhesion on Fabrics. ACS Appl. Nano Mater. 2023, 6, 18518–18530. [Google Scholar] [CrossRef]
- Mir, I.S.; Riaz, A.; Fréchette, J.; Roy, J.S.; McElhinney, J.; Pu, S.; Faraz, M.; Tardy, B.L.; Greener, J.; Dumée, L.F.; et al. Functionalization of bacterial cellulose-based nanofibrous surfaces with antibacterial moieties for membrane biofouling mitigation. J. Water Process Eng. 2025, 77, 108609. [Google Scholar] [CrossRef]
- Ishak, N.I.I.; Seng, O.B.; Chieh, D.C.J.; Yao, A.K.Z.; Shi, C.Y.; Hwa, N.Q. Treatment of river water using modular gravity-driven ultrafiltration (GDU) for individual contingency water supply. Water Supply 2022, 22, 5618–5637. [Google Scholar] [CrossRef]
- Qin, Q.; Lu, H.; Zhu, Z.; Qiu, Y.; Liu, X.; Yin, D. Safety and security of household water purifiers against pathogenic microbial contamination and bio-risk evaluation of their microbial community structures. Sep. Purif. Technol. 2025, 357, 130012. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Mishra, P.K.; Ekielski, A. A Review on Polyacrylonitrile as an Effective and Economic Constituent of Adsorbents for Wastewater Treatment. Molecules 2022, 27, 8689. [Google Scholar] [CrossRef]
- Alsubei, M.D.; Reid, B.; Aljlil, S.A.; Coppens, M.-O.; Campos, L.C. Fabrication and characterization of coated ceramic membranes from natural sources for water treatment applications. J. Membr. Sci. 2024, 690, 122226. [Google Scholar] [CrossRef]
| Membrane | Cellulose Fiber (%) | Polycaprolactone (%) |
|---|---|---|
| Cellulose | 100 | 0 |
| PCL | 0 | 100 |
| PCL/Cellulose | 50 | 50 |
| Membrane | Number of Pores (cm−2) | Pores Diameter (µm) | Fiber Diameter (µm) |
|---|---|---|---|
| Cellulose | 6263.87 | 26.63 ± 11.64 | 14.63 ± 5.82 |
| PCL | 277,791.29 | 1.96 ± 0.74 | 0.43 ± 0.16 |
| PCL/Cellulose | 20,657.35 | 4.46 ± 1.20 | 2.37 ± 0.84 |
| pH | Conductivity (µs·cm−1) | Turbidity (NTU) | Color (Pt-Co) | Nitrate (mg L−1) | Phosphorus (mg L−1) | Total Coliforms (MPN *) | E. coli (MPN *) |
|---|---|---|---|---|---|---|---|
| 7.72 | 146.1 | 6.25 | 123.33 | 2.57 | 4.83 | 707.93 | 1215.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulbarán-Rangel, B.; Palacios-Hinestroza, H.; Arreaga-Cancino, A.; Santos-Ventura, E.M.; Hernández-Cristóbal, O.; Zurita, F. Preliminary Study of Cellulose and Polycaprolactone-Based Materials for Enhancing Bacteriological and Physicochemical Quality of Contaminated Water. Environments 2025, 12, 355. https://doi.org/10.3390/environments12100355
Sulbarán-Rangel B, Palacios-Hinestroza H, Arreaga-Cancino A, Santos-Ventura EM, Hernández-Cristóbal O, Zurita F. Preliminary Study of Cellulose and Polycaprolactone-Based Materials for Enhancing Bacteriological and Physicochemical Quality of Contaminated Water. Environments. 2025; 12(10):355. https://doi.org/10.3390/environments12100355
Chicago/Turabian StyleSulbarán-Rangel, Belkis, Hasbleidy Palacios-Hinestroza, Anahí Arreaga-Cancino, Edgar Mauricio Santos-Ventura, Orlando Hernández-Cristóbal, and Florentina Zurita. 2025. "Preliminary Study of Cellulose and Polycaprolactone-Based Materials for Enhancing Bacteriological and Physicochemical Quality of Contaminated Water" Environments 12, no. 10: 355. https://doi.org/10.3390/environments12100355
APA StyleSulbarán-Rangel, B., Palacios-Hinestroza, H., Arreaga-Cancino, A., Santos-Ventura, E. M., Hernández-Cristóbal, O., & Zurita, F. (2025). Preliminary Study of Cellulose and Polycaprolactone-Based Materials for Enhancing Bacteriological and Physicochemical Quality of Contaminated Water. Environments, 12(10), 355. https://doi.org/10.3390/environments12100355











