Sedimentation Rate and Contamination Levels Profile of Potentially Toxic Elements in the Limoncocha Lagoon RAMSAR Wetland in the Ecuadorian Amazon
Abstract
1. Introduction
2. Materials and Methods
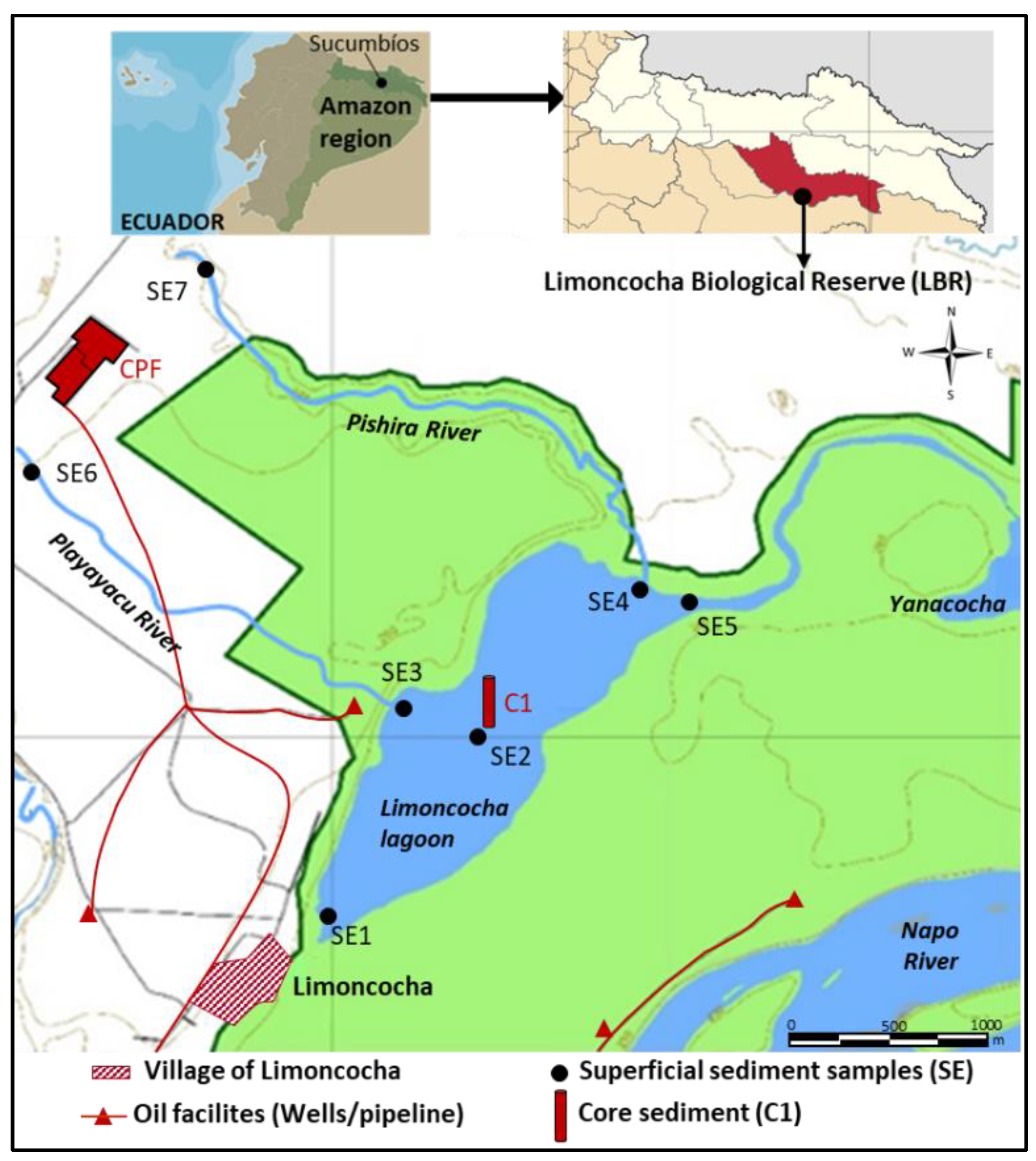
2.1. Study Area: The Limoncocha Lagoon
2.2. Sampling and Analytical Methods
2.3. Contamination Assessment Methodology
3. Results and Discussion
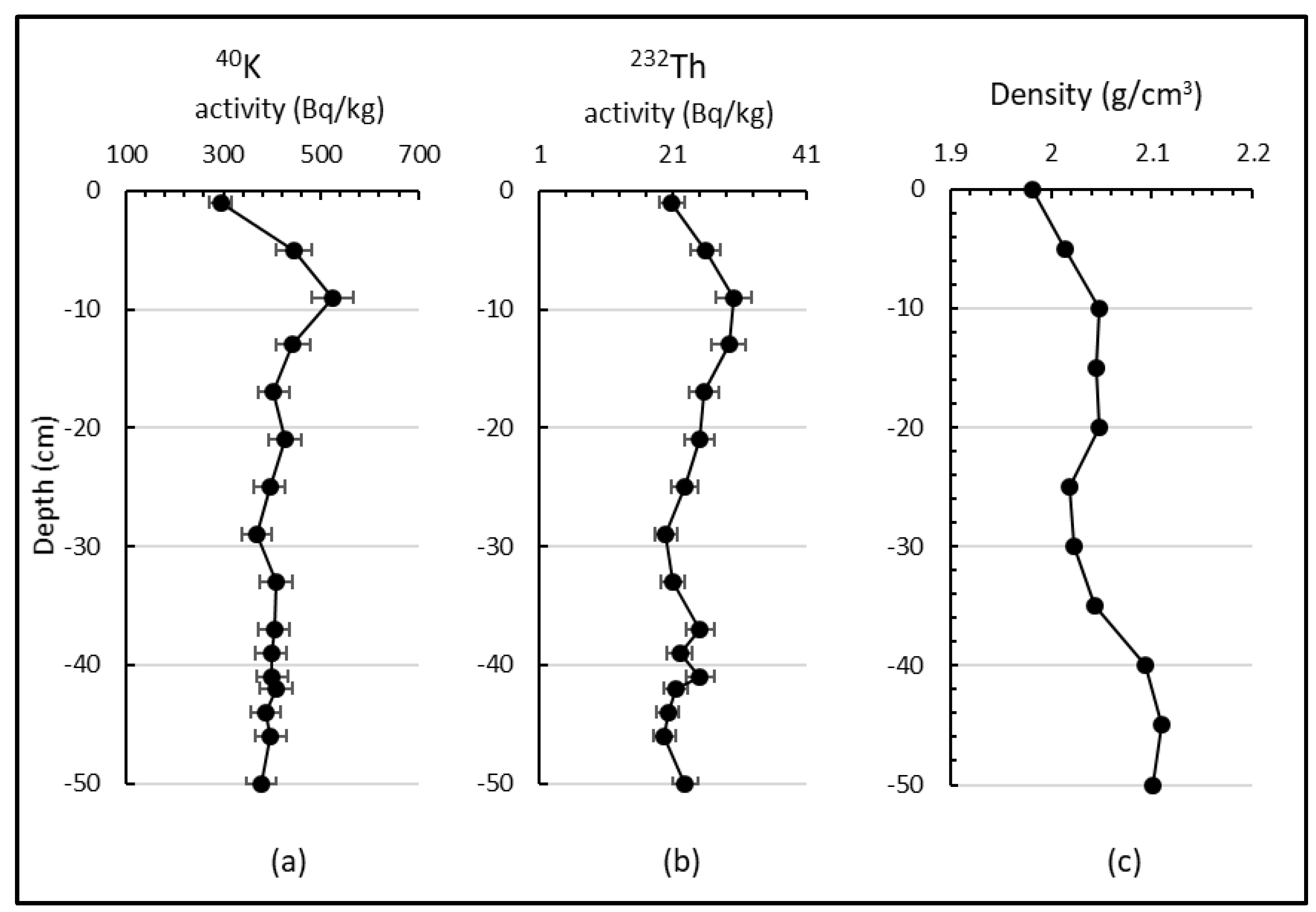
3.1. Density Profile
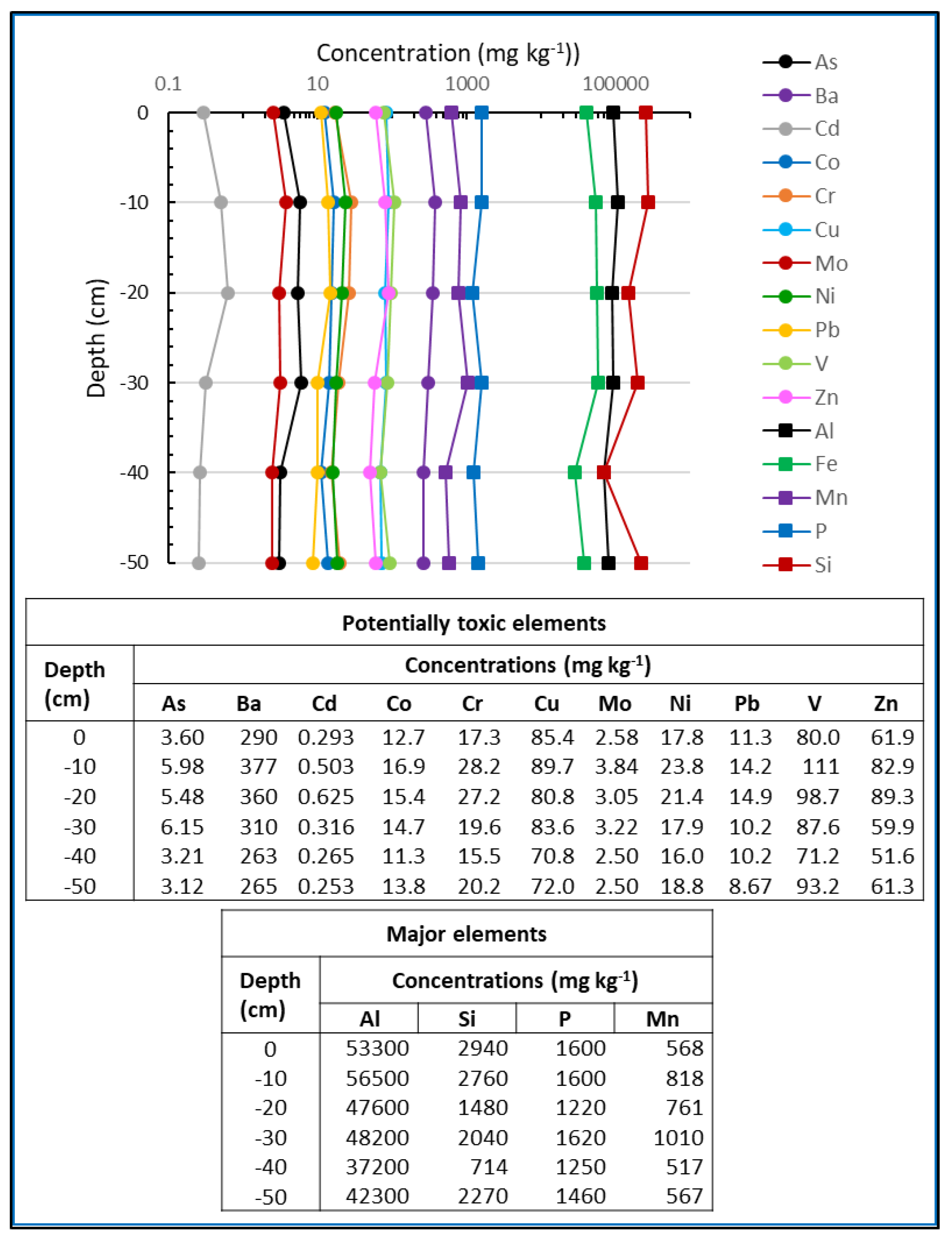
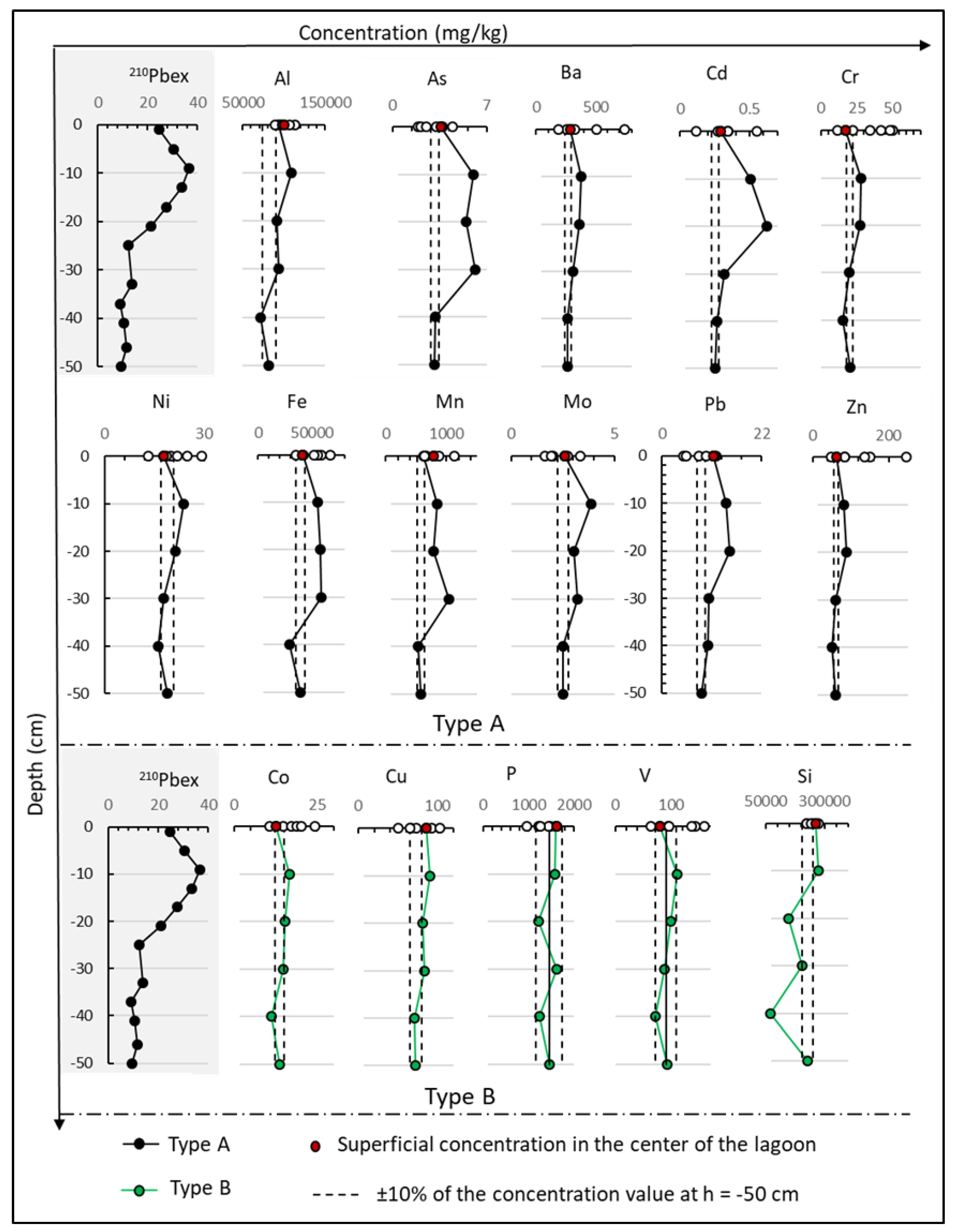
3.2. Trace Elements Concentration Profiles
3.3. Pollution Indices Profiles
3.3.1. Selection of Geochemical Baseline and Reference Element
3.3.2. Contamination and Potential Risk Assessment Based on Pollution Indices
3.4. Radionuclides and Sedimentation Rate in the Sediment Core
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Audry, S.; Schäfer, J.; Blanc, G.; Bossy, C.; Lavaux, G. Anthropogenic Components of Heavy Metal (Cd, Zn, Cu, Pb) Budgets in the Lot-Garonne Fluvial System (France). Appl. Geochem. 2004, 19, 769–786. [Google Scholar] [CrossRef]
- Chatterjee, M.; Silva Filho, E.V.; Sarkar, S.K.; Sella, S.M.; Bhattacharya, A.; Satpathy, K.K.; Prasad, M.V.R.; Chakraborty, S.; Bhattacharya, B.D. Distribution and Possible Source of Trace Elements in the Sediment Cores of a Tropical Macrotidal Estuary and Their Ecotoxicological Significance. Environ. Int. 2007, 33, 346–356. [Google Scholar] [CrossRef]
- Torres, E.; Ayora, C.; Canovas, C.R.; García-Robledo, E.; Galván, L.; Sarmiento, A.M. Metal Cycling during Sediment Early Diagenesis in a Water Reservoir Affected by Acid Mine Drainage. Sci. Total Environ. 2013, 461–462, 416–429. [Google Scholar] [CrossRef] [PubMed]
- Arcega-Cabrera, F.; Noreña-Barroso, E.; Oceguera-Vargas, I. Lead from Hunting Activities and Its Potential Environmental Threat to Wildlife in a Protected Wetland in Yucatan, Mexico. Ecotoxicol. Environ. Saf. 2014, 100, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zaaboub, N.; Martins, M.V.A.; Dhib, A.; Béjaoui, B.; Galgani, F.; el Bour, M.; Aleya, L. Accumulation of Trace Metals in Sediments in a Mediterranean Lagoon: Usefulness of Metal Sediment Fractionation and Elutriate Toxicity Assessment. Environ. Pollut. 2015, 207, 226–237. [Google Scholar] [CrossRef]
- López-Blanco, C.; Kenney, W.F.; Varas, A. Recent Flood Management Efforts Obscure the Climate Signal in a Sediment Record from a Tropical Lake. J. Paleolimnol. 2017, 58, 467–478. [Google Scholar] [CrossRef]
- Kutlu, B. Dissemination of Heavy-Metal Contamination in Surface Sediments of the Uzunçayır Dam Lake, Tunceli, Turkey. Hum. Ecol. Risk Assess. 2018, 24, 2182–2194. [Google Scholar] [CrossRef]
- Aghadadashi, V.; Neyestani, M.R.; Mehdinia, A.; Riyahi Bakhtiari, A.; Molaei, S.; Farhangi, M.; Esmaili, M.; Rezai Marnani, H.; Gerivani, H. Spatial Distribution and Vertical Profile of Heavy Metals in Marine Sediments around Iran’s Special Economic Energy Zone; Arsenic as an Enriched Contaminant. Mar. Pollut. Bull. 2019, 138, 437–450. [Google Scholar] [CrossRef]
- Chai, M.; Li, R.; Ding, H.; Zan, Q. Occurrence and Contamination of Heavy Metals in Urban Mangroves: A Case Study in Shenzhen, China. Chemosphere 2019, 219, 165–173. [Google Scholar] [CrossRef]
- Mao, L.; Ye, H.; Li, F.; Yang, M.; Tao, H.; Wen, H. Enrichment Assessment of Sb and Trace Metals in Sediments with Significant Variability of Background Concentration in Detailed Scale. Environ. Sci. Pollut. Res. 2019, 26, 2794–2805. [Google Scholar] [CrossRef]
- Skowronek, F.; Sagemann, J.; Stenzel Schulz, F.H. Evolution of Heavy-Metal Profiles in River Weser Estuary Sediments, Germany; Springer: Berlin/Heidelberg, Germany, 1994; Volume 24. [Google Scholar]
- Hamzeh, M.; Ouddane, B.; El-Daye, M.; Halwani, J. Profile of Trace Metals Accumulation in Core Sediment from Seine River Estuary (Docks Basin). Environ. Technol 2013, 34, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Crespo, C.; Martín, M. Determination of Background Levels and Pollution Assessment for Seven Metals (Cd, Cu, Ni, Pb, Zn, Fe, Mn) in Sediments of a Mediterranean Coastal Lagoon. Catena 2015, 133, 206–214. [Google Scholar] [CrossRef]
- Bárcena, J.F.; Claramunt, I.; García-Alba, J.; Pérez, M.L.; García, A. A Method to Assess the Evolution and Recovery of Heavy Metal Pollution in Estuarine Sediments: Past History, Present Situation and Future Perspectives. Mar. Pollut. Bull. 2017, 124, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Davoodi, H.; Gharibreza, M.; Negarestan, H.; Mortazavi, M.S.; Lak, R. Ecological Risk Assessment of the Assaluyeh and Bassatin Estuaries (Northern Persian Gulf) Using Sediment Quality Indices. Estuar. Coast. Shelf Sci. 2017, 192, 17–28. [Google Scholar] [CrossRef]
- Wang, G.; Hu, X.; Zhu, Y.; Jiang, H.; Wang, H. Historical Accumulation and Ecological Risk Assessment of Heavy Metals in Sediments of a Drinking Water Lake. Environ. Sci. Pollut. Res. 2018, 25, 24882–24894. [Google Scholar] [CrossRef]
- Bai, J.; Zhao, Q.; Wang, W.; Wang, X.; Jia, J.; Cui, B.; Liu, X. Arsenic and Heavy Metals Pollution along a Salinity Gradient in Drained Coastal Wetland Soils: Depth Distributions, Sources and Toxic Risks. Ecol. Indic. 2019, 96, 91–98. [Google Scholar] [CrossRef]
- Fernandes, F.; Poleto, C. Geochemistry in Sediment Core for Zinc and Nickel Metals and Comparison between Indexes of Environmental References. J. Environ. Eng. 2019, 145, 04018146. [Google Scholar] [CrossRef]
- Huang, B.; Guo, Z.; Xiao, X.; Zeng, P.; Peng, C. Changes in Chemical Fractions and Ecological Risk Prediction of Heavy Metals in Estuarine Sediments of Chunfeng Lake Estuary, China. Mar. Pollut. Bull. 2019, 138, 575–583. [Google Scholar] [CrossRef]
- Li, R.; Tang, C.; Li, X.; Jiang, T.; Shi, Y.; Cao, Y. Reconstructing the Historical Pollution Levels and Ecological Risks over the Past Sixty Years in Sediments of the Beijiang River, South China. Sci. Total Environ. 2019, 649, 448–460. [Google Scholar] [CrossRef]
- Meng, K.; Xu, M.; Zhao, Y.; Li, F.; Xu, W.; Chen, Y. Accumulation, Sources and Pollution of Heavy Metals in the Sediments of Coastal Tidal Flats in the North Jiangsu Radial Sand Ridges, China. Environ. Earth Sci. 2019, 78, 128. [Google Scholar] [CrossRef]
- Orani, A.M.; Vassileva, E.; Renac, C.; Schmidt, S.; Angelidis, M.O.; Rozmaric, M.; Louw, D. First Assessment on Trace Elements in Sediment Cores from Namibian Coast and Pollution Sources Evaluation. Sci. Total Environ. 2019, 669, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.; Rizzi, J.; do Couto, E.V.; Souza, D.C.; Ferreira, P.A.L.; Figueira, R.; Froehner, S. Historical Pollution of an Urban Watershed Based in Geochemical, Geoacumulation, and EROD Activity in PLHC-1 Analyses in Sediment Cores. Arch. Environ. Contam. Toxicol. 2019, 76, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Fural, Ş.; Kükrer, S.; Cürebal, İ. Geographical Information Systems Based Ecological Risk Analysis of Metal Accumulation in Sediments of İkizcetepeler Dam Lake (Turkey). Ecol. Indic. 2020, 119, 106784. [Google Scholar] [CrossRef]
- Li, J.; Zuo, Q.; Feng, F.; Jia, H. Occurrence and Ecological Risk Assessment of Heavy Metals from Wuliangsuhai Lake, Yellow River Basin, China. Water 2022, 14, 1264. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Viguri, J.R.; Casado-Martínez, M.C.; DelValls, T.A. Sediment quality assessment and dredged material management in Spain: Part I, application of sediment quality guidelines in the bay of Santander. Integr. Environ. Assess. 2007, 3, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Guerra, M.; Viguri, J.R.; Casado-Martínez, M.C.; DelValls, T.A. Sediment quality assessment and dredged material management in Spain: Part II, analysis of action levels for dredged material management and application to the bay of Cadiz. Integr. Environ. Assess 2007, 3, 539–551. [Google Scholar] [CrossRef]
- Dung, T.T.T.; Cappuyns, V.; Swennen, R.; Phung, N.K. From Geochemical Background Determination to Pollution Assessment of Heavy Metals in Sediments and Soils. Rev. Environ. Sci. Biotechnol. 2013, 12, 335–353. [Google Scholar] [CrossRef]
- Sakan, S.; Dević, G.; Relić, D.; Anđelković, I.; Sakan, N.; Đorđević, D. Evaluation of Sediment Contamination with Heavy Metals: The Importance of Determining Appropriate Background Content and Suitable Element for Normalization. Environ. Geochem. Health 2015, 37, 97–113. [Google Scholar] [CrossRef]
- Saddik, M.; Fadili, A.; Makan, A. Assessment of Heavy Metal Contamination in Surface Sediments along the Mediterranean Coast of Morocco. Environ. Monit. Assess. 2019, 191, 197. [Google Scholar] [CrossRef]
- Cáceres Choque, L.F.; Ramos Ramos, O.E.; Valdez Castro, S.N.; Choque Aspiazu, R.R.; Choque Mamani, R.G.; Fernández Alcazar, S.G.; Sracek, O.; Bhattacharya, P. Fractionation of Heavy Metals and Assessment of Contamination of the Sediments of Lake Titicaca. Environ. Monit. Assess. 2013, 185, 9979–9994. [Google Scholar] [CrossRef]
- Colizza, E.; Fontolan, G.; Brambati, A. Impact of a Coastal Disposal Site for Inert Wastes on the Physical Marine Environment, Barcola, Bovedo, Trieste, Italy. Environ. Geol. 1996, 27, 270–285. [Google Scholar] [CrossRef]
- Adamo, P.; Arienzo, M.; Imperato, M.; Naimo, D.; Nardi, G.; Stanzione, D. Distribution and Partition of Heavy Metals in Surface and Sub-Surface Sediments of Naples City Port. Chemosphere 2005, 61, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Abrahim, G.M.S.; Parker, R.J. Assessment of Heavy Metal Enrichment Factors and the Degree of Contamination in Marine Sediments from Tamaki Estuary, Auckland, New Zealand. Environ Monit Assess. 2008, 136, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Irabien, M.J.; Cearreta, A.; Leorri, E.; Gómez, J.; Viguri, J. A 130 Year Record of Pollution in the Suances Estuary (Southern Bay of Biscay): Implications for Environmental Management. Mar. Pollut. Bull. 2008, 56, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z. Geochemical Background-an Environmental Perspective. Mineralogia 2011, 42, 7–17. [Google Scholar] [CrossRef]
- Wei, C.; Wen, H. Geochemical Baselines of Heavy Metals in the Sediments of Two Large Freshwater Lakes in China: Implications for Contamination Character and History. Environ. Geochem. Health 2012, 34, 737–748. [Google Scholar] [CrossRef]
- Viguri, J.R.; Irabien, M.J.; Yusta, I.; Soto, J.; Gómez, J.; Rodriguez, P.; Martinez-Madrid, M.; Irabien, J.A.; Coz, A. Physico-Chemical and Toxicological Characterization of the Historic Estuarine Sediments: A Multidisciplinary Approach. Environ. Int. 2007, 33, 436–444. [Google Scholar] [CrossRef]
- Costa, E.S.; Grilo, C.F.; Wolff, G.A.; Thompson, A.; Figueira, R.C.L.; Sá, F.; Neto, R.R. Geochemical Records in Sediments of a Tropical Estuary (Southeastern Coast of Brazil). Reg. Stud. Mar. Sci. 2016, 6, 49–61. [Google Scholar] [CrossRef]
- Birch, G.F. Determination of Sediment Metal Background Concentrations and Enrichment in Marine Environments—A Critical Review. Sci. Total Environ. 2017, 580, 813–831. [Google Scholar] [CrossRef]
- Dung, T.T.T.; Linh, T.M.; Chau, T.B.; Hoang, T.M.; Swennen, R.; Cappuyns, V. Contamination Status and Potential Release of Trace Metals in a Mangrove Forest Sediment in Ho Chi Minh City, Vietnam. Environ. Sci. Pollut. Res. 2019, 26, 9536–9551. [Google Scholar] [CrossRef]
- Guzmán, H.M.; Gómez-Álvarez, A.; Valenzuela-García, J.L.; Encinas-Romero, M.A.; Villalba-Atondo, A.I.; Encinas-Soto, K.K. Assessment of the Impact on Sediment Quality from Abandoned Artisanal Mine Runoffs in a Semi-Arid Environment (the Sonora River Basin—Northwestern Mexico). Environ. Earth Sci. 2019, 78, 145. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Chen, J.; Jiang, X.; Zheng, B. Combined Use of Diffusive Gradients in Thin Film, High-Resolution Dialysis Technique and Traditional Methods to Assess Pollution and Bioavailability of Sediment Metals of Lake Wetlands in Taihu Lake Basin. Sci. Total Environ. 2019, 671, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Birch, G.; Lee, J.H. The Use of Sedimentary Metal Data in Predictive Modelling of Estuarine Contamination, Assessment of Environmental Condition and Pollutant Source Identification (Narrabeen Lagoon, Sydney, Australia). Environ. Sci. Pollut. Res. 2020, 27, 43685–43699. [Google Scholar] [CrossRef] [PubMed]
- Tnoumi, A.; Angelone, M.; Armiento, G.; Caprioli, R.; Crovato, C.; de Cassan, M.; Montereali, M.R.; Nardi, E.; Parrella, L.; Proposito, M.; et al. Heavy Metal Content and Potential Ecological Risk Assessment of Sediments from Khnifiss Lagoon National Park (Morocco). Environ. Monit. Assess. 2022, 194, 356. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, J.; Guo, J.; Hu, X.; Bao, H.; Chen, J. Record of Heavy Metals in Huguangyan Maar Lake Sediments: Response to Anthropogenic Atmospheric Pollution in Southern China. Sci. Total Environ. 2022, 831, 154829. [Google Scholar] [CrossRef]
- Meybeck, M.; Lestel, L.; Bonté, P.; Moilleron, R.; Colin, J.L.; Rousselot, O.; Hervé, D.; de Pontevès, C.; Grosbois, C.; Thévenot, D.R. Historical Perspective of Heavy Metals Contamination (Cd, Cr, Cu, Hg, Pb, Zn) in the Seine River Basin (France) Following a DPSIR Approach (1950-2005). Sci. Total Environ. 2007, 375, 204–231. [Google Scholar] [CrossRef]
- Soto-Torres, J.; Soto-Velloso, J.A.; Ródenas, G.; Gelen, A.; Díaz, O.; Viguri, J. Estimación de las tasas de erosión en torno a la bahía de Santander, España. Cienc Tierra Espacio 2007, 7, 1031–1036. [Google Scholar]
- Irabien, M.J.; Rada, M.; Gómez, J.; Soto, J.; Mañanes, A.; Viguri, J. An Assessment of Anthropogenic Impact in a Nature Reserve: The Santoña Marshes (Northern Spain). J. Iber. Geol. 2008, 34, 235–242. [Google Scholar]
- Bonachea, J.; Bruschi, V.M.; Hurtado, M.A.; Forte, L.M.; da Silva, M.; Etcheverry, R.; Cavallotto, J.L.; Dantas, M.F.; Pejon, O.J.; Zuquette, L.V.; et al. Natural and Human Forcing in Recent Geomorphic Change; Case Studies in the Rio de La Plata Basin. Sci. Total Environ. 2010, 408, 2674–2695. [Google Scholar] [CrossRef]
- Navas, A.; Valero-Garcés, B.; Gaspar, L.; Palazón, L. Radionuclides and Stable Elements in the Sediments of the Yesa Reservoir, Central Spanish Pyrenees. J. Soils Sediments 2011, 11, 1082–1098. [Google Scholar] [CrossRef]
- Song, Y.; Choi, M.S.; Lee, J.Y.; Jang, D.J. Regional Background Concentrations of Heavy Metals (Cr, Co, Ni, Cu, Zn, Pb) in Coastal Sediments of the South Sea of Korea. Sci. Total Environ. 2014, 482–483, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.S.; Ferreira, P.A.; Rizzi, J.; Figueira, R.; Froehner, S. Spatial and Temporal Variation of Heavy Metals Contamination in Recent Sediments from Barigui River Basin, South Brazil. Environ. Pollut. Clim. Change 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Pratte, S.; Bao, K.; Shen, J.; de Vleeschouwer, F.; le Roux, G. Centennial Records of Cadmium and Lead in NE China Lake Sediments. Sci. Total Environ. 2019, 657, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.G.; Oldfieldz, F. The Assessment of 210Pb Data from Sites with Varying Sediment Accumulation Rates. Hydrobiologia 1983, 103, 29–35. [Google Scholar] [CrossRef]
- Appleby, P.G.; Nolan, P.J.; Oldfield, F.; Richardson, N.; Higgitt, S.R. 210Pb Dating of Lake Sediments and Ombrotrophic Peats by Gamma Essay. Sci. Total Environ. 1988, 69, 157–177. [Google Scholar] [CrossRef]
- Appleby, P.G.; Oldfield, F. The Calculation of Lead-210 Dates Assuming a Constant Rate of Supply of Unsupported 210Pb to the Sediment. Catena 1978, 5, 1–8. [Google Scholar] [CrossRef]
- Mabit, L.; Benmansour, M.; Abril, J.M.; Walling, D.E.; Meusburger, K.; Iurian, A.R.; Bernard, C.; Tarján, S.; Owens, P.N.; Blake, W.H.; et al. Fallout 210Pb as a Soil and Sediment Tracer in Catchment Sediment Budget Investigations: A Review. Earth Sci. Rev. 2014, 138, 335–351. [Google Scholar] [CrossRef]
- Sha, Z.; Wang, Q.; Wang, J.; Du, J.; Hu, J.; Ma, Y.; Kong, F.; Wang, Z. Regional Environmental Change and Human Activity over the Past Hundred Years Recorded in the Sedimentary Record of Lake Qinghai, China. Environ. Sci. Pollut. Res. 2017, 24, 9662–9674. [Google Scholar] [CrossRef]
- Du, J.; Wang, Z.; Du, J.; Lin, W.; Lu, B.; Qi, Y.; Gao, H.; Wang, Y.; Yao, Z. Radionuclides in Sediment as Tracers for Evolution of Modern Sedimentary Processes in the Bohai Sea. Reg. Stud. Mar. Sci. 2021, 48, 102061. [Google Scholar] [CrossRef]
- Luo, M.; Kang, X.; Liu, Q.; Yu, H.; Tao, Y.; Wang, H.; Niu, Y.; Niu, Y. Research on the Geochemical Background Values and Evolution Rules of Lake Sediments for Heavy Metals and Nutrients in the Eastern China Plain from 1937 to 2017. J. Hazard. Mater. 2022, 436, 129136. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Lal, D.; Martin, J.M.; Meybeck, M. Geochronology of lake sediments. Earth Planet. Sci. Lett. 1971, 11, 407–414. [Google Scholar] [CrossRef]
- Robbins, J.; Edgington, D.; Kemp, A. Comparative 210Pb, 137Cs, and Pollen Geochronologies of Sediments from Lakes Ontario and Erie. Quat. Res. 1978, 10, 256–278. [Google Scholar] [CrossRef]
- Sanchez-Cabeza, J.A.; Ruiz-Fernández, A.C. 210Pb sediment radiochronology: An integrated formulation and classification of dating models. Geochim. Cosmochim. Acta 2012, 82, 183–200. [Google Scholar] [CrossRef]
- Ramsar The List of Wetlands of International Importance. p. 55. Available online: https://www.Ramsar.Org/Sites/Default/Files/Documents/Library/Sitelist.Pdf. (accessed on 2 February 2020).
- Lessmann, J.; Fajardo, J.; Muñoz, J.; Bonaccorso, E. Large Expansion of Oil Industry in the Ecuadorian Amazon: Biodiversity Vulnerability and Conservation Alternatives. Ecol. Evol. 2016, 6, 4997–5012. [Google Scholar] [CrossRef]
- Valdiviezo-Rivera, J.; Carrillo-Moreno, C.; Gea-Izquierdo, E. Annotated List of Freshwater Fishes of the Limoncocha Lagoon, Napo River Basin, Northern Amazon Region of Ecuador. Check List 2018, 14, 55–75. [Google Scholar] [CrossRef]
- Anderson, E.P.; Osborne, T.; Maldonado-Ocampo, J.A.; Mills-Novoa, M.; Castello, L.; Montoya, M.; Encalada, A.C.; Jenkins, C.N. Energy Development Reveals Blind Spots for Ecosystem Conservation in the Amazon Basin. Front. Ecol. Environ. 2019, 17, 521–529. [Google Scholar] [CrossRef]
- Carrillo, K.C.; Drouet, J.C.; Rodríguez-Romero, A.; Tovar-Sánchez, A.; Ruiz-Gutiérrez, G.; Viguri Fuente, J.R. Spatial Distribution and Level of Contamination of Potentially Toxic Elements in Sediments and Soils of a Biological Reserve Wetland, Northern Amazon Region of Ecuador. J. Environ. Manag. 2021, 289, 112495. [Google Scholar] [CrossRef]
- Carrillo, K.C.; Rodríguez-Romero, A.; Tovar-Sánchez, A.; Ruiz-Gutiérrez, G.; Fuente, J.R.V. Geochemical Baseline Establishment, Contamination Level and Ecological Risk Assessment of Metals and As in the Limoncocha Lagoon Sediments, Ecuadorian Amazon Region. J. Soils Sediments 2022, 22, 293–315. [Google Scholar] [CrossRef]
- Neira, F.; Gómez, S.; Pérez, G. Sostenibilidad de los usos de subsistencia de la biodiversidad en un Área Protegida de la Amazonia Ecuatoriana: Un análisis biofísico. Ecuad. Debate 2006, 67, 155–164. [Google Scholar]
- Neira, F.; Younes, N. Evaluación multicriterial de los usos de subsistencia de la biodiversidad por parte de una comunidad kichwa en la Reserva Biológica Limoncocha. In Amenazas y Retos en la Gestión de la Reserva de Biosfera Yasuní, 1st ed.; Krainer, A., Mora, M.F., Eds.; FLACSO-Sede Ecuador: Quito, Ecuador, 2011; pp. 137–152. ISBN 978-9978-67-304-1. [Google Scholar]
- Neira, F.; Suza, M.; Robles, K. Usos sostenibles de la biodiversidad en un área protegida de laAmazonía ecuatoriana (2006-2011) (Sustainable uses of biodiversity in a protected area of Ecuadorian Amazonia (2006–2011)). Let. Verdes. Rev. Latinoam. De Estud. Socioambientales 2013, 14, 338–357. [Google Scholar]
- GAD, Gobierno Autonomo Descrentralizado Limoncocha (Autonomous Government Decrentralized Limoncocha). Actualización del plan de desarrollo y ordenamiento territorial de la parroquia Limoncocha (Updating of the development plan and territorial ordering of the Limoncocha parish). Available online: https://gadlimoncocha.gob.ec (accessed on 4 June 2022).
- ISO 17892-3:2015; Geotechnical Investigation and Testing—Laboratory Testing of Soil—Part 3: Determination of Particle Density. ISO: Geneva, Switzerland.
- Gelen, A.; Soto, J.; Gómez, J.; Díaz, O. Sediment dating of Santander Bay, Spain. J. Radioanal. Nucl. Chem. 2004, 261, 437–441. [Google Scholar] [CrossRef]
- Maanan, M.; Saddik, M.; Maanan, M.; Chaibi, M.; Assobhei, O.; Zourarah, B. Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecol. Indic. 2015, 48, 616–626. [Google Scholar] [CrossRef]
- Kowalska, J.B.; Mazurek, R.; Gąsiorek, M.; Zaleski, T. Pollution indices as useful tools for the comprehensive evaluation of the degree of soil contamination—A review. Environ. Geochem. Health 2018, 40, 2395–2420. [Google Scholar] [CrossRef] [PubMed]
- Håkanson, L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Müller, G. Index of geo-accumulation in sediments of the Rhine River. GeoJournal 1969, 2, 108–118. [Google Scholar]
- Chen, C.W.; Kao, C.M.; Chen, C.F.; Di, D.C. Distribution and accumulation of heavy metals in the sediments of Kaohsiung Harbor, Taiwan. Chemosphere 2007, 66, 1431–1440. [Google Scholar] [CrossRef]
- Birch, G.F.; Olmos, M.A. Sediment-bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES J. Mar. Sci. 2008, 65, 1407–1413. [Google Scholar] [CrossRef]
- Birch, G.F.; Gunns, T.J.; Olmos, M. Sediment-bound metals as indicators of anthropogenic change in estuarine environments. Mar. Pollut. Bull. 2015, 101, 243–257. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Li, S.; Chen, J. Bioavailability and toxicity of trace metals (Cd, Cr, Cu, Ni, and Zn) in sediment cores from the Shima River, South China. Chemosphere 2018, 192, 31–42. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, J.; Zhao, Q. Heavy metals in wetland soils along a wetland forming chronosequence in the Yellow River Delta of China: Levels, sources and toxic risks. Ecol. Indic 2016, 69, 331–339. [Google Scholar] [CrossRef]
- Jarrín, A.E.; Salazar, J.G.; Martínez-Fresneda, M. Pollution risk evaluation of the aquifers of the Limoncocha Biological Reserve, Ecuadorian Amazon. Rev. Ambient. Agua 2017, 12, 652–665. (In Spanish) [Google Scholar] [CrossRef][Green Version]
- Wittmann, H.; von Blanckenburg, F.; Guyot, J.L.; Laraque, A.; Bernal, C.; Kubik, P.W. Sediment production and transport from in situ-produced cosmogenic 10Be and river loads in the Napo River basin, an upper Amazon tributary of Ecuador and Peru. J. South Am. Earth Sci. 2011, 31, 45–53. [Google Scholar] [CrossRef]
- Agah, H.; Hashtroudi, M.S.; Baeyens, W. Trace metals and major elements in sediments of the northern Persian gulf. J. Persian Gulf 2012, 3, 45–58. [Google Scholar]
- Yang, J.; Wang, W.; Zhao, M. Spatial distribution and historical trends of heavy metals in the sediments of petroleum producing regions of the Beibu Gulf, China. Mar. Pollut. Bull. 2015, 91, 87–95. [Google Scholar] [CrossRef]
- Sadeghi, P.; Loghmani, M.; Afsa, E. Trace element concentrations, ecological and health risk assessment in sediment and marine fish Otolithes ruber in Oman Sea. Iran Mar Pollut Bull 2019, 140, 248–254. [Google Scholar] [CrossRef]
- Ogunlaja, A.; Ogunlaja, O.O.; Okewole, D.M.; Morenikeji, O.A. Risk assessment and source identification of heavy metal contamination by multivariate and hazard index analyses of a pipeline vandalised area in Lagos State, Nigeria. Sci. Total Environ. 2019, 651, 2943–2952. [Google Scholar] [CrossRef]
- Ghanavati, N.; Nazarpour, A.; De Vivo, B. Ecological and human health risk assessment of toxic metals in street dusts and surface soils in Ahvaz, Iran. Environ. Geochem. Health 2019, 41, 875–891. [Google Scholar] [CrossRef]
- Bissen, M.; Frimmel, F.H. Arsenic—A Review. Part I: Occurrence, Toxicity, Speciation, Mobility. Acta Hydroch. Hydrob. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.; Li, Y.; Baligar, V.C. Evaluation of soil amendments as a remediation alternative for cadmium-contaminated soils under cacao plantations. Environ. Sci. Pollut. Res. 2016, 23, 17571–17580. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, D.; Li, Y. Environmental behaviors and potential ecological risks of heavy metals (Cd, Cr, Cu, Pb, and Zn) in multimedia in an oilfield in China. Environ. Sci. Pollut. Res. 2016, 23, 13964–13972. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Pinedo-Hernández, J.; Díez, S. Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinú River Basin, Colombia. Environ. Res. 2017, 154, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, L.; Liang, T.; Zhang, Y.; Li, J.; Xiao, J.; Dong, L.; Zhang, H. Geostatistical analyses and co-occurrence correlations of heavy metals distribution with various types of land use within a watershed in eastern QingHai-Tibet Plateau, China. Sci. Total Environ. 2019, 653, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Mestanza, C.; Ubidia, M.; Figueroa, H.; Logroño, S.; Pozo, E.; Vizuete, M. Limoncocha Biological Reserve lagoon: Meanings from an anthropic perspective. Int. J. Eng. Sci. Res. Technol. 2019, 6, 137–142. [Google Scholar] [CrossRef]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Report to the General Assembly: Sources and Effects of Ionizing Radiation—Exposures from Natural Radiation Sources, Annex B; United Nations: New York, NY, USA, 2000.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Report to General Assembly, with Scientific Annexes. Exposure of the Public and Workers from Various Sources of Radiation; United Nations: New York, NY, USA, 2008.
- Bowen, H.J.M. Environmental Chemistry of the Elements; Academic Press: London, UK, 1979. [Google Scholar]
- Rudnik, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 4. [Google Scholar]
- Koide, M.; Bruland, K.W.; Goldberg, E.D. Th-228/Th-232 and Pb-210 geochronologies in marine and lake sediments. Geochim. Cosmochim. Ac 1973, 37, 1171–1187. [Google Scholar] [CrossRef]
- Robbins, J.A.; Edgington, D. Determination of Recent Sedimentation Rates in Lake Michigan Using Pb-210 and Cs-137. Geochim. Cosmochim. Ac 1975, 39, 285–304. [Google Scholar] [CrossRef]
- Eakins, J.A.; Morrison, R.T. A new procedure for the determination of lead-210 in lake and marine sediments. The International J. Appl. Radiat. Isot. 1978, 29, 531–536. [Google Scholar] [CrossRef]
- Baskaran, M.; Naidu, A.S. 210Pb-derived chronology and the fluxes of 210Pb and 137Cs isotopes into continental shelf sediments, East Chukchi Sea, Alaskan Arctic. Geochim. Cosmochim. Acta 1995, 59, 4435–4448. [Google Scholar] [CrossRef]
- Turner, L.J.; Delorme, L.D. Assessment of 210Pb data from Canadian lakes using the CIC and CRS models. Environ. Geol. 1996, 28, 78–87. [Google Scholar] [CrossRef]
- Santschi, P.H.; Presley, B.J.; Wade, T.L.; Garcia-Romero, B.; Baskaran, M. Historical contamination of PAHs, PCBs, DDTs, and heavy metals in Mississippi River Delta, Galveston Bay and Tampa Bay sediment cores. Mar. Environ. Res. 2001, 52, 51–79. [Google Scholar] [CrossRef]
- San Miguel, E.G.; Bolívar, J.P.; Garcia-Tenorio, R. Vertical distribution of Th-isotope ratios, 210Pb, 226Ra and 137Cs in sediment cores from an estuary affected by anthropogenic releases. Sci. Total Environ. 2004, 318, 143–157. [Google Scholar] [CrossRef]
- Harb, S. Natural radioactivity and external gamma radiation exposure at the coastal Red Sea in Egypt. Radiat. Prot. Dosim. 2008, 130, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Matamet, F.R.M.; Bonotto, D.M. Identifying sedimentation processes in the Coata River, Altiplano of the Puno department, Peru, by the 210Pb method. Environ. Earth Sci. 2019, 78, 641. [Google Scholar] [CrossRef]
- Cigagna, C.; Bonotto, D.M.; Camargo, A.F.M. Sedimentation rates by the 210Pb chronological method in Itanhaém river watershed, southeast Brazil. Environ. Monit. Assess. 2021, 193, 819. [Google Scholar] [CrossRef] [PubMed]
- Pesantes, A.A.; Carpio, E.P.; Vitvar, T.; Lopez, M.M.M.; Menendez-Aguado, J.M.M. A multi-index analysis approach to heavy metal pollution assessment in river sediments in the Ponce Enriquez area, Ecuador. Water 2019, 11, 590. [Google Scholar] [CrossRef]
- Garreaud, R.D.; Vuille, M.; Compagnucci, R.; Marengo, J. Present-day South American climate. Palaeogeogr. Palaeocl. 2009, 281, 180–195. [Google Scholar] [CrossRef]
 Cf < 1 low pollution,
Cf < 1 low pollution,  1 < Cf < 3 moderate contamination; Igeo:
1 < Cf < 3 moderate contamination; Igeo:  Igeo < 0 uncontaminated,
Igeo < 0 uncontaminated,  0 < Igeo < 1 uncontaminated-moderately contaminated; Ef:
0 < Igeo < 1 uncontaminated-moderately contaminated; Ef:  Ef < 1 no enrichment,
Ef < 1 no enrichment,  1 < Ef < 3 minor enrichment. Pollutants:
1 < Ef < 3 minor enrichment. Pollutants:  As,
As,  Ba,
Ba,  Cd,
Cd,  Co,
Co,  Cr,
Cr,  Cu,
Cu,  Mo,
Mo,  Ni,
Ni,  Pb,
Pb,  V,
V,  Zn. At each depth, the bar graph is shown with the values of each element. The same color code has been used to represent the studied elements.
Zn. At each depth, the bar graph is shown with the values of each element. The same color code has been used to represent the studied elements.
 Cf < 1 low pollution,
Cf < 1 low pollution,  1 < Cf < 3 moderate contamination; Igeo:
1 < Cf < 3 moderate contamination; Igeo:  Igeo < 0 uncontaminated,
Igeo < 0 uncontaminated,  0 < Igeo < 1 uncontaminated-moderately contaminated; Ef:
0 < Igeo < 1 uncontaminated-moderately contaminated; Ef:  Ef < 1 no enrichment,
Ef < 1 no enrichment,  1 < Ef < 3 minor enrichment. Pollutants:
1 < Ef < 3 minor enrichment. Pollutants:  As,
As,  Ba,
Ba,  Cd,
Cd,  Co,
Co,  Cr,
Cr,  Cu,
Cu,  Mo,
Mo,  Ni,
Ni,  Pb,
Pb,  V,
V,  Zn. At each depth, the bar graph is shown with the values of each element. The same color code has been used to represent the studied elements.
Zn. At each depth, the bar graph is shown with the values of each element. The same color code has been used to represent the studied elements.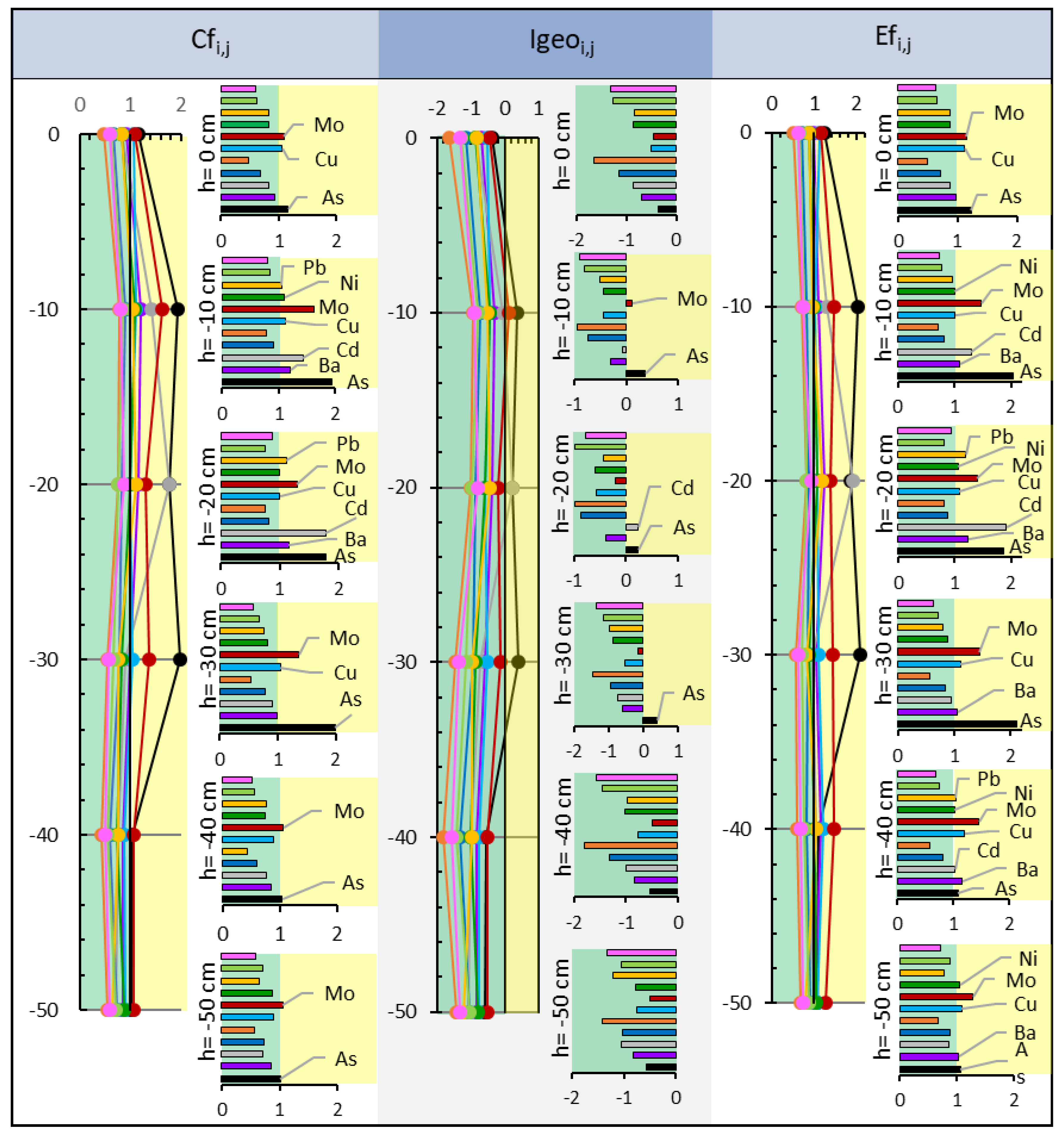
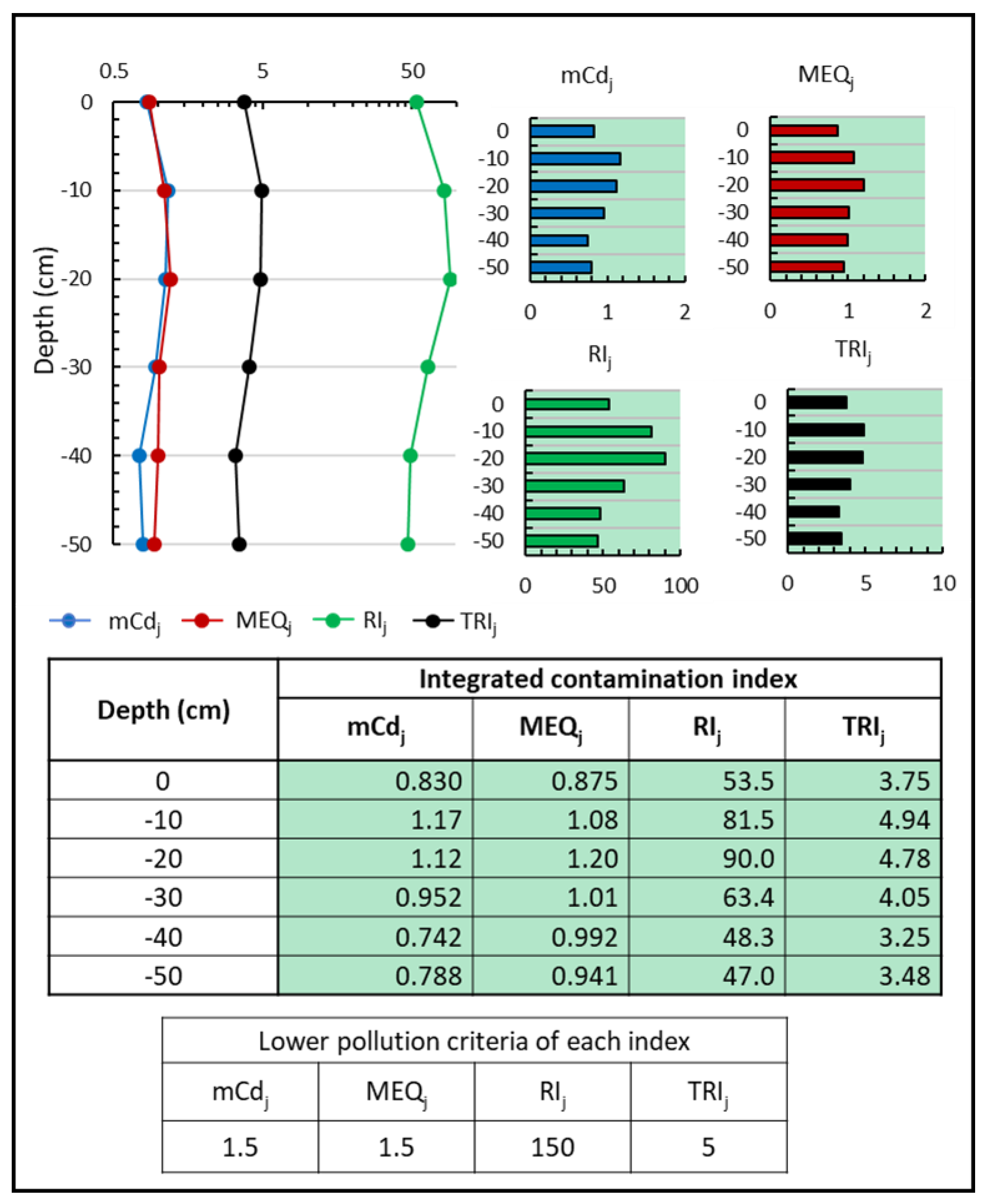


| Indexes | Equations | Criteria | Classification |
|---|---|---|---|
| Contamination factor, [79] | contaminant baseline concentration i | < 1 | Low contamination |
| 1 < < 3 | Moderate contamination | ||
| 3 < < 6 | Considerable contamination | ||
| 6 < < 12 | Very high contamination | ||
| > 12 | Extreme contamination | ||
| Geoaccumul-ation index, [80] | contaminant baseline concentration i and 1.5 is the correction factor of the bottom matrix due to lithogenic effects | Class 0, uncontaminated | |
| Class 1, uncontaminated to moderately contaminated | |||
| Class 2, moderately polluted | |||
| Class 3, moderately polluting to heavily polluted | |||
| Class 4, heavily polluted | |||
| Class 5, heavily polluted to extremely polluted | |||
| Class 6, extremely polluted | |||
| Enrichment factor, [81] | concentration of the reference element (R) in sample j, baseline concentration of reference element | < 1 | not enriched |
| 1 < <3 | slightly enriched | ||
| 3 < <5 | moderately enriched | ||
| Moderately to severely enriched | |||
| severely enriched | |||
| very severely spiked | |||
| extremely enriched | |||
| Modified pollution degree, [34] | is the contamination factor i at site j and n is the number of contaminants studied | Without pollution | |
| Low pollution | |||
| Moderate pollution | |||
| High pollution | |||
| Very high pollution | |||
| Extremely high pollution | |||
| Ultra-high pollution | |||
| Average enrichment ratio, [82,83] | is the enrichment factor of element i at site j and n the number of contaminants. | Category 1 (No enrichment) | |
| Category 2 (Minor Enrichment) | |||
| Category 3 (Moderate Enrichment) | |||
| Category 4 (Extensive Enrichment) | |||
| Category 5 (Severe Enrichment) | |||
| Potential ecological risk index, [79] | is the ecological risk factor of element i at site j, n number of elements and the toxic response factor for element i. Tri has a value of 30 for Cd, 10 for As, 5 for Cu, Ni, and Pb, 2 for Cr and 1 for Zn. | <150 <40 | Low potential risk |
| 150<<300 40<<80 | Moderate potential risk | ||
| 300<<600 80<<160 | Considerable potential risk | ||
| >600 160<<320 | High potential risk | ||
| >320 | Very high potential risk | ||
| Toxic risk index (). [84,85] | is the concentration of elements i at site j, is the likely effect level of item i, is the threshold effect level of element i and is the toxic risk index of element i at site j. | No toxic risk | |
| Low toxic risk | |||
| Moderate toxic risk | |||
| Considerable toxic risk | |||
| Very high toxic risk |
| Element | Minimum Concentration (mg kg−1) | Sampling Point of Surface Sediment | |
|---|---|---|---|
| Core in the Centre of the Lagoon | Surface Sediment Samples [69,70] | ||
| As | 3.12 | 1.92 | S3-Mouth of the Playayacu river |
| Ba | 263 | 188 | S5-Caño |
| Cd | 0.253 | 0.118 | S5-Caño |
| Co | 11.3 | 10.7 | S5-Caño |
| Cr | 15.5 | 11.4 | S5-Caño |
| Cu | 70.8 | 50.6 | S3-Mouth of the Playayacu river |
| Mo | 2.5 | 1.65 | S3-Mouth of the Playayacu river |
| Ni | 16.0 | 13.2 | S5-Caño |
| Pb | 8.7 | 4.62 | S3-Mouth of the Playayacu river |
| V | 71.2 | 61.8 | S5-Caño |
| Zn | 51.6 | 48.4 | S5-Caño |
| Al | 71887 | 89600 | S4-Mouth of the Pishira river |
| Fe | 28990 | 34800 | S5-Caño |
| Mn | 532 | 643 | S7-Pishira River |
| P | 1221 | 961 | S6-Playayacu River |
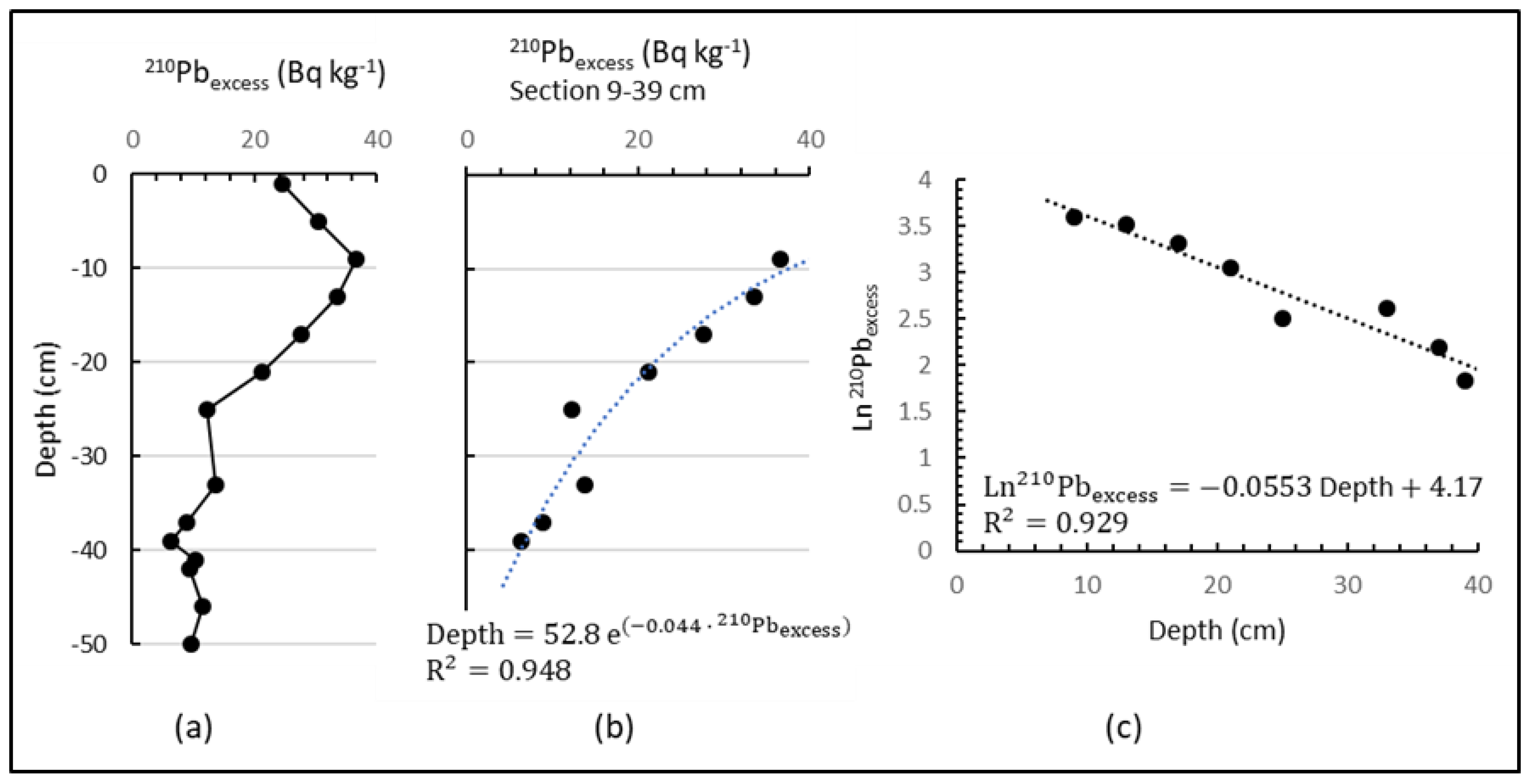
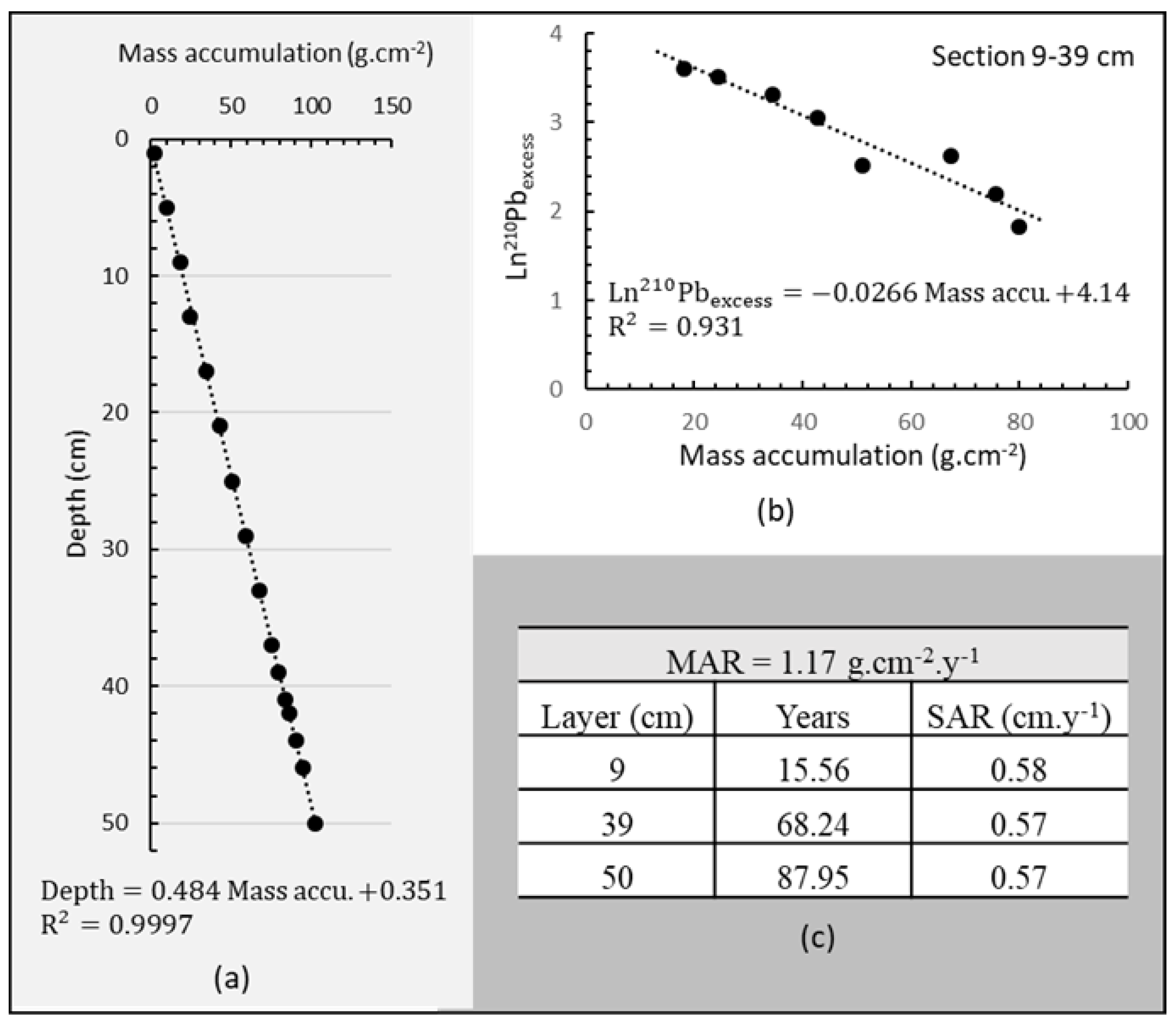
| Depth cm | 210PbTotal | 226Ra | 210Pbexcess | 40K | 232Th |
|---|---|---|---|---|---|
| Bq kg−1 | |||||
| 1 | 49.8 ± 10.4 | 25.1 ± 4.1 | 24.7 ± 10.4 | 293.9 ± 23.8 | 20.9 ± 1.8 |
| 5 | 61.9 ± 13.5 | 31.3 ± 3.6 | 30.5 ± 13.5 | 445.1 ± 35.9 | 26.0 ± 2.2 |
| 9 | 69.3 ± 9.9 | 32.7 ± 3.6 | 36.5 ± 9.9 | 525.0 ± 42.5 | 30.2 ± 2.7 |
| 13 | 69.0 ± 15.1 | 35.4 ± 4.4 | 33.6 ± 15.1 | 442.4 ± 35.7 | 29.4 ± 2.5 |
| 17 | 59.8 ±12.6 | 32.3 ± 3.8 | 27.6 ± 12.6 | 402.7 ± 32.4 | 25.7 ± 2.2 |
| 21 | 55.8 ± 12.9 | 34.6 ± 4.0 | 21.2 ± 12.9 | 426.8 ± 34.4 | 25.0 ± 2.2 |
| 25 | 45.6 ± 9.8 | 33.3 ± 3.9 | 12.3 ± 9.8 | 395.7 ± 31.9 | 22.9 ± 2.0 |
| 33 | 44.5 ± 9.6 | 30.8 ± 3.6 | 13.7 ± 9.6 | 408.8 ± 32.9 | 20.9 ± 1.8 |
| 37 | 42.7 ± 9.6 | 33.8 ± 3.8 | 8.9 ± 9.6 | 403.8 ± 32.5 | 25.1 ± 2.1 |
| 39 | 41.0 ± 10.1 | 34.8 ± 3.9 | 6.3 ± 9.6 | 398.6 ± 32.1 | 22.1 ± 1.9 |
| 41 | 47.3 ± 10.3 | 36.9 ± 4.0 | 10.4 ± 9. | 399.9 ± 32.2 | 25.0 ± 2.1 |
| 42 | 41.9 ± 10.9 | 32.6 ± 3.7 | 9.4 ± 10.3 | 407.7 ± 32.8 | 21.5 ± 1.9 |
| 44 | --- | 32.0 ± 3.6 | --- | 386.9 ± 31.2 | 20.2 ± 1.8 |
| 46 | 38.4 ± 8.6 | 26.8 ± 3.3 | 11.6 ± 8.6 | 397.3 ± 32.0 | 19.7 ± 1.7 |
| 50 | 42.0 ± 9.4 | 32.5 ± 3.4 | 9.6 ± 9.4 | 379.1 ± 30.5 | 22.9 ± 2.0 |
| Range this work | 38.4–69 | 25–37 | --- | 294–525 | 20–30 |
| Average this work | 47.8 | 32.3 | --- | 407.6 | 23.8 |
| World average [99,100] | --- | 32 (17–60) | --- | 420 (140–850) | 45 (11–64) |
| World soil [101] | --- | 30 (7–180) | --- | 440 (0.2–1200) | 37 (4–78) |
| UCC [102] | --- | 33 | --- | 720 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coral-Carrillo, K.; Ruiz-Gutiérrez, G.; Gómez-Arozamena, J.; Viguri, J.R. Sedimentation Rate and Contamination Levels Profile of Potentially Toxic Elements in the Limoncocha Lagoon RAMSAR Wetland in the Ecuadorian Amazon. Environments 2023, 10, 2. https://doi.org/10.3390/environments10010002
Coral-Carrillo K, Ruiz-Gutiérrez G, Gómez-Arozamena J, Viguri JR. Sedimentation Rate and Contamination Levels Profile of Potentially Toxic Elements in the Limoncocha Lagoon RAMSAR Wetland in the Ecuadorian Amazon. Environments. 2023; 10(1):2. https://doi.org/10.3390/environments10010002
Chicago/Turabian StyleCoral-Carrillo, Katty, Gema Ruiz-Gutiérrez, José Gómez-Arozamena, and Javier R. Viguri. 2023. "Sedimentation Rate and Contamination Levels Profile of Potentially Toxic Elements in the Limoncocha Lagoon RAMSAR Wetland in the Ecuadorian Amazon" Environments 10, no. 1: 2. https://doi.org/10.3390/environments10010002
APA StyleCoral-Carrillo, K., Ruiz-Gutiérrez, G., Gómez-Arozamena, J., & Viguri, J. R. (2023). Sedimentation Rate and Contamination Levels Profile of Potentially Toxic Elements in the Limoncocha Lagoon RAMSAR Wetland in the Ecuadorian Amazon. Environments, 10(1), 2. https://doi.org/10.3390/environments10010002







