Emotion Evaluation and Response Slowing in a Non-Human Primate: New Directions for Cognitive Bias Measures of Animal Emotion?
Abstract
:1. Introduction
2. Methods
2.1. Participants and Housing
2.2. Stimuli and Apparatus
2.3. Procedure
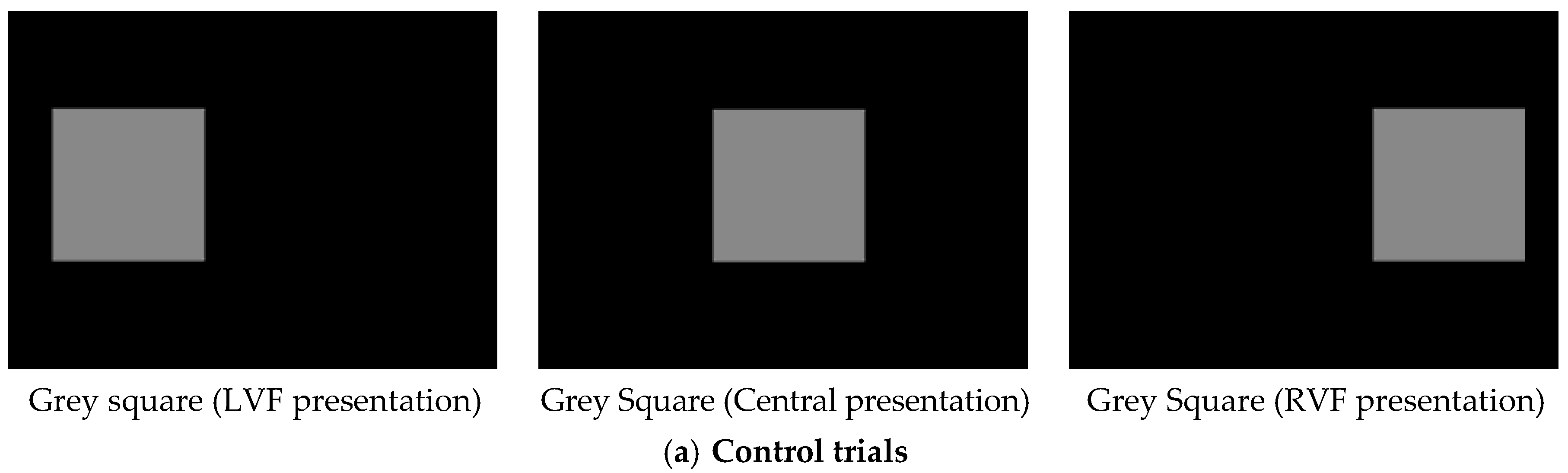
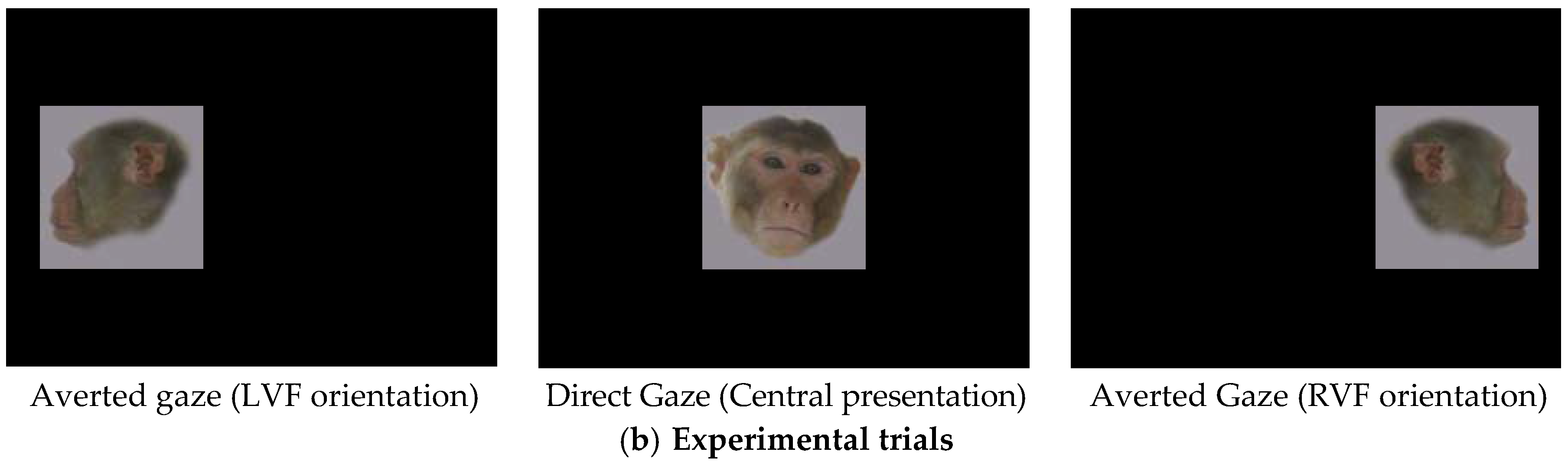
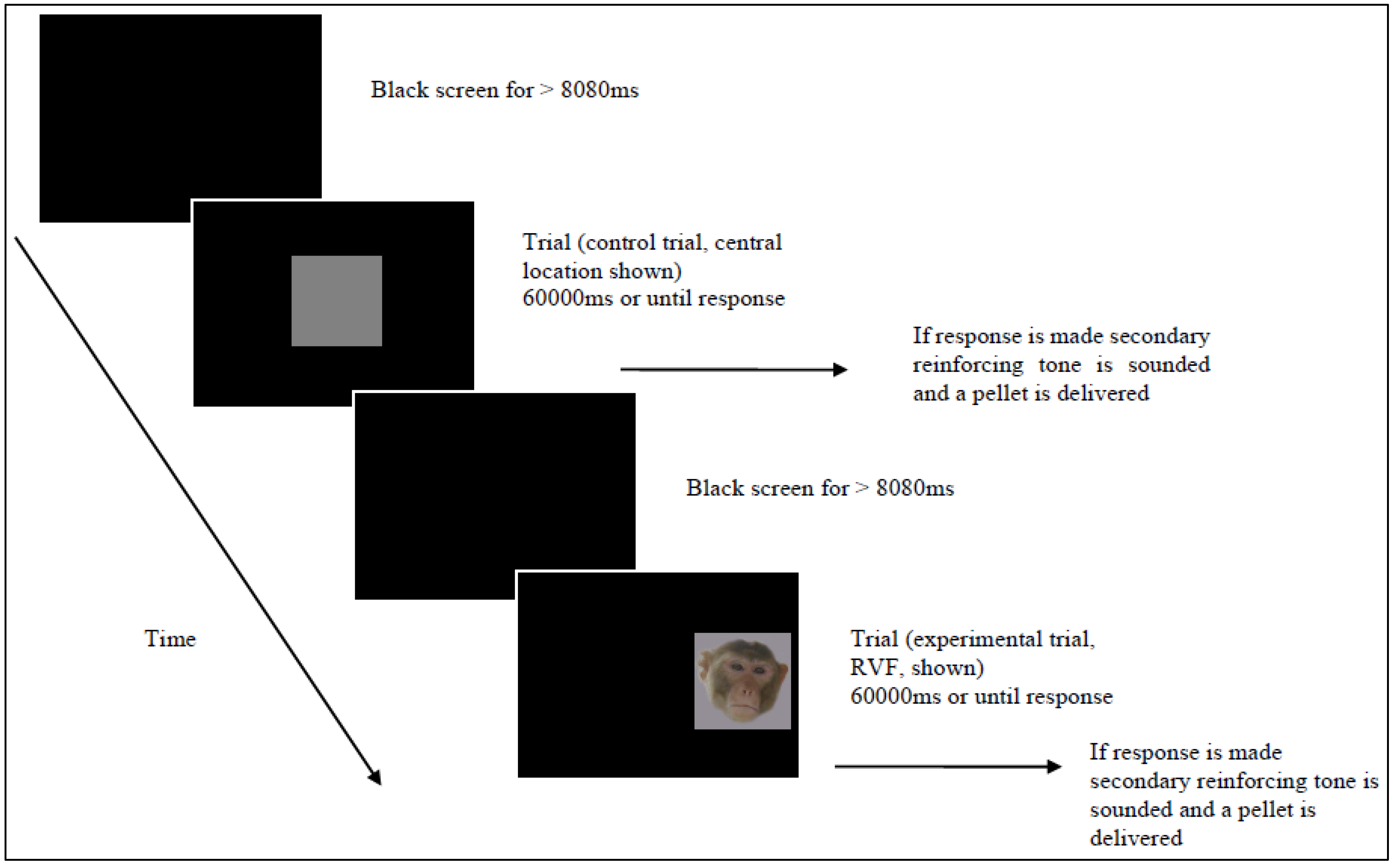

2.4. Data Treatment and Statistical Analysis
3. Results
3.1. Emotion State and Emotional Distractor Interact to Impair Performance
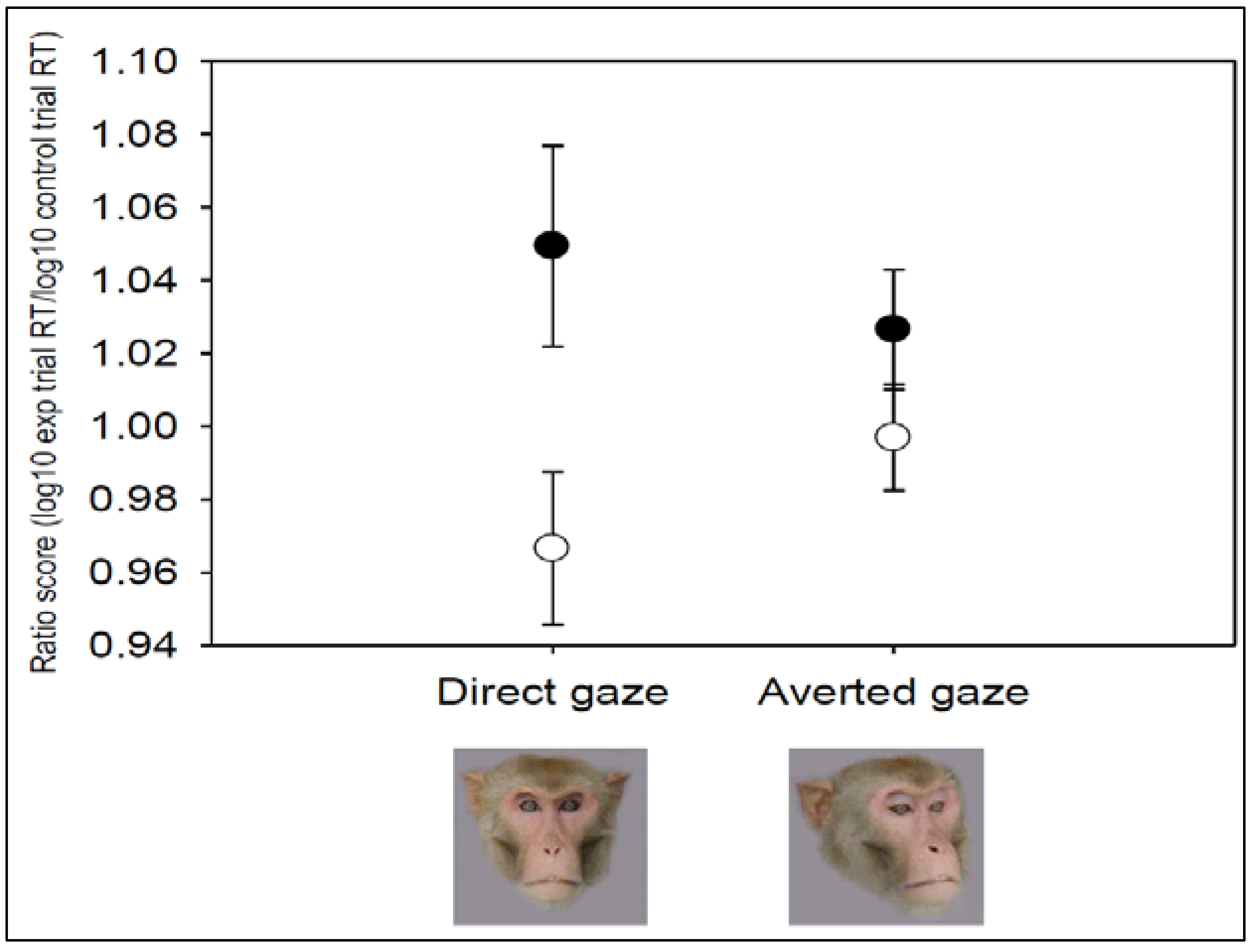
3.2. Additional Tests
3.2.1. Previous Stimulus Seen Does Not Affect Latency to Respond in Current Trials
3.2.2. Stress-Related Arousal Speeds Responses on the Basic Operant Tasks
3.2.3. Food Motivation Does Not Differ between Conditions
4. Discussion
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harding, E.J.; Paul, E.S.; Mendl, M. Animal behaviour: Cognitive bias and affective state. Nature 2004, 427, 312–312. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Burman, O.H.P.; Parker, R.M.A.; Paul, E.S. Cognitive bias as an indicator of animal emotion and welfare: Emerging evidence and underlying mechanisms. Appl. Anim. Behav. Sci. 2009, 118, 161–181. [Google Scholar] [CrossRef]
- Hales, C.A.; Stuart, S.A.; Anderson, M.H.; Robinson, E.S.J. Modelling cognitive affective biases in major depressive disorder using rodents. Br. J. Pharmacol. 2014, 171, 4524–4538. [Google Scholar] [CrossRef] [PubMed]
- Bethell, E.J. A “how-to” guide for designing judgment bias studies to assess captive animal welfare. J. Appl. Anim. Welf. Sci. 2015, 18, S18–S42. [Google Scholar] [CrossRef] [PubMed]
- Bethell, E.J.; Holmes, A.; MacLarnon, A.; Semple, S. Cognitive bias in a non-human primate: Husbandry procedures influence cognitive indicators of psychological well-being in captive rhesus macaques. Anim. Welf. 2012, 21, 185–195. [Google Scholar] [CrossRef]
- Bar-Haim, Y.; Lamy, D.; Pergamin, L.; Bakermans-Kranenburg, M.J.; van Ijzendoorn, M.H. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol. Bull. 2007, 133, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Macleod, C.; Mathews, A.; Tata, P. Attentional bias in emotional disorders. J. Abnorm. Psychol. 1986, 95, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Bethell, E.J.; Holmes, A.; MacLarnon, A.; Semple, S. Evidence that emotion mediates social attention in rhesus macaques. PLoS ONE 2012, 7, e44387. [Google Scholar] [CrossRef] [PubMed]
- Paul, E.S.; Harding, E.J.; Mendl, M. Measuring emotional processes in animals: The utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005, 29, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, E.; Ferguson, D.; Lee, C. Are hungry sheep more pessimistic? The effects of food restriction on cognitive bias and the involvement of ghrelin in its regulation. Physiol. Behav. 2014, 123, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Brilot, B.O.; Normandale, C.L.; Parkin, A.; Bateson, M. Can we use starlings’ aversion to eyespots as the basis for a novel ‘cognitive bias’ task? Appl. Anim. Behav. Sci. 2009, 118, 182–190. [Google Scholar] [CrossRef]
- Garner, M.; Mogg, K.; Bradley, B.P. Orienting and maintenance of gaze to facial expressions in social anxiety. J. Abnorm. Psychol. 2006, 115, 760–770. [Google Scholar] [CrossRef] [PubMed]
- Mogg, K.; Garner, M.; Bradley, B.P. Anxiety and orienting of gaze to angry and fearful faces. Biol. Psychol. 2007, 76, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Öhman, A. Automaticity and the amygdala: Nonconscious responses to emotional faces. Curr. Dir. Psychol. Sci. 2002, 11, 62–66. [Google Scholar] [CrossRef]
- Ohman, A.; Mineka, S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol. Rev. 2001, 108, 483–522. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H.; Larson, C.; Shelton, S.E.; Davidson, R.J. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav. Neurosci. 1998, 112, 286–292. [Google Scholar] [CrossRef] [PubMed]
- LeDoux, J. The Emotional Brain; Simon & Schuster: New York, NY, USA, 1996. [Google Scholar]
- Kalin, N.H.; Shelton, S.E. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann. NY Acad. Sci. 2003, 1008, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H.; Shelton, S.E.; Rickman, M.; Davidson, R.J. Individual differences in freezing and cortisol in infant and mother rhesus monkeys. Behav. Neurosci. 1998, 112, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Algom, D.; Chajut, E.; Lev, S. A rational look at the emotional stroop phenomenon: A generic slowdown, not a stroop effect. J. Exp. Psychol. Gen. 2004, 133, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Russo, R.; Bowles, R.; Dutton, K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? J. Exp. Psychol.: Gen. 2001, 130, 681–700. [Google Scholar] [CrossRef]
- Frings, C.; Englert, J.; Wentura, D.; Bermeitinger, C. Decomposing the emotional stroop effect. Q. J. Exp. Psychol. 2010, 63, 42–49. [Google Scholar] [CrossRef] [PubMed]
- McKenna, F.P.; Sharma, D. Reversing the emotional stroop effect reveals that it is not what it seems: The role of fast and slow components. J. Exp. Psychol. Learn. Mem. Cognit. 2004, 30, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Mogg, K.; Holmes, A.; Garner, M.; Bradley, B.P. Effects of threat cues on attentional shifting, disengagement and response slowing in anxious individuals. Behav. Res. Therapy 2008, 46, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, R.J.; Blanchard, D.C. Attack and defense in rodents as ethoexperimental models for the study of emotion. Prog. Neuro Psychopharmacol. Biol. Psychiatr. 1989, 13, S3–S14. [Google Scholar] [CrossRef]
- Lang, P.J.; Bradley, M.M.; Cuthbert, B.N. Motivated attention: Affect, activation, and action. In Attention and orienting: Sensory and Motivational Processe; Lang, R.F.S., Balaban, M.T., Eds.; Erlbaum: Hillsdale, NJ, USA, 1997; pp. 97–135. [Google Scholar]
- Bradley, M.M. Natural selective attention: Orienting and emotion. Psychophysiology 2009, 46, 1–11. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, N.; Corr, P.J. A two-dimensional neuropsychology of defense: Fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004, 28, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Buss, K.A.; Davidson, R.J.; Kalin, N.H.; Goldsmith, H.H. Context-specific freezing and associated physiological reactivity as a dysregulated fear response. Dev. Psychol. 2004, 40, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Hagenaars, M.A.; Oitzl, M.; Roelofs, K. Updating freeze: Aligning animal and human research. Neurosci. Biobehav. Rev. 2014, 47, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.C.; Griebel, G.; Pobbe, R.; Blanchard, R.J. Risk assessment as an evolved threat detection and analysis process. Neurosci. Biobehav. Rev. 2011, 35, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Gallup, G.G. Tonic immobility: The role of fear and predation. Psychol. Rec. 1977, 27, 41–61. [Google Scholar]
- Oler, J.A.; Fox, A.S.; Shelton, S.E.; Rogers, J.; Dyer, T.D.; Davidson, R.J.; Shelledy, W.; Oakes, T.R.; Blangero, J.; Kalin, N.H. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 2010, 466, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Maestripieri, D. Gestural communication in macaques: Usage and meaning of nonvocal signals. Evolut. Commun. 1997, 1, 193–222. [Google Scholar] [CrossRef]
- Sato, W.; Aoki, S. Right hemispheric dominance in processing of unconscious negative emotion. Brain Cognit. 2006, 62, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Adolphs, R.; Damasio, H.; Tranel, D.; Damasio, A.R. Cortical systems for the recognition of emotion in facial expressions. J. Neurosci. 1996, 16, 7678–7687. [Google Scholar] [PubMed]
- Coulon, M.; Nowak, R.; Andanson, S.; Petit, B.; Levy, F.; Boissy, A. Effects of prenatal stress and emotional reactivity of the mother on emotional and cognitive abilities in lambs. Dev. Psychobiol. 2015, 57, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Mauer, N.; Borkenau, P. Temperament and early information processing: Temperament-related attentional bias in emotional stroop tasks. Personal. Individ. Differ. 2007, 43, 1063–1073. [Google Scholar] [CrossRef]
- Holmes, A.; Bradley, B.P.; Kragh Nielsen, M.; Mogg, K. Attentional selectivity for emotional faces: Evidence from human electrophysiology. Psychophysiology 2009, 46, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.; Duncan, J.; Brett, M.; Lawrence, A.D. Prefrontal cortical function and anxiety: Controlling attention to threat-related stimuli. Nat. Neurosci. 2004, 7, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.M.G.; Mathews, A.; MacLeod, C. The emotional stroop task and psychopathology. Psychol. Bull. 1996, 120, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Mathews, A.; Macleod, C. Selective processing of threat cues in anxiety-states. Behav. Res. Therapy 1985, 23, 563–569. [Google Scholar] [CrossRef]
- Reynolds, M.G.; Langerak, R.M. Emotional stroop dilution: The boundary conditions of attentional capture by threat words. Acta. Psychol. 2015, 159, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Allritz, M.; Call, J.; Borkenau, P. How chimpanzees (pan troglodytes) perform in a modified emotional stroop task. Anim. Cognit. 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Allritz, M.; (Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany). personal communication, 2015.
- Aston-Jones, G.; Cohen, J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Davis, M.; Öhman, A. Fear and anxiety: Animal models and human cognitive psychophysiology. J. Affect. Disord. 2000, 61, 137–159. [Google Scholar] [CrossRef]
- Pearson, J.M.; Watson, K.K.; Platt, M.L. Decision making: The neuroethological turn. Neuron 2014, 82, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Burman, O.H.; Parker, R.M.; Paul, E.S.; Mendl, M. Sensitivity to reward loss as an indicator of animal emotion and welfare. Biol. Lett. 2008, 4, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Mendl, M.; Paul, E.S. Consciousness, emotion and animal welfare: Insights from cognitive science. Anim. Welf. 2004, 13, S17–S25. [Google Scholar]
- NC3Rs. Primate accommodation, care and use. Available online: https://www.nc3rs.org.uk/non-human-primate-accommodation-care-and-use (accessed on 10 October 2015).
- NC3Rs. The macaque website, national centre for the replacement, refiunement and reduction of animal in research. Available online: http://www.nc3rs.org.uk/macaques/ (accessed on 4 September 2015).
- Psychology Software Tools Inc. [E-Prime 1.1]. Available online: http://www.Pstnet.Com.2012 (accessed on 7 July 2005).
- Chajut, E.; Mama, Y.; Levy, L.; Algom, D. Avoiding the approach trap: A response bias theory of the emotional stroop effect. J. Exp. Psychol. Learn. Memory Cognit. 2010, 36, 1567. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.P.; Mogg, K.; Falla, S.J.; Hamilton, L.R. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognit. Emot. 1998, 12, 737–753. [Google Scholar] [CrossRef]
- Hommer, R.E.; Meyer, A.; Stoddard, J.; Connolly, M.E.; Mogg, K.; Bradley, B.P.; Pine, D.S.; Leibenluft, E.; Brotman, M.A. Attention bias to threat faces in severe mood dysregulation. Depression & Anxiety (1091–4269) 2014, 31, 559–565. [Google Scholar] [PubMed]
- Ly, V.; Huys, Q.J.M.; Stins, J.F.; Roelofs, K.; Cools, R. Individual differences in bodily freezing predict emotional biases in decision making. Front. Behav. Neurosci. 2014, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Salthouse, T.A.; Hedden, T. Interpreting reaction time measures in between-group comparisons. J. Clin. Exp. Neuropsychol. 2002, 24, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Urry, K.; Burns, N.R.; Baetu, I. Accuracy-based measures provide a better measure of sequence learning than reaction time-based measures. Front. Psychol. 2015, 6, 1158. [Google Scholar] [CrossRef] [PubMed]
- Mayerl, J. Controlling the baseline speed of respondents: An empirical evaluation of data treatment methods of response latencies. In Proceedings of the Sixth international Conference on Logic and Methodology, Amsterdam, The Netherlands, 2005; Dijkum, C., Blasius, J., van Hilton, B., Eds.; 2005; pp. 1–20. [Google Scholar]
- Partan, S.R. Single and multichannel signal composition: Facial expressions and vocalizations of rhesus macaques (macaca mulatta). Behaviour 2002, 139, 993–1027. [Google Scholar] [CrossRef]
- Deaner, R.O.; Khera, A.V.; Platt, M.L. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Curr. Biol. 2005, 15, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.K.; Ghodasra, J.H.; Furlong, M.A.; Platt, M.L. Visual preferences for sex and status in female rhesus macaques. Anim. Cognit. 2012, 15, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Haude, R.H.; Graber, J.G.; Farres, A.G. Visual observing by rhesus-monkeys—Some relationships with social-dominance rank. Anim. Learn. Behav. 1976, 4, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Higham, J.P.; Hughes, K.D.; Brent, L.J.N.; Dubuc, C.; Engelhardt, A.; Heistermann, M.; Maestriperi, D.; Santos, L.R.; Stevens, M. Familiarity affects the assessment of female facial signals of fertility by free-ranging male rhesus macaques. Proc. R. Soc. B Biol. Sci. 2011, 278, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Mandalaywala, T.M.; Parker, K.J.; Maestripieri, D. Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychol. Sci. 2014, 25, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.K.; Ghodasra, J.H.; Platt, M.L. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.K.; Platt, M.L. Neuroethology of reward and decision making. Philosophical Transactions of the Royal Society B Biol. Sci. 2008, 363, 3825–3835. [Google Scholar] [CrossRef] [PubMed]
- Deaner, R.O.; Platt, M.L. Reflexive social attention in monkeys and humans. Curr. Biol. 2003, 13, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.V.; Deaner, R.O.; Platt, M.L. Social status gates social attention in monkeys. Curr. Biol. 2006, 16, R119–R120. [Google Scholar] [CrossRef] [PubMed]
- Higham, J.P.; Heistermann, M.; Maestripieri, D. The endocrinology of male rhesus macaque social and reproductive status: A test of the challenge and social stress hypotheses. Behav. Ecol. Sociobiol. 2013, 67, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, N.; Keil, A. Accelerative and decelerative effects of hedonic valence and emotional arousal during visual scene processing. Q. J. Exp. Psychol. 2013, 66, 1276–1301. [Google Scholar] [CrossRef] [PubMed]
- Bateson, M.; Nettle, D. Development of a cognitive bias methodology for measuring low mood in chimpanzees. Peerj. 2015, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.; (Lincoln Park Zoo, Chicago, USA). personal communication, 2015.
- Champoux, M.; Bennett, A.; Shannon, C.; Higley, J.D.; Lesch, K.P.; Suomi, S.J. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol. Psychiatr. 2002, 7, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, G.A.; Capaldo, T.; Lindner, L.; Grow, G. Building an inner sanctuary: Complex PTSD in chimpanzees. J. Trauma Dissociation 2008, 9, 9–34. [Google Scholar] [CrossRef] [PubMed]
- NC3Rs. Our vision 2015–2025, national centre for the replacement refinement and reduction of animals in research. Available online: https://www.nc3rs.org.uk/sites/default/files/documents/Corporate_publications/NC3Rs%20Our%20Vision%202015-2025.pdf (accessed on 10 October 2015).
- Animal Procedures Committee (A.P.C.). Review of the assessment of cumulative severity and lifetime experience in non-human primates used in neuroscience research. Available online: https://www.Gov.Uk/government/uploads/system/uploads/attachment_data/file/261687/cs_nhp_review_final_2013_corrected.Pdf (accessed on 10 October 2015).
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bethell, E.J.; Holmes, A.; MacLarnon, A.; Semple, S. Emotion Evaluation and Response Slowing in a Non-Human Primate: New Directions for Cognitive Bias Measures of Animal Emotion? Behav. Sci. 2016, 6, 2. https://doi.org/10.3390/bs6010002
Bethell EJ, Holmes A, MacLarnon A, Semple S. Emotion Evaluation and Response Slowing in a Non-Human Primate: New Directions for Cognitive Bias Measures of Animal Emotion? Behavioral Sciences. 2016; 6(1):2. https://doi.org/10.3390/bs6010002
Chicago/Turabian StyleBethell, Emily J., Amanda Holmes, Ann MacLarnon, and Stuart Semple. 2016. "Emotion Evaluation and Response Slowing in a Non-Human Primate: New Directions for Cognitive Bias Measures of Animal Emotion?" Behavioral Sciences 6, no. 1: 2. https://doi.org/10.3390/bs6010002
APA StyleBethell, E. J., Holmes, A., MacLarnon, A., & Semple, S. (2016). Emotion Evaluation and Response Slowing in a Non-Human Primate: New Directions for Cognitive Bias Measures of Animal Emotion? Behavioral Sciences, 6(1), 2. https://doi.org/10.3390/bs6010002






