Discrimination within Recognition Memory in Schizophrenia
Abstract
:1. Introduction
- 1)
- Probands with schizophrenia were expected to demonstrate significantly lower discrimination (d') on nonverbal and verbal tasks of recognition memory compared to nonpsychiatric controls and probands with bipolar disorder.
- 2)
- Relatives of patients with schizophrenia were expected to demonstrate significantly lower discrimination (d') compared to nonpsychiatric controls and relatives of patients with bipolar disorder, for both nonverbal and verbal material.
- 3)
- Recognition of geometric designs was expected to be higher than recognition of nonsense designs across all groups. However, probands with schizophrenia and relatives of patients with schizophrenia were expected to demonstrate a significantly lower benefit from geometric design types compared to nonpsychiatric controls, probands with bipolar disorder and relatives of patients with bipolar disorder.
- 4)
- In comparison to controls and probands with bipolar disorder, probands with schizophrenia were expected to demonstrate significantly greater use of a serial strategy (a less efficient strategy) and significantly lower use of a semantic strategy for recall of a list of words. A similar pattern was expected in first-degree biological relatives of patients with schizophrenia.
2. Method
2.1. Participants
2.1.1. Patient Probands
| Variable | Schizophrenia probands | Bipolar probands | Controls | Test value (df) | p-value |
|---|---|---|---|---|---|
| (N = 40) | (N = 30) | (N = 65) | |||
| Age (Years) | 44.75 (10.2) | 44.03 (9.8) | 44.57 (13.5) | F(2,132) = 0.03 | n.s. a |
| Gender (M/F) | 33/7 | 23/7 | 29/36 | χ2 (2,N = 145) = 18.4 | < 0.001 |
| Ethnicity | |||||
| Caucasian | 35/37 | 29/29 | 56/57 | NA | NA |
| African American | 2/1 | 0/0 | 5/5 | NA | NA |
| Hispanic | 1/0 | 0/0 | 0/0 | NA | NA |
| Asian | 1/1 | 0/0 | 0/0 | NA | NA |
| Native American | 1/1 | 1/1 | 2/1 | NA | NA |
| #x2003;Other | 0/0 | 0/0 | 2/2 | NA | NA |
| Education (Years) | 13.95 (2.3) | 15.17 (2.1) | 15.11 (1.9) | F(2,132) = 4.61 | 0.012 a |
| Estimated IQ | 96.75 (12.9) | 111.90 (16.1) | 109.71 (11.0) | F(2,132) = 16.19 | < 0.001 a |
| SAPS Mean Score | 0.54 (0.5) | 0.18 (0.2) | NA | t(57.24) = 4.30 | < 0.001 b,c |
| SANS Mean Score | 0.91 (0.5) | 0.33 (0.3) | NA | t(61.57) = 6.49 | < 0.001 b,c |
| BPRS Mean Score | 1.79 (0.5)N = 39 | 1.49 (0.4)N = 29 | NA | t(65.99) = 2.80 | 0.007 b,c |
| CPZ (mg) | 646 (442)N = 14 | 316.67 (76.4)N = 3 | NA | t(14.98) = 2.61 | 0.020 b,c |
2.1.2. First-Degree Biological Relatives
2.1.3. Nonpsychiatric Controls
| Variable | Schizophrenia relatives | Bipolar relatives | Controls | Test value (df) | p-value |
|---|---|---|---|---|---|
| (N = 52) | (N = 31) | (N = 65) | |||
| Age (Years) | 49.85 (11.1) | 46.45 (14.3) | 44.57 (13.5) | F(2,145) = 2.45 | n.s. a |
| Gender (M/F) | 20/32 | 19/12 | 29/36 | χ2(2,N = 148) = 4.2 | n.s. |
| Ethnicity | |||||
| Caucasian | 46/47 | 30/30 | 56/57 | NA | NA |
| African American | 2/2 | 0/0 | 5/5 | NA | NA |
| Hispanic | 4/1 | 0/0 | 0/0 | NA | NA |
| Asian | 0/2 | 0/0 | 0/0 | NA | NA |
| Native American | 0/0 | 0/1 | 2/1 | NA | NA |
| Other | 0/0 | 1/0 | 2/2 | NA | NA |
| Education (Years) | 14.62 (2.3) | 14.32 (3.1) | 15.11 (1.9) | F(2, 145) = 1.37 | n.s. a |
| Estimated IQ | 105.17 (14.0) | 108.97 (15.3) | 109.71 (11.0) | F(2, 145) = 1.85 | n.s. a |
| SPQ Total Score | 14.85 (10.0)N = 48 | 16.39 (15.1)N = 28 | 10.38 (6.2)N = 64 | F(2, 137) = 4.76 | 0.010 a |
2.2. Procedures
2.2.1. Recurring Figures Test
2.2.2. California Verbal Learning Test (CVLT)
2.3. Measure of Discrimination in Recognition Memory
2.3.1. Recurring Figures Test
2.3.2. CVLT
3. Results
| Variable | Schizophrenia probands | Bipolar probands | Controls | Schizophrenia relatives | Bipolar relatives |
|---|---|---|---|---|---|
| (N = 40) | (N = 30) | (N = 65) | (N = 52) | (N = 31) | |
| RFT: d' Total | 1.53 (0.76) a,c | 2.05 (0.91) c | 2.16 (0.71) a | 2.18 (0.65) | 2.16 (0.55) |
| (Trials 2–6) | |||||
| RFT: d' Total | 1.48 (0.93) a,b | 2.24 (1.10) b | 2.39 (0.76) a | 2.19 (0.88) | 2.20 (0.74) |
| (Trials 7–8) | |||||
| RFT: d' Geometric | 2.53 (1.16) a,c | 3.17 (1.21)c | 3.45 (0.95) a | 3.55 (0.87) | 3.33 (0.88) |
| Designs (Trials 2–6) | |||||
| RFT: d' Geometric | 2.82 (1.43) a | 3.24 (1.31) | 3.83 (0.95) a | 3.70 (0.90) | 3.71 (0.99) |
| Designs (Trials 7–8) | |||||
| RFT: d' Nonsense | 1.05 (0.73) b,c | 1.54 (0.94)c | 1.59 (0.82) b | 1.55 (0.73) | 1.58 (0.75) |
| Designs (Trials 2–6) | |||||
| RFT: d' Nonsense | 0.92 (1.03) a,b | 1.89 (1.17)b | 1.79 (1.07) a | 1.62 (1.26) | 1.47 (1.19) |
| Designs (Trials 7–8) | |||||
| CVLT: d' Recognition | 2.63 (0.99) b1,b2 | 3.61 (0.86)b1,N = 29 | 3.54 (0.86) b2 | 3.42 (0.93) | 3.64 (0.79) |
3.1. Recurring Figures Test
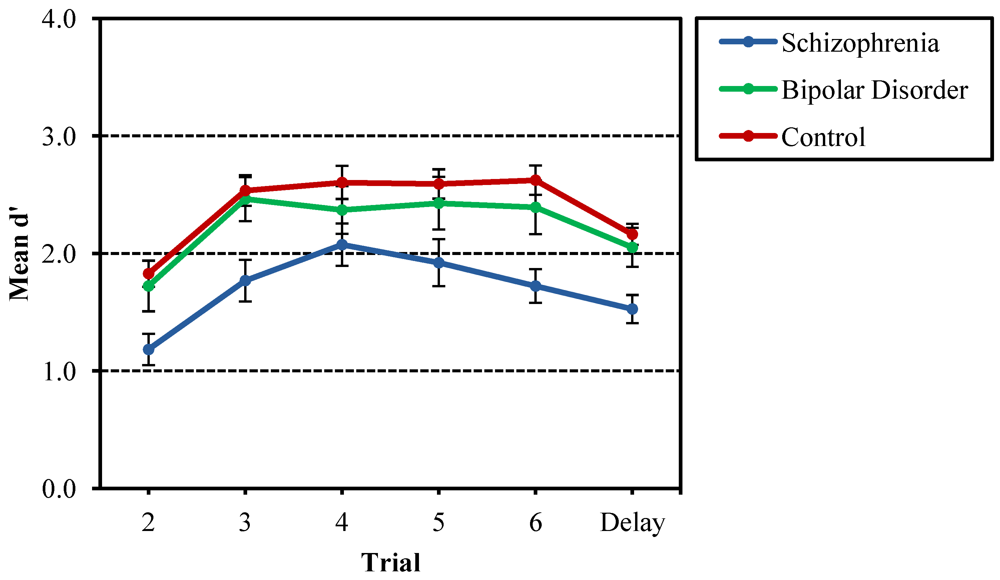
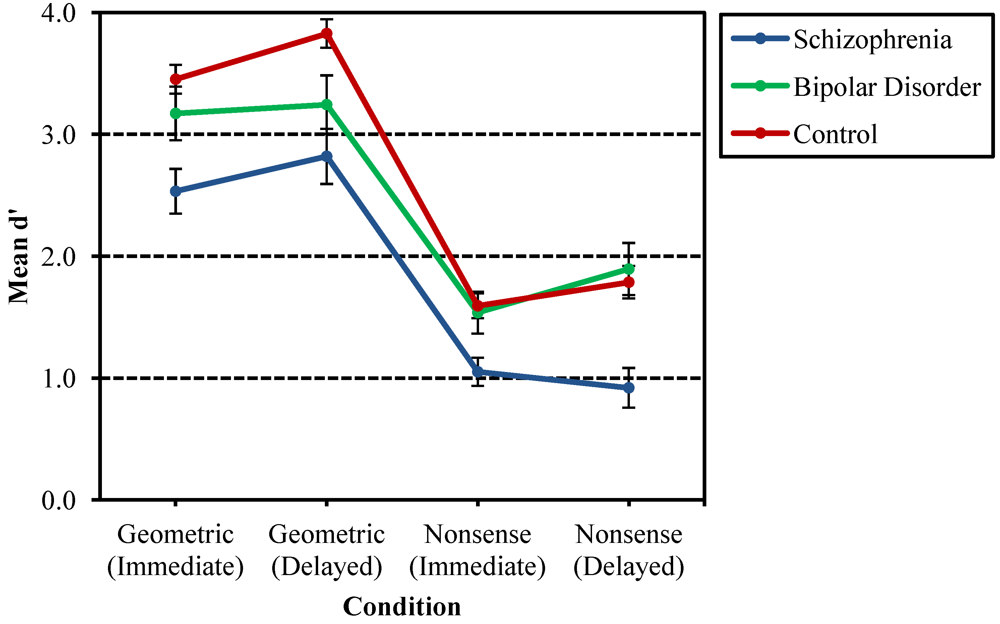
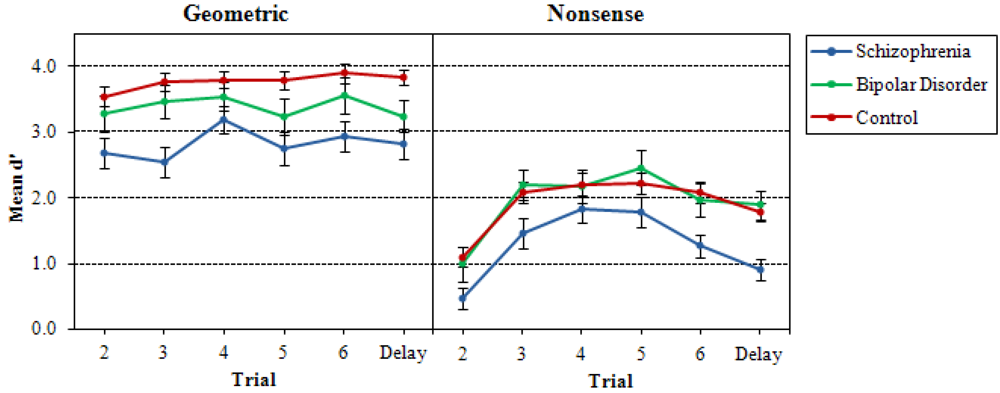
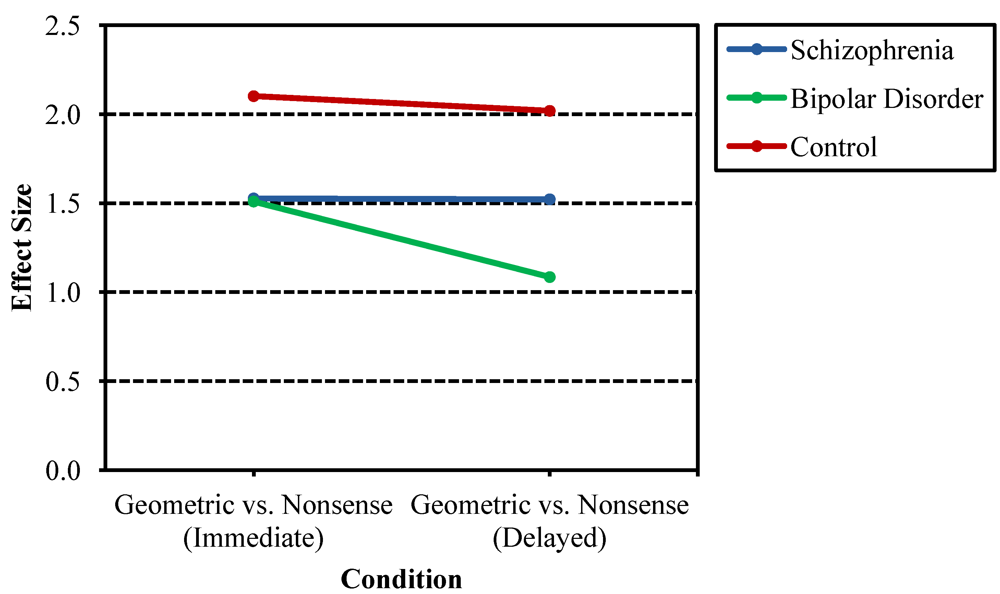
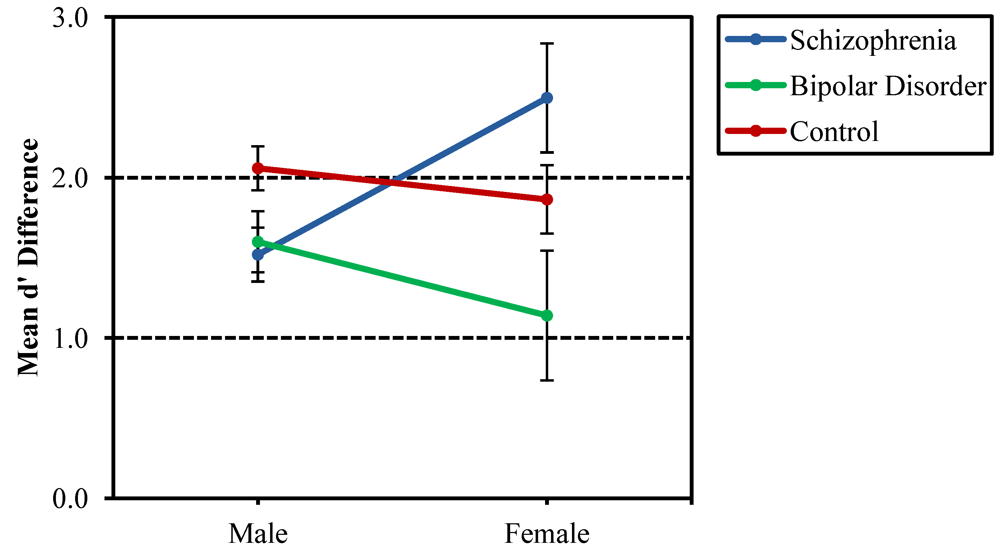
3.2. CVLT
3.3. Correlation Analysis
3.4. Supplemental Analyses
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Kraepelin, E. Psychiatrie: Ein Kurzes Lehrbuch fur Studirende und Aerzte, 4th ed.; Abel: Barth, Germany, 1893. [Google Scholar]
- Rund, B.R. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr. Bull. 1998, 24, 425–434. [Google Scholar] [CrossRef]
- Cannon, T.D.; Huttunen, M.O.; Lonnqvist, J.; Tuulio-Henriksson, A.; Pirkola, T.; Glahn, D.; Finkelstein, J.; Hietanen, M.; Kaprio, J.; Koskenvuo, M. The inheritance of neuropsychological dysfunction in twins discordant for schizophrenia. Am. J. Hum. Genet. 2000, 67, 369–382. [Google Scholar] [CrossRef]
- Faraone, S.V.; Seidman, L.J.; Kremen, W.S.; Pepple, J.R.; Lyons, M.J.; Tsuang, M.T. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: A diagnostic efficiency analysis. J. Abnorm. Psychol. 1995, 104, 286–304. [Google Scholar] [CrossRef]
- Greenwood, T.A.; Swerdlow, N.R.; Gur, R.E.; Cadenhead, K.S.; Calkins, M.E.; Dobie, D.J.; Freedman, R.; Green, M.F.; Gur, R.C.; Lazzeroni, L.C.; et al. Genome-wide linkage analyses of 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophrenia. Am. J. Psychiat. 2013, 170, 521–532. [Google Scholar]
- LeBlanc, M.; Kulle, B.; Sundet, K.; Agartz, I.; Melle, I.; Djurovic, S.; Frigessi, A.; Andreassen, O.A. Genome-wide study identifies PTPRO and WDR72 and FOXQ1-SUMO1P1 interaction associated with neurocognitive function. J. Psychiatr. Res. 2012, 46, 271–278. [Google Scholar] [CrossRef]
- Porteous, D.J.; Thompson, P.; Brandon, N.J.; Millar, J.K. The genetics and biology of DISC1—An emerging role in psychosis and cognition. Biol. Psychiat. 2006, 60, 123–131. [Google Scholar] [CrossRef]
- Toomey, R.; Faraone, S.V.; Seidman, L.J.; Kremen, W.S.; Pepple, J.R.; Tsuang, M.T. Association of neuropsychological vulnerability markers in relatives of schizophrenic patients. Schizophr. Res. 1998, 31, 89–98. [Google Scholar] [CrossRef]
- Tuulio-Henriksson, A.T.; Haukka, J.; Partonen, T.; Varilo, T.; Paunio, T.; Ekelund, J.; Cannon, T.D.; Meyer, J.M.; Lonnqvist, J. Heritability and number of quantitative trait loci of neurocognitive functions in families with schizophrenia. Am. J. Med. Genet. B 2002, 114, 483–490. [Google Scholar] [CrossRef]
- Egan, M.F.; Goldberg, T.E.; Kolachana, B.S.; Callicott, J.H.; Mazzanti, C.M.; Straub, R.E.; Goldman, D.; Weinberger, D.R. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. USA 2001, 98, 6917–6922. [Google Scholar] [CrossRef]
- Eisenberg, D.P.; Berman, K.F. Executive function, Neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology 2010, 35, 258–277. [Google Scholar] [CrossRef]
- Lawrie, S.M.; Whalley, H.C.; Abukmeil, S.S.; Kestelman, J.N.; Donnelly, L.; Miller, P.; Best, J.J.K.; Cunningham Owens, D.G.; Johnstone, E.C. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol. Psychiat. 2001, 49, 811–823. [Google Scholar]
- Seidman, L.J.; Faraone, S.V.; Goldstein, J.M.; Goodman, J.M.; Kremen, W.S.; Matsuda, G.; Hoge, E.A.; Kennedy, D.; Makris, N.; Caviness, V.S.; et al. Reduced subcortical brain volumes in nonpsychotic siblings of schizophrenic patients: A pilot magnetic resonance imaging study. Am. J. Med. Genet. B 1997, 74, 507–514. [Google Scholar] [CrossRef]
- Staal, W.G.; Hulshoff Pol, H.E.; Schnack, H.G.; Hoogendoorn, M.L.C.; Jellema, K.; Kahn, R.S. Structural brain abnormalities in patients with schizophrenia and their healthy siblings. Am. J. Psychiat. 2000, 153, 416–421. [Google Scholar]
- Green, M.F.; Kern, R.S.; Braff, D.L.; Mintz, J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophr. Bull. 2000, 26, 119–136. [Google Scholar] [CrossRef]
- Calkins, M.E.; Gur, R.C.; Ragland, J.D.; Gur, R.E. Face recognition memory deficits and visual object memory performance in patients with schizophrenia and their relatives. Am. J. Psychiat. 2005, 162, 1963–1966. [Google Scholar] [CrossRef]
- Conklin, H.M.; Curtis, C.E.; Calkins, M.E.; Iacono, W.G. Working memory functioning in schizophrenia patients and their first-degree relatives: Cognitive functioning shedding light on etiology. Neuropsychologia 2005, 43, 930–942. [Google Scholar] [CrossRef]
- Robles, O.; Blaxton, T.; Adami, H.; Arango, C.; Thaker, G.; Gold, J. Nonverbal delayed recognition in the relatives of schizophrenia patients with or without schizophrenia spectrum. Biol. Psychiat. 2008, 63, 498–504. [Google Scholar] [CrossRef]
- Giakoumaki, S.G.; Roussos, P.; Pallis, E.G.; Bitsios, P. Sustained attention and working memory deficits follow a familial pattern in schizophrenia. Arch. Clin. Neuropsych. 2011, 26, 687–695. [Google Scholar] [CrossRef]
- Hill, S.K.; Harris, M.S.; Herbener, E.S.; Pavuluri, M.; Sweeney, J.A. Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr. Bull. 2008, 34, 743–759. [Google Scholar]
- Keshavan, M.S.; Kulkami, S.; Bhojraj, T.; Francis, A.; Diwadkar, V.; Montrose, D.M.; Seidman, L.J.; Sweeney, J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front. Hum. Neurosci. 2010, 3, 62. [Google Scholar]
- Zalla, T.; Joyce, C.; Szoke, A.; Schurhoff, F.; Pillon, B.; Komano, O.; Perez-Diaz, F.; Bellivier, F.; Alter, C.; Dubois, B.; Rouillon, F.; et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiat. Res. 2004, 121, 207–217. [Google Scholar] [CrossRef]
- Cannon, T.D.; Zorrilla, L.E.; Shtasel, D.; Gur, R.E.; Gur, R.C.; Marco, E.J.; Mobert, P.; Price, A. Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch. Gen. Psychiat. 1994, 51, 651–660. [Google Scholar] [CrossRef]
- Gottesman, I.I.; Gould, T.D. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am. J. Psychiat. 2003, 160, 636–645. [Google Scholar] [CrossRef]
- Faraone, S.V.; Seidman, L.J.; Kremen, W.S.; Toomey, R.; Pepple, J.R.; Tsuang, M.T. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: The effects of genetic loading. Biol. Psychiat. 2000, 48, 120–126. [Google Scholar] [CrossRef]
- Aleman, A.; Hijman, R.; deHaan, E.H.F.; Kahn, R.S. Memory impairment in schizophrenia: A meta-analysis. Am. J. Psychiat. 1999, 159, 1358–1366. [Google Scholar]
- Fioravanti, M.; Carlone, O.; Vitale, B.; Cinti, M.E.; Clare, L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol. Rev. 2005, 15, 73–95. [Google Scholar] [CrossRef]
- Gold, J.M.; Randolph, C.; Carpenter, C.J.; Goldberg, T.E.; Weinberger, D.R. Forms of memory failure in schizophrenia. J. Abnorm. Psychol. 1992, 101, 487–494. [Google Scholar] [CrossRef]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef]
- Leavitt, V.M.; Goldberg, T.E. Episodic memory in schizophrenia. Neuropsychol. Rev. 2009, 19, 312–323. [Google Scholar] [CrossRef]
- Mesholam-Gately, R.I.; Guiliano, A.J.; Faraone, S.V.; Goff, K.P.; Seidman, L.J. Neurocognition in first-episode schizophrenia: A meta-analytic review. Neuropsychology 2009, 23, 315–336. [Google Scholar] [CrossRef]
- Paulsen, J.S.; Heaton, R.K.; Sadek, J.R.; Perry, W.; Delis, D.C.; Braff, D.; Kuck, J.; Zisook, S.; Jeste, D.V. The nature of learning and memory impairments in schizophrenia. J. Int. Neuropsych. Soc. 1995, 1, 88–89. [Google Scholar] [CrossRef]
- Stone, M.; Gabrieli, J.D.E.; Stebbins, G.T.; Sullivan, E.V. Working and strategic memory deficits in schizophrenia. Neuropsychology 1998, 12, 278–288. [Google Scholar] [CrossRef]
- Palmer, B.W.; Savla, G.N.; Fellows, I.E.; Twamley, E.W.; Jeste, D.V.; Lacro, J.P. Do people with schizophrenia have differential impairment in episodic memory and/or working memory relative to other cognitive abilities? Schizophr. Res. 2010, 116, 259–265. [Google Scholar] [CrossRef]
- Egan, M.F.; Goldberg, T.E.; Gscheidle, T.; Weirich, M.; Rawlings, R.; Hyde, T.M.; Bigelow, L.; Weinberger, D.R. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol. Psychiat. 2001, 50, 98–107. [Google Scholar] [CrossRef]
- Faraone, S.V.; Seidman, L.J.; Kremen, W.S.; Toomey, R.; Pepple, J.R.; Tsuang, M.T. Neuropsychological functioning among the nonpsychotic relatives of schizophrenic patients: A 4-year follow-up study. J. Abnorm. Psychol. 1999, 108, 176–181. [Google Scholar] [CrossRef]
- Goldberg, T.E.; Ragland, J.D.; Torrey, E.F.; Gold, J.M.; Bigelow, L.B.; Weinberger, D.R. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch. Gen. Psychiat. 1990, 47, 1066–1072. [Google Scholar] [CrossRef]
- Kremen, W.S.; Seidman, L.J.; Pepple, J.R.; Lyons, M.J.; Tsuang, M.T.; Faraone, S.V. Neuropsychological risk indicators for schizophrenia: A review of family studies. Schizophr. Bull. 1994, 20, 103–119. [Google Scholar] [CrossRef]
- Laurent, A.; Moreaud, O.; Bosson, J.L.; Naegele, B.; Boucharlat, J.; Saoud, M.; Dalery, J.; d’Amato, T. Neuropsychological functioning among non-psychotic siblings and parents of schizophrenic patients. Psychiat. Res. 1999, 87, 147–157. [Google Scholar] [CrossRef]
- Lyons, M.J.; Toomey, R.; Seidman, L.J.; Kremen, W.S.; Faraone, S.V.; Tsuang, M.T. Verbal learning and memory in relatives of schizophrenics: Preliminary findings. Biol. Psychiat. 1995, 37, 750–753. [Google Scholar] [CrossRef]
- Toulopoulou, T.; Morris, R.G.; Rabe-Hesketh, S.; Murray, R.M. Selectivity of verbal memory deficit in schizophrenic patients and their relatives. Am. J. Med. Genet. 2003, 116B, 1–7. [Google Scholar] [CrossRef]
- Toulopoulou, T.; Rabe-Hesketh, S.; King, H.; Murray, R.M.; Morris, R.G. Episodic memory in schizophrenic patients and their relatives. Schizophr. Res. 2003, 63, 261–271. [Google Scholar] [CrossRef]
- Whyte, M.C.; McIntosh, A.M.; Johnstone, E.C.; Lawrie, S.M. Declarative memory in unaffected adult relatives of patients with schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2005, 78, 13–26. [Google Scholar] [CrossRef]
- Barch, D.M.; Csernansky, J.G.; Conturo, T.; Snyder, A.Z. Working and long-term memory deficits in schizophrenia: Is there a common prefrontal mechanism? J. Abnorm. Psychol. 2002, 111, 478–494. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Cognition in schizophrenia: Impairments, Determinants, and functional importance. Psychiat. Clin. N. Am. 2005, 28, 613–633. [Google Scholar] [CrossRef]
- Grillon, M.L.; Krebs, M.O.; Gourevitch, R.; Giersch, A.; Huron, C. Episodic memory and impairment of an early encoding process in schizophrenia. Neuropsychology 2010, 24, 101–108. [Google Scholar] [CrossRef]
- Fiszdon, J.M.; Silverstein, S.M.; Buchwald, J.; Hull, J.W.; Smith, T.E. Verbal memory in schizophrenia: sex differences over repeated assessments. Schizophr. Res. 2003, 61, 235–243. [Google Scholar] [CrossRef]
- Tsai, P.C.; McDowd, J.; Tang, T.C.; Su, C.Y. Processing speed mediates gender differences in memory in schizophrenia. Clin. Neuropsychol. 2012, 26, 626–640. [Google Scholar] [CrossRef]
- Vaskinn, A.; Sundet, K.; Simonsen, C.; Hellvin, T.; Melle, I.; Andreassen, O.A. Sex differences in neuropsychological performance and social functioning in schizophrenia and bipolar disorder. Neuropsychology 2011, 25, 499–510. [Google Scholar] [CrossRef]
- Goldberg, T.E. Some fairly obvious distinctions between schizophrenia and bipolar disorder. Schizophr. Res. 1999, 39, 127–132. [Google Scholar] [CrossRef]
- Seidman, L.J.; Kremen, W.S.; Koren, D.; Faraone, S.V.; Goldstein, J.M.; Tsuang, M.T. A comparative profile analysis of neuropsychological functioning in patients with schizophrenia and bipolar psychoses. Schizophr. Res. 2002, 53, 31–44. [Google Scholar] [CrossRef]
- Lewis, R. Should cognitive deficit be a diagnostic criterion for schizophrenia? J. Psychiatr. Neurosci. 2004, 29, 102–113. [Google Scholar]
- Green, M.F.; Nuechterlein, K.H.; Gold, J.M.; Barch, D.M.; Cohen, J.; Essock, S.; Fenton, W.S.; Frese, F.; Goldberg, T.E.; Heaton, R.K.; et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiat. 2004, 56, 301–307. [Google Scholar] [CrossRef]
- Davidson, M.; Reichenberg, A.; Rabinowitz, J.; Weiser, M.; Kaplan, Z.; Mark, M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am. J. Psychiat. 1999, 156, 1328–1335. [Google Scholar]
- Reichenberg, A.; Weiser, M.; Rapp, M.A.; Rabinowitz, J.; Caspi, A.; Schmeidler, J.; Knobler, H.Y.; Lubin, G.; Nahon, D.; Harvey, P.D.; et al. Elaboration on premorbid intellectual performance in schizophrenia: Premorbid intellectual decline and risk for schizophrenia. Arch. Gen. Psychiat. 2005, 62, 1297–1304. [Google Scholar] [CrossRef]
- Tiihonen, J.; Haukka, J.; Henriksson, M.; Cannon, M.; Kieseppa, T.; Laaksonen, I.; Sinivuo, J.; Lonnqvist, J. Premorbid intellectual functioning in bipolar disorder and schizophrenia: Results from a cohort study of male conscripts. Am. J. Psychiat. 2005, 162, 1904–1910. [Google Scholar] [CrossRef]
- Zammit, S.; Allebeck, P.; David, A.S.; Dalman, C.; Hemmingsson, T.; Lundberg, I.; Lewis, G. A longitudinal study of premorbid IQ score and risk of developing schizophrenia, bipolar disorder, severe depression, and other nonaffective psychoses. Arch. Gen. Psychiat. 2004, 61, 354–360. [Google Scholar] [CrossRef]
- Asarnow, R.F.; Nuechterlein, K.H.; Asamen, J.; Fogelson, D.; Subotnik, K.L.; Zaucha, K.; Guthrie, D. Neurocognitive functioning and schizophrenia spectrum disorders can be independent expressions of familial liability for schizophrenia in community control children: The UCLA family study. Schizophr. Res. 2002, 54, 111–120. [Google Scholar] [CrossRef]
- Asarnow, R.F.; MacCrimmon, D.J. Attention/information processing, neuropsychological functioning, and thought disorder during the acute and partial recovery phases of schizophrenia: A longitudinal study. Psychiat. Res. 1982, 7, 309–319. [Google Scholar] [CrossRef]
- Frame, C.L.; Oltmanns, T.F. Serial recall by schizophrenic and affective patients during and after psychotic episodes. J. Abnorm. Psychol. 1982, 91, 311–318. [Google Scholar] [CrossRef]
- Nuechterlein, K.H.; Dawson, M.E. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr. Bull. 1984, 10, 160–203. [Google Scholar] [CrossRef]
- Pelletier, M.; Achim, A.M.; Montoya, A.; Lal, S.; Lepage, M. Cognitive and clinical moderators of recognition memory in schizophrenia: A meta-analysis. Schizophr. Res. 2005, 74, 233–252. [Google Scholar] [CrossRef]
- Weiss, A.P.; Ellis, C.B.; Roffman, J.L.; Stufflebeam, S.; Hamalainen, M.S.; Duff, M.; Goff, D.C.; Schacter, D.L. Aberrant frontoparietal function during recognition memory in schizophrenia: A multimodal neuroimaging investigation. J. Neurosci. 2009, 29, 11347–11359. [Google Scholar] [CrossRef]
- Weiss, A.P.; Goff, D.C.; Duff, M.; Roffman, J.L.; Schacter, D.L. Distinguishing familiarity-based from source-based memory performance in patients with schizophrenia. Schizophr. Res. 2008, 99, 208–217. [Google Scholar] [CrossRef]
- Nurnberger, J.I., Jr.; Blehar, M.C.; Kaufmann, C.A.; York-Cooler, C.; Simpson, S.G.; Harkavy-Friedman, J.; Severe, J.B.; Malaspina, D.; Reich, T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch. Gen. Psychiat. 1994, 51, 849–859. [Google Scholar] [CrossRef]
- Andreasen, N.C. Scale for the Assessment of Positive Symptoms (SAPS); University of Iowa: Iowa City, IA, USA, 1983. [Google Scholar]
- Andreasen, N.C. Scale for the Assessment of Negative Symptoms (SANS); University of Iowa: Iowa City, IA, USA, 1981. [Google Scholar]
- Ventura, J.; Lukoff, D.; Nuechterlein, K.H.; Liberman, R.P.; Green, M.F.; Shaner, A. Manual for the expanded Brief Psychiatric Rating Scale. Int. J. Method. Psych. 1993, 3, 221–224. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Sprague, R.L.; Kalachnik, J.E. Reliability, validity, and a total score cutoff for the dyskinesia identification system: Condensed user scale (DISCUS) with mentally ill and mentally retarded populations. Psychopharmacol. Bull. 1991, 27, 51–58. [Google Scholar]
- Brooker, B.H.; Cyr, J.J. Tables for clinicians to use to convert WAIS-R short forms. J. Clin. Psychol. 1986, 42, 983. [Google Scholar]
- Raine, A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 1991, 17, 555–564. [Google Scholar] [CrossRef]
- American Psychiatric Association. Structured Clinical Interview for DSM-IV (SCID); American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- American Psychiatric Association. Structured Clinical interview for DSM-IV Axis II (SCID-II); American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Kendler, K.S.; Lieberman, J.A.; Walsh, D. The Structured Interview for Schizotypy (SIS): A preliminary report. Schizophr. Bull. 1989, 15, 559–571. [Google Scholar] [CrossRef]
- Delis, D.C.; Kramer, J.H.; Kaplan, E.; Ober, B.A. California Verbal Learning Test (CVLT) Manual; The Psychological Corporation: New York, NY, USA, 1987. [Google Scholar]
- Kimura, D. Right temporal-lobe damage. Arch. Neurol. 1963, 8, 264–271. [Google Scholar] [CrossRef]
- MacMillan, N.A.; Creelman, D.C. Detection Theory: A User’s Guide; Cambridge University Press: New York, NY, USA, 1991. [Google Scholar]
- Snodgrass, J.G.; Corwin, J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Q. J. Exp. Psychol. 1988, 117, 34–50. [Google Scholar] [CrossRef]
- Skelley, S.L.; Goldberg, T.E.; Egan, M.F.; Weinberger, D.R.; Gold, J.M. Verbal and visual memory: Characterizing the clinical and intermediate phenotype in schizophrenia. Schizophr. Res. 2008, 105, 78–85. [Google Scholar] [CrossRef]
- Sitskoorn, M.M.; Aleman, A.; Ebisch, S.J.; Appels, M.C.; Kahn, R.S. Cognitive deficits in relatives of patients with schizophrenia: A meta-analysis. Schizophr. Res. 2004, 71, 285–295. [Google Scholar] [CrossRef]
- Snitz, B.E.; Macdonald, A.W., III; Carter, C.S. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: A meta-analytic review of putative endophenotypes. Schizophr. Bull. 2006, 32, 179–194. [Google Scholar]
- Herlitz, A.; Airaksinen, E.; Nordstrom, E. Sex differences in episodic memory: The impact of verbal and visuospatial ability. Neuropsychology 1999, 13, 590–597. [Google Scholar] [CrossRef]
- Kramer, J.H.; Yaffe, K.; Lengenfelder, J.; Delis, D.C. Age and gender interactions on verbal memory performance. J. Int. Neuropsychol. Soc. 2003, 9, 97–102. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McGuire, K.A.; Blahnik, M.M.; Sponheim, S.R. Discrimination within Recognition Memory in Schizophrenia. Behav. Sci. 2013, 3, 273-297. https://doi.org/10.3390/bs3020273
McGuire KA, Blahnik MM, Sponheim SR. Discrimination within Recognition Memory in Schizophrenia. Behavioral Sciences. 2013; 3(2):273-297. https://doi.org/10.3390/bs3020273
Chicago/Turabian StyleMcGuire, Kathryn A., Melanie M. Blahnik, and Scott R. Sponheim. 2013. "Discrimination within Recognition Memory in Schizophrenia" Behavioral Sciences 3, no. 2: 273-297. https://doi.org/10.3390/bs3020273
APA StyleMcGuire, K. A., Blahnik, M. M., & Sponheim, S. R. (2013). Discrimination within Recognition Memory in Schizophrenia. Behavioral Sciences, 3(2), 273-297. https://doi.org/10.3390/bs3020273




