The Impact of Continuous and Partial Reinforcement on the Acquisition and Generalization of Human-Conditioned Fear
Abstract
1. Introduction
2. Methods and Materials
2.1. Participants
2.2. Stimuli and Measures
2.2.1. Stimuli
2.2.2. Online Expectancy Rating of US
2.2.3. Skin Conductance Response
2.3. Experimental Procedure
2.3.1. Habituation
2.3.2. Acquisition
2.3.3. Generalization
2.4. Statistical Analysis
3. Results
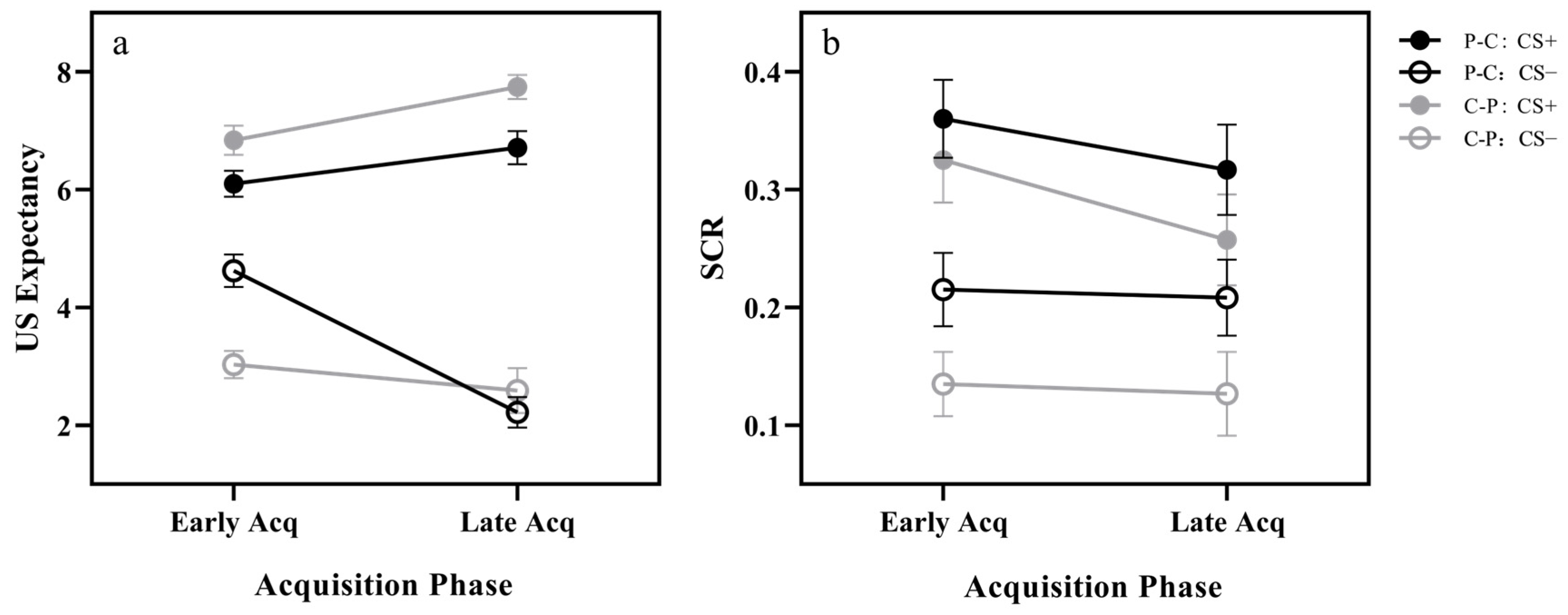
3.1. Acquisition
3.1.1. US Expectancy
3.1.2. Skin Conductance Response
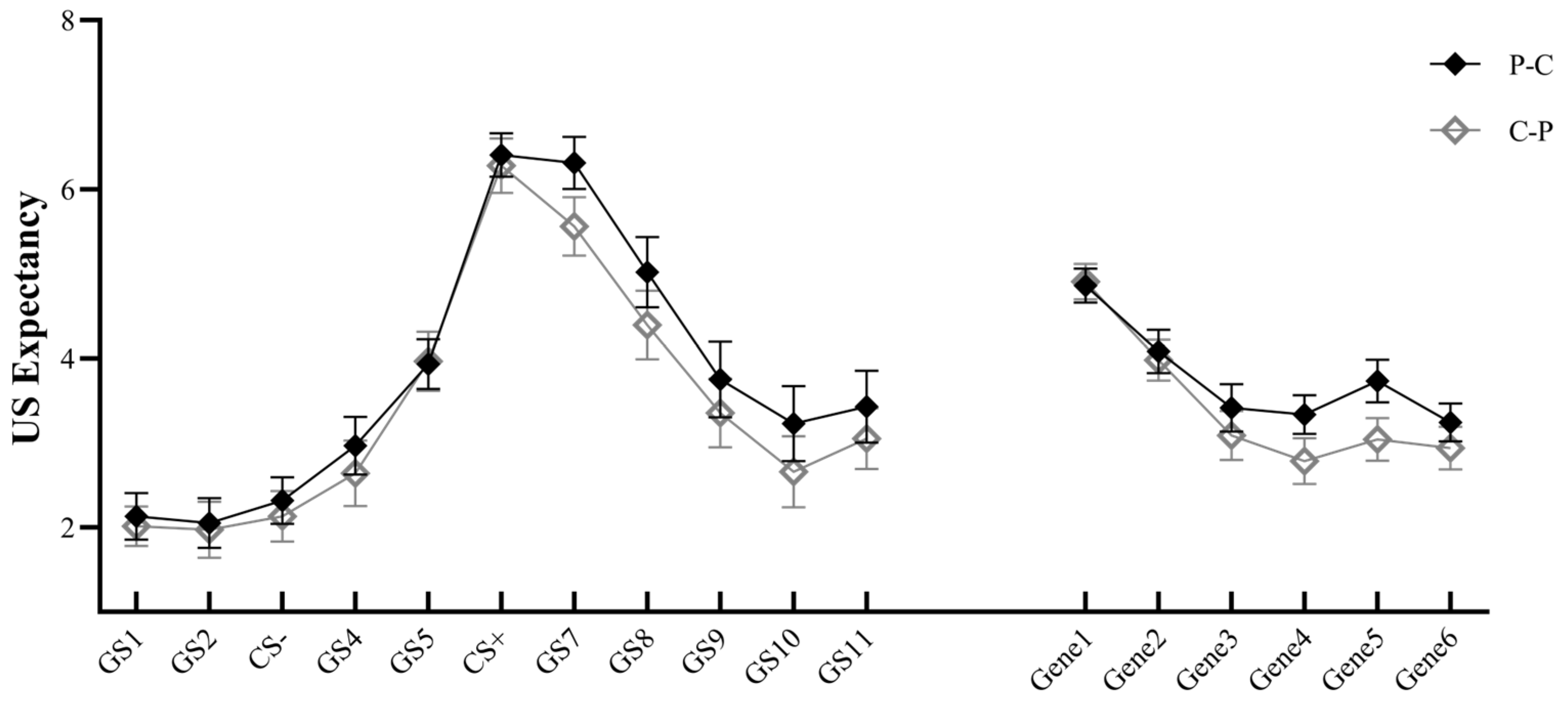
3.2. Generalization
3.2.1. US Expectancy
3.2.2. Skin Conductance Response
4. Discussion
4.1. CS–US Pairing Affects the Discrimination of Conditioned Fear Responses
4.2. Mixed Partial-then-Continuous Reinforcement Enhances Fear Generalization
4.3. Limitations of the Study
- Do you think there is a relationship between the graphs presented in the experiment and the electric shocks?
- What was the relationship?
- How certain are you about this relationship?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hovland, C.I. The generalization of conditioned responses: I. The sensory generalization of conditioned responses with varying frequencies of tone. J. Gen. Psychol. 1937, 17, 125–148. [Google Scholar] [CrossRef]
- Dunsmoor, J.E.; Martin, A.; LaBar, K.S. Role of conceptual knowledge in learning and retention of conditioned fear. Biol. Psychol. 2012, 89, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Kaczkurkin, A.N.; Rabin, S.; Geraci, M.; Pine, D.S.; Grillon, C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry 2014, 75, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, L.M.; Pauli, P.; Reif, A.; Mühlberger, A.; Langs, G.; Aalderink, T.; Wieser, M.J. Fear conditioning and stimulus generalization in patients with social anxiety disorder. J. Anxiety Disord. 2016, 44, 36–46. [Google Scholar] [CrossRef]
- Lissek, S.; Rabin, S.; Heller, R.E.; Lukenbaugh, D.; Geraci, M.; Pine, D.S.; Grillon, C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry 2010, 167, 47–55. [Google Scholar] [CrossRef]
- Lissek, S.; van Meurs, B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. Int. J. Psychophysiol. 2015, 98, 594–605. [Google Scholar] [CrossRef]
- Lissek, S.; Biggs, A.L.; Rabin, S.J.; Cornwell, B.R.; Alvarez, R.P.; Pine, D.S.; Grillon, C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behav. Res. Ther. 2008, 46, 678–687. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Enquist, M. A century of generalization. Anim. Behav. 2003, 66, 15–36. [Google Scholar] [CrossRef]
- Haubrich, J.; Bernabo, M.; Baker, A.G.; Nader, K. Impairments to consolidation, reconsolidation, and long-term memory maintenance lead to memory erasure. Annu. Rev. Neurosci. 2020, 43, 297–314. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, W.; Jie, J.; Fan, M.; Li, J.; Rong, M.; Yang, Z.; Zheng, X. The effect of partial and continuous reinforcement on the generalization of conditioned fear in humans. Learn. Motiv. 2022, 78, 101812. [Google Scholar] [CrossRef]
- Grant, D.A.; Schipper, L.M. The acquisition and extinction of conditioned eyelid responses as a function of the percentage of fixed-ratio random reinforcement. J. Exp. Psychol. 1952, 43, 313. [Google Scholar] [CrossRef] [PubMed]
- Merz, C.J.; Hermann, A.; Stark, R.; Wolf, O.T. Cortisol modifies extinction learning of recently acquired fear in men. Soc. Cogn. Affect. Neurosci. 2014, 9, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Phelps, E.A.; Delgado, M.R.; Nearing, K.I.; LeDoux, J.E. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron 2004, 43, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.W. Partial reinforcement effects in classical aversive conditioning in rabbits and human beings. J. Comp. Physiol. Psychol. 1975, 88, 596. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Wagner, A.R. Partial reinforcement of the classically conditioned eyelid response in the rabbit. J. Comp. Physiol. Psychol. 1964, 58, 157. [Google Scholar] [CrossRef]
- Dieterich, R.; Endrass, T.; Kathmann, N. Uncertainty is associated with increased selective attention and sustained stimulus processing. Cogn.Affect. Behav. Neurosci. 2016, 16, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Grupe, D.W.; Nitschke, J.B. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion 2011, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; Bandettini, P.A.; Knight, D.C. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav. Neurosci. 2007, 121, 635. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.M. Resistance to extinction when partial reinforcement is followed by regular reinforcement. J. Exp. Psychol. 1962, 64, 441. [Google Scholar] [CrossRef]
- Perry, S.L.; Moore, J.W. The partial-reinforcement effect sustained through blocks of continuous reinforcement in classical eyelid conditioning. J. Exp. Psychol. 1965, 69, 158. [Google Scholar] [CrossRef]
- Grady, A.K.; Bowen, K.H.; Hyde, A.T.; Totsch, S.K.; Knight, D.C. Effect of continuous and partial reinforcement on the acquisition and extinction of human conditioned fear. Behav. Neurosci. 2016, 130, 36. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor, J.E.; Prince, S.E.; Murty, V.P.; Kragel, P.A.; LaBar, K.S. Neurobehavioral mechanisms of human fear generalization. Neuroimage 2011, 55, 1878–1888. [Google Scholar] [CrossRef] [PubMed]
- Vervliet, B.; Geens, M. Fear generalization in humans: Impact of feature learning on conditioning and extinction. Neurobiol. Learn. Mem. 2014, 113, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Vervliet, B.; Kindt, M.; Vansteenwegen, D.; Hermans, D. Fear generalization in humans: Impact of verbal instructions. Behav. Res. Ther. 2010, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, W.; Homan, P.; Wang, P.; Zheng, X.; Schiller, D. Reminder duration determines threat memory modification in humans. Sci. Rep. 2018, 8, 8848. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, W.; Caoyang, J.; Wu, W.; Jie, J.; Xu, L.; Zheng, X. Moderate partially reduplicated conditioned stimuli as retrieval cue can increase effect on preventing relapse of fear to compound stimuli. Front. Hum. Neurosci. 2017, 11, 575. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Kanen, J.W.; LeDoux, J.E.; Monfils, M.-H.; Phelps, E.A. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proc. Natl. Acad. Sci. USA 2013, 110, 20040–20045. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Monfils, M.-H.; Raio, C.M.; Johnson, D.C.; LeDoux, J.E.; Phelps, E.A. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 2010, 463, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Lee, J.C.; Hayes, B.K.; Lovibond, P.F. Peak shift and rules in human generalization. J. Exp.Psychol. Learn. Mem. Cogn. 2018, 44, 1955. [Google Scholar] [CrossRef]
- Ahmed, O.; Lovibond, P.F. Rule-based processes in generalisation and peak shift in human fear conditioning. Q. J. Exp. Psychol. 2019, 72, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Lovibond, P.F.; Hayes, B.K.; Navarro, D.J. Negative evidence and inductive reasoning in generalization of associative learning. J. Exp.Psychol. Gen. 2019, 148, 289. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lee, J.C.; Hayes, B.K. Stimulus discriminability and induction as independent components of generalization. J. Exp. Psychol. Learn. Mem. Cogn. 2020, 46, 1106. [Google Scholar] [CrossRef] [PubMed]
- Mertens, G.; Bouwman, V.; Engelhard, I.M. Conceptual fear generalization gradients and their relationship with anxious traits: Results from a Registered Report. Int. J. Psychophysiol. 2021, 170, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kindt, M.; Soeter, M. Reconsolidation in a human fear conditioning study: A test of extinction as updating mechanism. Biol. Psychol. 2013, 92, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Society for Psychophysiological Research Ad Hoc Committee on Electrodermal Measures; Boucsein, W.; Fowles, D.C.; Grimnes, S.; Ben-Shakhar, G.; Roth, W.T.; Dawson, M.E.; Filion, D.L. Publication recommendations for electrodermal measurements. Psychophysiology 2012, 49, 1017–1034. [Google Scholar]
- Fan, M.; Zhang, D.; Zhao, S.; Xie, Q.; Chen, W.; Jie, J.; Wang, Y.; Zheng, X. Stimulus diversity increases category-based fear generalization and the effect of intolerance of uncertainty. Behav. Res. Ther. 2022, 159, 104201. [Google Scholar] [CrossRef]
- Feng, B.; Xu, L.; Zhang, W.; Chen, T.; Wang, W.; Zheng, X. The inhibitive effect of positive emotions on fear generalization. Acta Psychol. Sin. 2017, 49, 317. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Zhang, X.; Dong, Y.; Shi, P.; Luo, P.; Zheng, X. Retrieval-extinction as a reconsolidation-based treatment for emotional disorders: Evidence from an extinction retention test shortly after intervention. Behav. Res. Ther. 2021, 139, 103831. [Google Scholar] [CrossRef]
- Dai Yuqian, D.H.; Yi, L. The pure presence of others enhanced fear generalization. J. Psychol. Sci. 2023, 46, 752. [Google Scholar]
- Dunsmoor, J.E.; LaBar, K.S. Effects of discrimination training on fear generalization gradients and perceptual classification in humans. Behav. Neurosci. 2013, 127, 350. [Google Scholar] [CrossRef] [PubMed]
- Balderston, N.L.; Helmstetter, F.J. Conditioning with masked stimuli affects the timecourse of skin conductance responses. Behav. Neurosci. 2010, 124, 478. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F. Cognitive processes in extinction. Learn. Mem. 2004, 11, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Madaboosi, S.; Grasser, L.R.; Chowdury, A.; Javanbakht, A. Neurocircuitry of contingency awareness in Pavlovian fear conditioning. Cogn.Affect. Behav. Neurosci. 2021, 21, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.T.; Frank, M.J. A cholinergic feedback circuit to regulate striatal population uncertainty and optimize reinforcement learning. Elife 2015, 4, e12029. [Google Scholar] [CrossRef] [PubMed]
- Lamba, A.; Frank, M.J.; FeldmanHall, O. Anxiety impedes adaptive social learning under uncertainty. Psychol. Sci. 2020, 31, 592–603. [Google Scholar] [CrossRef]
- Behrens, T.E.; Hunt, L.T.; Woolrich, M.W.; Rushworth, M.F. Associative learning of social value. Nature 2008, 456, 245–249. [Google Scholar] [CrossRef]
- Dickinson, A. Contemporary Animal Learning Theory; Cambridge University: Cambridge, UK, 1980. [Google Scholar]
- Xu, L.; Ou, S.; Zheng, X.; Chen, T.; Feng, B.; Yan, P. The impact of state anxiety on fear generalization. Acta Psychol. Sin. 2016, 48, 1507. [Google Scholar] [CrossRef]
- Angela, J.Y.; Dayan, P. Uncertainty, neuromodulation, and attention. Neuron 2005, 46, 681–692. [Google Scholar]
- Spence, K.W. Cognitive and drive factors in the extinction of the conditioned eye blink in human subjects. Psychol. Rev. 1966, 73, 445. [Google Scholar] [CrossRef]
- Lissek, S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: The case for conditioned overgeneralization. Depress. Anxiety 2012, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Lissek, S.; Bradford, D.E.; Alvarez, R.P.; Burton, P.; Espensen-Sturges, T.; Reynolds, R.C.; Grillon, C. Neural substrates of classically conditioned fear-generalization in humans: A parametric fMRI study. Soc. Cogn. Affect. Neurosci. 2014, 9, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, T.; Carlson, J.M.; Cha, J.; Hajcak, G.; Mujica-Parodi, L.R. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress. Anxiety 2013, 30, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Bechara, A.; Tranel, D.; Damasio, H.; Adolphs, R.; Rockland, C.; Damasio, A.R. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 1995, 269, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- David, C.K.; Hanh, T.N.; Peter, A.B. The role of awareness in delay and trace fear conditioning in humans. Cogn. Affect. Behav. Neurosci. 2006, 6, 157–162. [Google Scholar] [CrossRef]
- Knight, D.C.; Nguyen, H.T.; Bandettini, P.A. Expression of conditional fear with and without awareness. Proc. Natl. Acad. Sci. USA 2003, 100, 15280–15283. [Google Scholar] [CrossRef] [PubMed]
- Manns, J.R.; Clark, R.E.; Squire, L. Single-cue delay eyeblink conditioning is unrelated to awareness. Cogn. Affect. Behav. Neurosci. 2001, 1, 192. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J. Mechanisms of exposure therapy: How neuroscience can improve psychological treatments for anxiety disorders. Clin. Psychol. Rev. 2007, 27, 750–759. [Google Scholar] [CrossRef]
- McGuire, J.F.; Lewin, A.B.; Storch, E.A. Enhancing exposure therapy for anxiety disorders, obsessive-compulsive disorder and post-traumatic stress disorder. Expert Rev. Neurother. 2014, 14, 893–910. [Google Scholar] [CrossRef]
- Forbes, D.; Bisson, J.I.; Monson, C.M.; Berliner, L. Effective Treatments for PTSD; Guilford Publications: New York, NY, USA, 2020. [Google Scholar]


| Group | Habituation | Acquisition | Generalization | |
|---|---|---|---|---|
| Block1 | Block2 | |||
| P–C group | 3(L1), 3(L2) | 4(CS+) (2US) 4CS− | 4(CS+) (4US) 4CS− | 6(GS1), 6(GS2), 12(CS−), 6(GS4), 6(GS5), 12(CS+), 6(GS7), 6(GS8), 6(GS9), 6(GS10), 6(GS11) |
| C–P group | 3(L1), 3(L2) | 4(CS+) (4US) 4CS− | 4(CS+) (2US) 4CS− | 6(GS1), 6(GS2), 12(CS−), 6(GS4), 6(GS5), 12(CS+), 6(GS7), 6(GS8), 6(GS9), 6(GS10), 6(GS11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Zhao, S.; Rong, M.; Liu, Y.; Gao, Y.; Chen, W.; Zhang, D.; Zheng, X. The Impact of Continuous and Partial Reinforcement on the Acquisition and Generalization of Human-Conditioned Fear. Behav. Sci. 2024, 14, 630. https://doi.org/10.3390/bs14080630
Song Y, Zhao S, Rong M, Liu Y, Gao Y, Chen W, Zhang D, Zheng X. The Impact of Continuous and Partial Reinforcement on the Acquisition and Generalization of Human-Conditioned Fear. Behavioral Sciences. 2024; 14(8):630. https://doi.org/10.3390/bs14080630
Chicago/Turabian StyleSong, Yidan, Shaochen Zhao, Muxin Rong, Ying Liu, Yu Gao, Wei Chen, Donghuan Zhang, and Xifu Zheng. 2024. "The Impact of Continuous and Partial Reinforcement on the Acquisition and Generalization of Human-Conditioned Fear" Behavioral Sciences 14, no. 8: 630. https://doi.org/10.3390/bs14080630
APA StyleSong, Y., Zhao, S., Rong, M., Liu, Y., Gao, Y., Chen, W., Zhang, D., & Zheng, X. (2024). The Impact of Continuous and Partial Reinforcement on the Acquisition and Generalization of Human-Conditioned Fear. Behavioral Sciences, 14(8), 630. https://doi.org/10.3390/bs14080630





