Neural Signals Associated with Orienting Response and Arousal Inhibition in Concealed Information Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
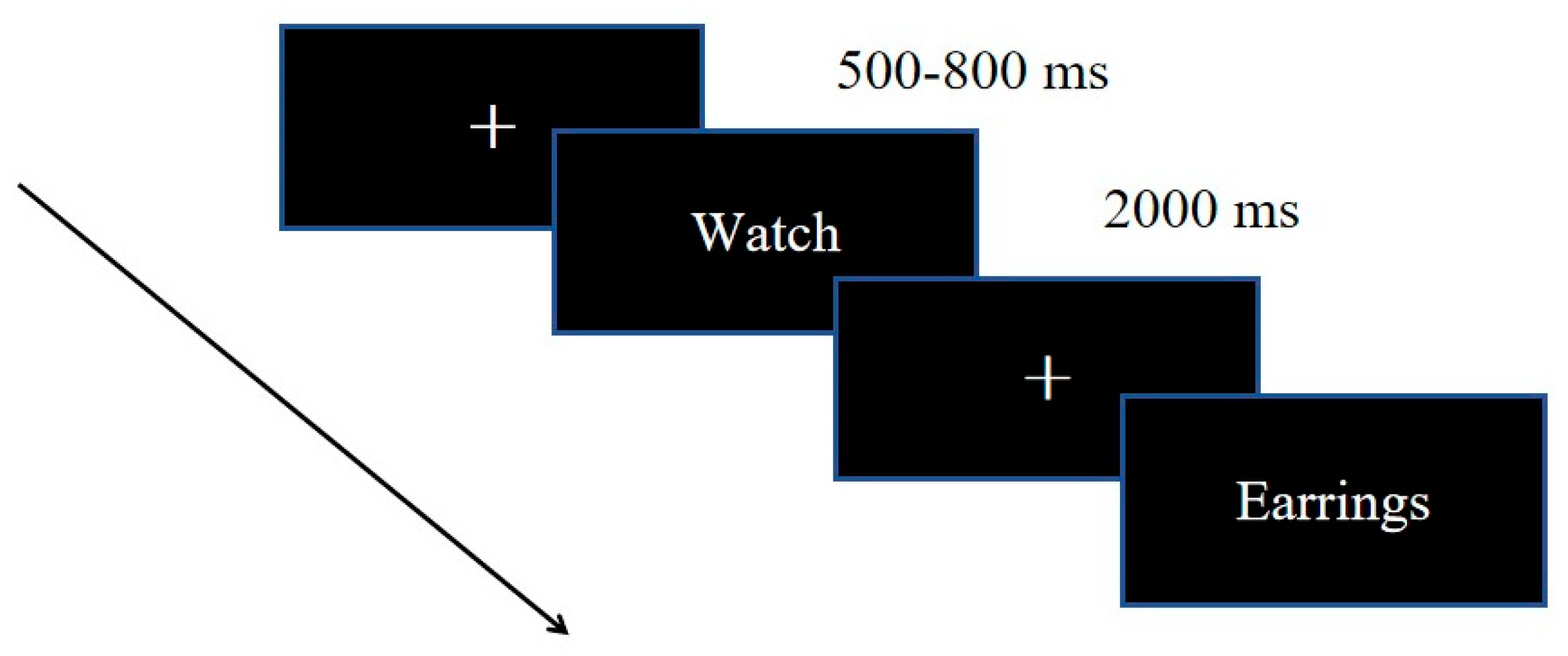
2.2. Procedure
2.3. EEG Data Acquisition
2.4. Statistical Analysis
3. Results
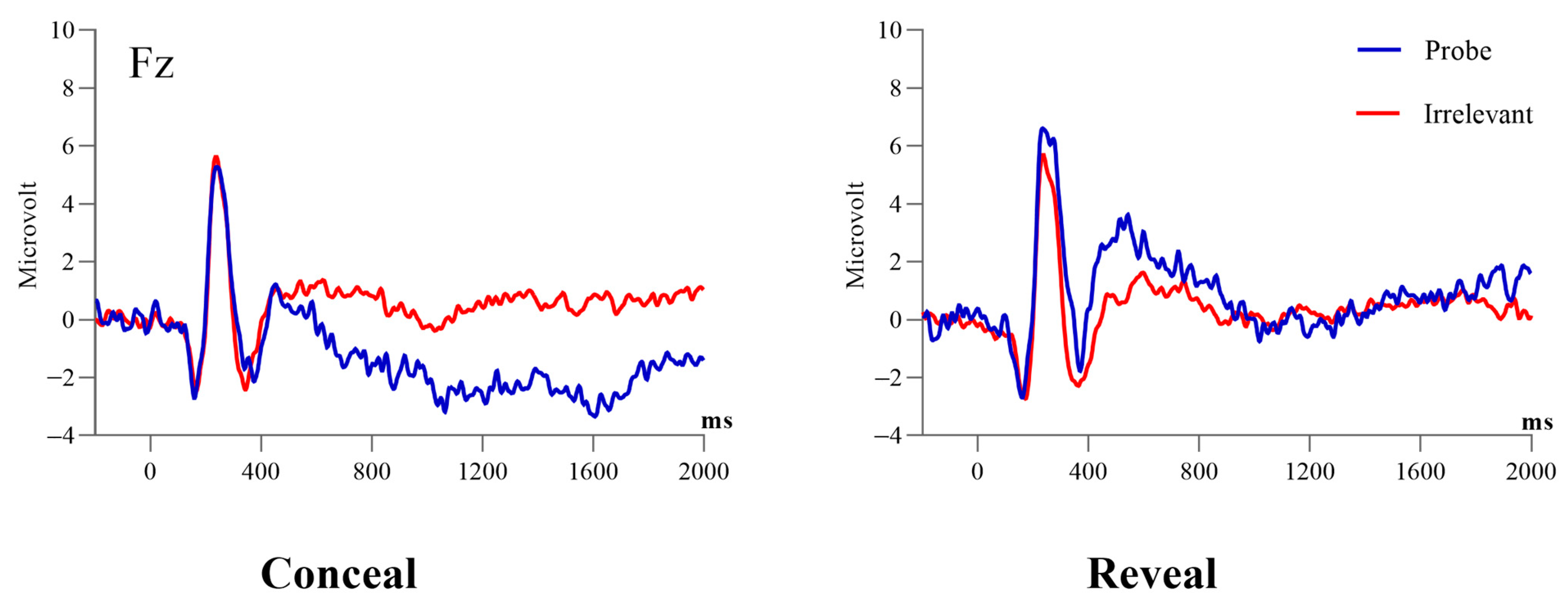
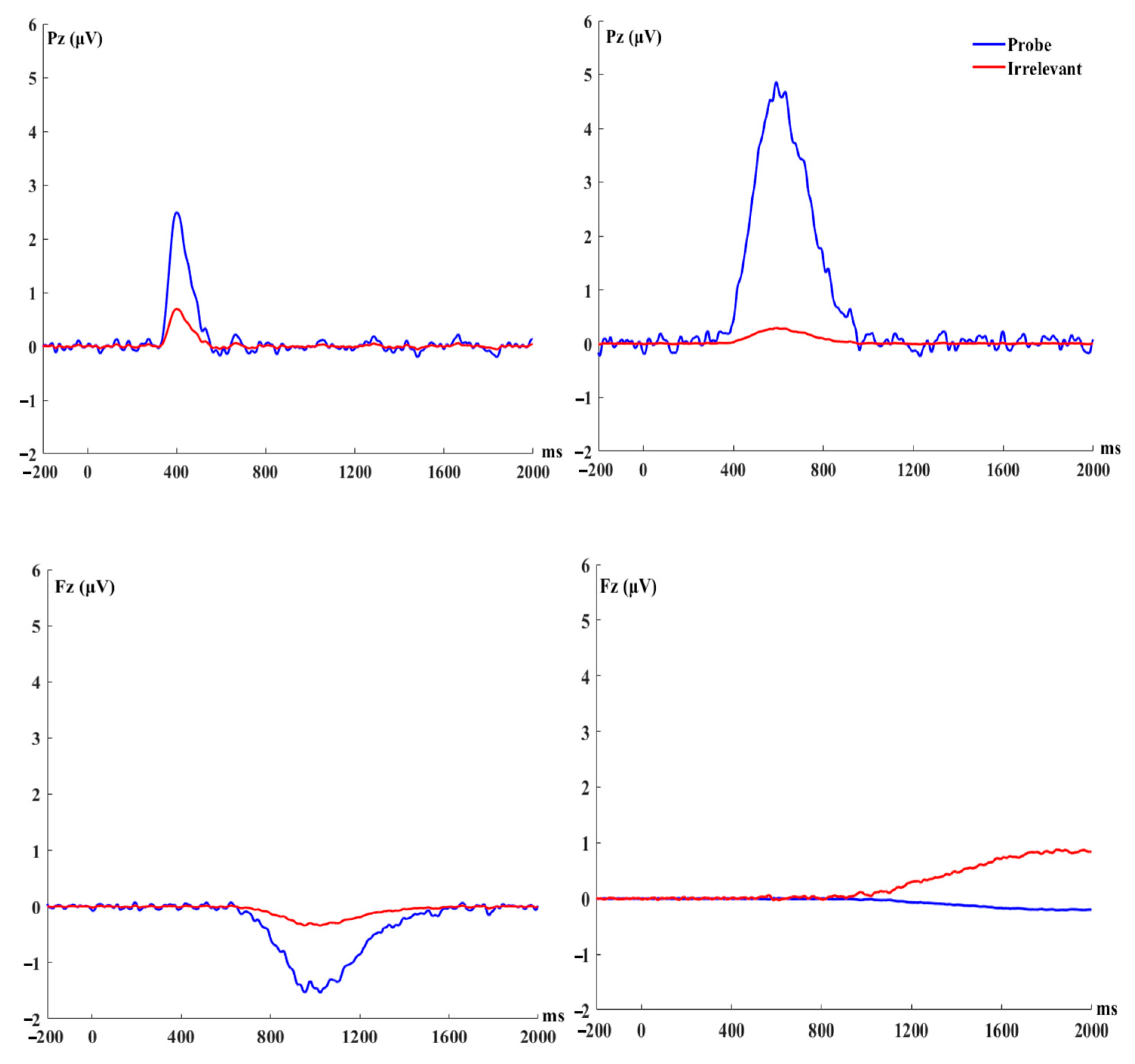
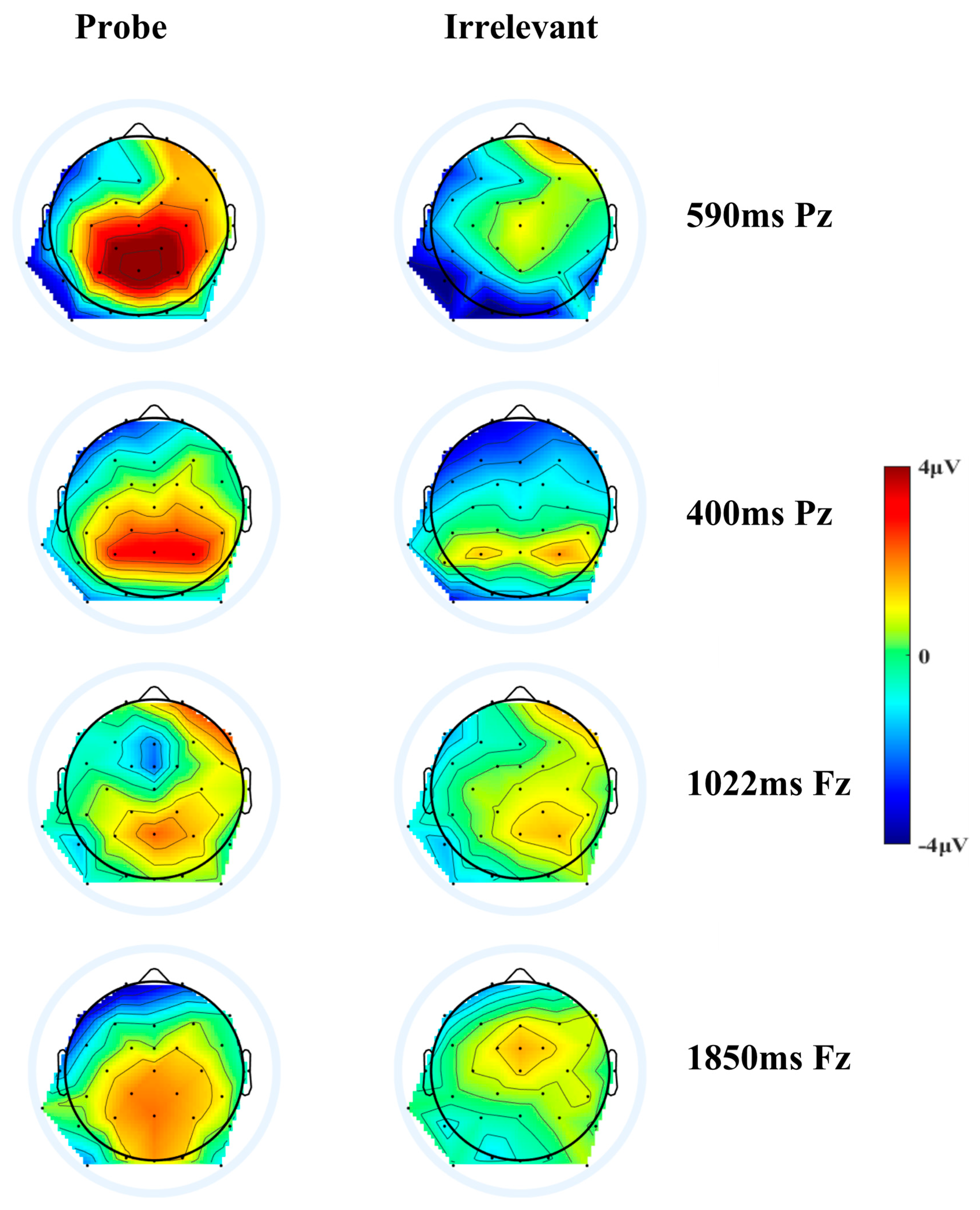
3.1. P300 to Probe vs. Irrelevant (The Parietal Positivity Peaking at 400 ms)
3.2. P300 to Probe vs. Irrelevant (The Parietal Positivity Peaking at 590 ms)
3.3. Exploratory Analyses
3.3.1. The Frontal Negativity Peaking at 1022 ms
3.3.2. The Frontal Negativity Peaking at 1850 ms
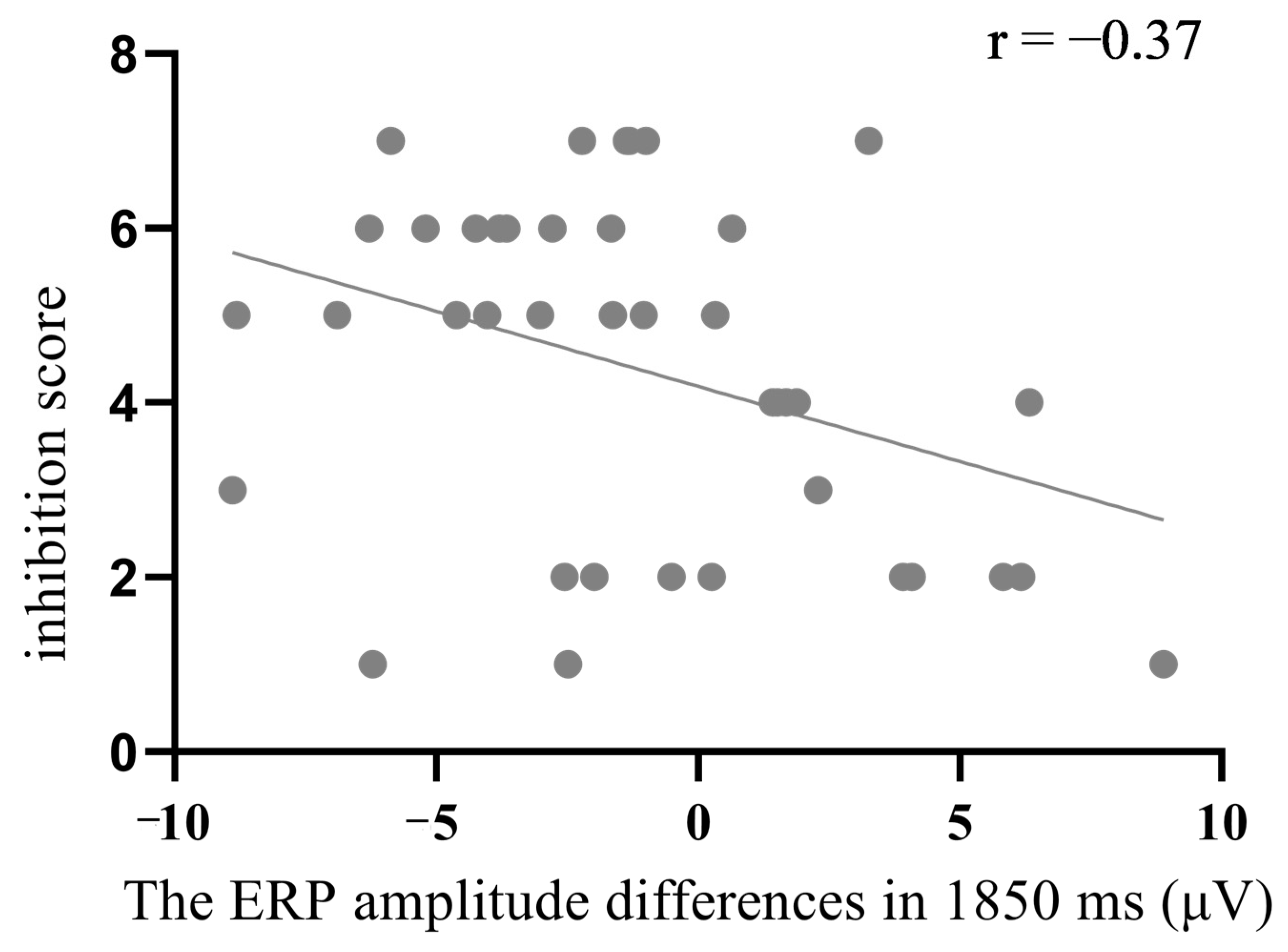
3.3.3. Was the Negativity at 1850 ms Related to Arousal Inhibition?
4. Discussion
4.1. Neural Signatures Associated with Orienting Response
4.2. Neural Signatures Associated with Arousal Inhibition
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ambach, W.; Stark, R.; Peper, M.; Vaitl, D. Separating deceptive and orienting components in a Concealed Information Test. Int. J. Psychophysiol. 2008, 70, 95–104. [Google Scholar] [CrossRef]
- Peth, J.; Vossel, G.; Gamer, M. Emotional arousal modulates the encoding of crime-related details and corresponding physiological responses in the Concealed Information Test: Arousal and memory in a CIT. Psychophysiology 2012, 49, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, I.; Nittono, H.; Hirota, A.; Ogawa, T.; Takasawa, N. Event-related brain potentials during the standard autonomic-based concealed information test. Int. J. Psychophysiol. 2009, 74, 58–68. [Google Scholar] [CrossRef]
- Verschuere, B.; Ben-Shakhar, G. Theory of the concealed Information Test. In Memory Detection: Theory and Application of the Concealed Information Test; Cambridge University Press: Cambridge, UK, 2011; pp. 128–148. [Google Scholar] [CrossRef]
- Sokolov, É.N. Perception and the Conditioned Reflex. Med. J. Aust. 1965, 1, 983. [Google Scholar] [CrossRef]
- Gamer, M.; Gödert, H.W.; Keth, A.; Rill, H.-G.; Vossel, G. Electrodermal and phasic heart rate responses in the Guilty Actions Test: Comparing guilty examinees to informed and uninformed innocents. Int. J. Psychophysiol. 2008, 69, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, B.; Crombez, G.; Koster EH, W.; Van Bockstaele, B.; De Clercq, A. Startling secrets: Startle eye blink modulation by concealed crime information. Biol. Psychol. 2007, 76, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Klein Selle, N.; Verschuere, B.; Kindt, M.; Meijer, E.; Ben-Shakhar, G. Orienting versus inhibition in the Concealed Information Test: Different cognitive processes drive different physiological measures: Orienting and inhibition processes in the CIT. Psychophysiology 2016, 53, 579–590. [Google Scholar] [CrossRef]
- Klein Selle, N.; Verschuere, B.; Kindt, M.; Meijer, E.; Ben-Shakhar, G. Unraveling the roles of orienting and inhibition in the Concealed Information Test: Orienting and inhibition in the CIT. Psychophysiology 2017, 54, 628–639. [Google Scholar] [CrossRef]
- Klein Selle, N.; Agari, N.; Ben-Shakhar, G. Hide or Seek? Physiological Responses Reflect Both the Decision and the Attempt to Conceal Information. Psychol. Sci. 2019, 30, 1424–1433. [Google Scholar] [CrossRef]
- Kubo, K.; Nittono, H. The Role of Intention to Conceal in the P300-based Concealed Information Test. Appl. Psychophysiol. Biofeedback 2009, 34, 227–235. [Google Scholar] [CrossRef]
- Rosenfeld, J.P.; Ozsan, I.; Ward, A.C. P300 amplitude at Pz and N200/N300 latency at F3 differ between participants simulating suspect versus witness roles in a mock crime: P300 and N200/N300 in a CIT. Psychophysiology 2017, 54, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, I.; Nittono, H. A concealment-specific frontal negative slow wave is generated from the right prefrontal cortex in the Concealed Information Test. Biol. Psychol. 2018, 135, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Klein Selle, N.; Gueta, C.; Harpaz, Y.; Deouell, L.Y.; Ben-Shakhar, G. Brain-based concealed memory detection is driven mainly by orientation to salient items. Cortex 2021, 136, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. For Distinguished Early Career Contribution to Psychophysiology: Award Address, 1985: A Triarchic Model of P300 Amplitude. Psychophysiology 1986, 23, 367–384. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300 An integrative theory of P3a and P3. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Leue, A.; Beauducel, A. A meta-analysis of the P3 amplitude in tasks requiring deception in legal and social contexts. Brain Cogn. 2019, 135, 103564. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, I.; Nittono, H. The intention to conceal activates the right prefrontal cortex: An event-related potential study. NeuroReport 2015, 26, 223–227. [Google Scholar] [CrossRef]
- Birbaumer, N.; Elbert, T.; Canavan, A.G.; Rockstroh, B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990, 70, 1–41. [Google Scholar] [CrossRef]
- Rösler, F.; Heil, M.; Röder, B. Slow negative brain potentials as reflections of specific modular resources of cognition. Biol. Psychol. 1997, 45, 109–141. [Google Scholar] [CrossRef]
- Paul, S.; Simon, D.; Kniesche, R.; Kathmann, N.; Endrass, T. Timing effects of antecedent- and response-focused emotion regulation strategies. Biol. Psychol. 2013, 94, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Sai, L.; Cheng, J.; Shang, S.; Fu, G.; Verschuere, B. Does deception involve more cognitive control than truth-telling? Meta-analyses of N2 and MFN ERP studies. Psychophysiology 2023, 60, e14333. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Dien, J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. J. Neurosci. Methods 2010, 187, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dien, J.; Khoe, W.; Mangun, G.R. Evaluation of PCA and ICA of simulated ERPs: Promax vs. infomax rotations. Hum. Brain Mapp. 2007, 28, 742–763. [Google Scholar] [CrossRef]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.D.; Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Kass, R.E.; Raftery, A.E. Bayes Factors. J. Am. Stat. Assoc. 1995, 90, 773–795. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B. Life-span changes in P3a. Psychophysiology 2004, 41, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Stige, S.; Fjell, A.M.; Smith, L.; Lindgren, M.; Walhovd, K.B. The Development of Visual P3a and P3b. Dev. Neuropsychol. 2007, 32, 563–584. [Google Scholar] [CrossRef]
- Alperin, B.R.; Mott, K.K.; Holcomb, P.J.; Daffner, K.R. Does the age-related “anterior shift” of the P3 reflect an inability to habituate the novelty response? Neurosci. Lett. 2014, 577, 6–10. [Google Scholar] [CrossRef]
- Porcaro, C.; Balsters, J.H.; Mantini, D.; Robertson, I.H.; Wenderoth, N. P3b amplitude as a signature of cognitive decline in the older population: An EEG study enhanced by Functional Source Separation. NeuroImage 2019, 184, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Alho, K.; Winkler, I.; Escera, C.; Huotilainen, M.; Virtanen, J.; Jääskeläinen, I.P.; Pekkonen, E.; Ilmoniemi, R.J. Processing of novel sounds and frequency changes in the human auditory cortex: Magnetoencephalographic recordings. Psychophysiology 1998, 35, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Gumenyuk, V.; Korzyukov, O.; Alho, K.; Escera, C.; Näätänen, R. Effects of auditory distraction on electrophysiological brain activity and performance in children aged 8–13 years. Psychophysiology 2004, 41, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Squires, N.K.; Squires, K.C.; Hillyard, S.A. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalogr. Clin. Neurophysiol. 1975, 38, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Strobel, A.; Debener, S.; Sorger, B.; Peters, J.C.; Kranczioch, C.; Hoechstetter, K.; Engel, A.K.; Brocke, B.; Goebel, R. Novelty and target processing during an auditory novelty oddball: A simultaneous event-related potential and functional magnetic resonance imaging study. NeuroImage 2008, 40, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, J.P.; Shue, E.; Singer, E. Single versus multiple probe blocks of P300-based concealed information tests for self-referring versus incidentally obtained information. Biol. Psychol. 2007, 74, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Koeckritz, R.; Beauducel, A.; Hundhausen, J.; Redolfi, A.; Leue, A. Does concealing familiarity evoke other processes than concealing untrustworthiness?—Different forms of concealed information modulate P3 effects. Personal. Neurosci. 2019, 2, e2. [Google Scholar] [CrossRef][Green Version]
- Liesefeld, H.R.; Zimmer, H.D. Think spatial: The representation in mental rotation is nonvisual. J. Exp. Psychol. Learn. Mem. Cogn. 2013, 39, 167–182. [Google Scholar] [CrossRef]

| Temporal Factors | Peak Loading (ms) | Variance (%) | Polarity |
|---|---|---|---|
| TF01 | 1850 | 25.3 | − |
| TF02 | 590 | 17.9 | + |
| TF03 | 1022 | 17.3 | − |
| TF04 | 400 | 6.2 | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, W.; Wang, F.; Zhu, H.; Jiang, C.; Sai, L. Neural Signals Associated with Orienting Response and Arousal Inhibition in Concealed Information Test. Behav. Sci. 2024, 14, 627. https://doi.org/10.3390/bs14080627
Feng W, Wang F, Zhu H, Jiang C, Sai L. Neural Signals Associated with Orienting Response and Arousal Inhibition in Concealed Information Test. Behavioral Sciences. 2024; 14(8):627. https://doi.org/10.3390/bs14080627
Chicago/Turabian StyleFeng, Wang, Fei Wang, Hongyi Zhu, Chen Jiang, and Liyang Sai. 2024. "Neural Signals Associated with Orienting Response and Arousal Inhibition in Concealed Information Test" Behavioral Sciences 14, no. 8: 627. https://doi.org/10.3390/bs14080627
APA StyleFeng, W., Wang, F., Zhu, H., Jiang, C., & Sai, L. (2024). Neural Signals Associated with Orienting Response and Arousal Inhibition in Concealed Information Test. Behavioral Sciences, 14(8), 627. https://doi.org/10.3390/bs14080627





