Social Interoception and Autonomic System Reactivity during Synchronization Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
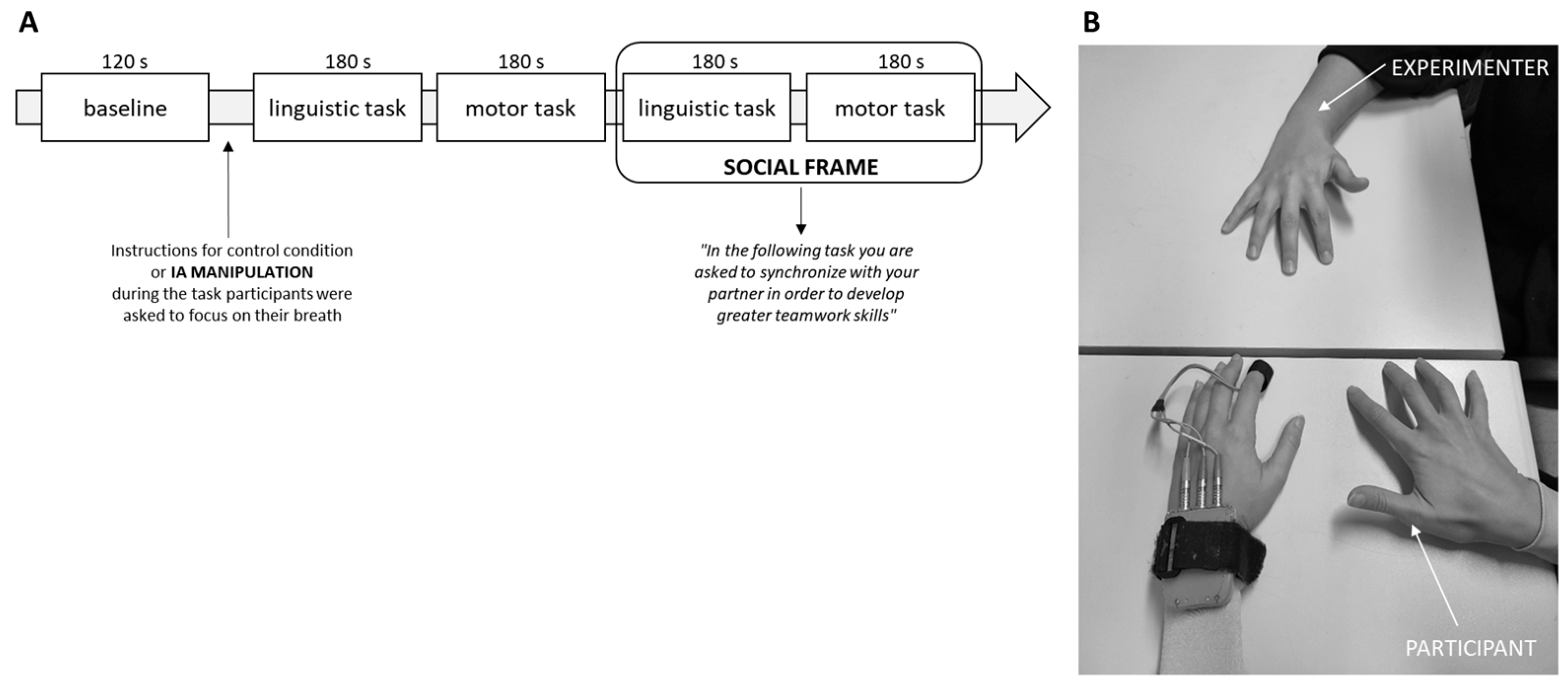
2.2. Joint Synchronization Tasks
2.3. Procedure
2.4. Autonomic Data Recording
2.5. Statistical Data Analysis
3. Results
3.1. First Order of Results
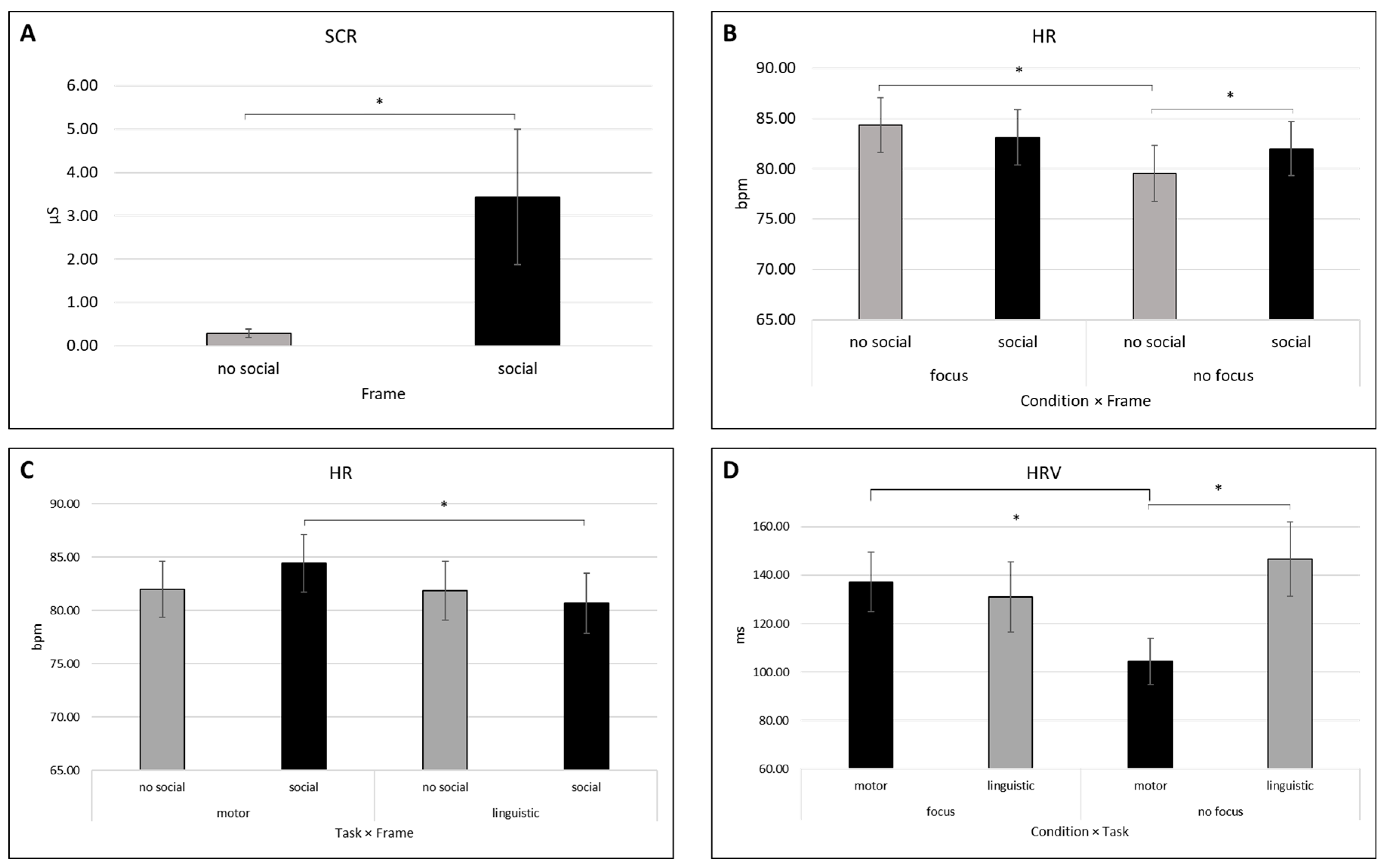
3.1.1. SCL and SCR
3.1.2. HR
3.1.3. HRV
3.2. Second Order of Results
3.2.1. BVP
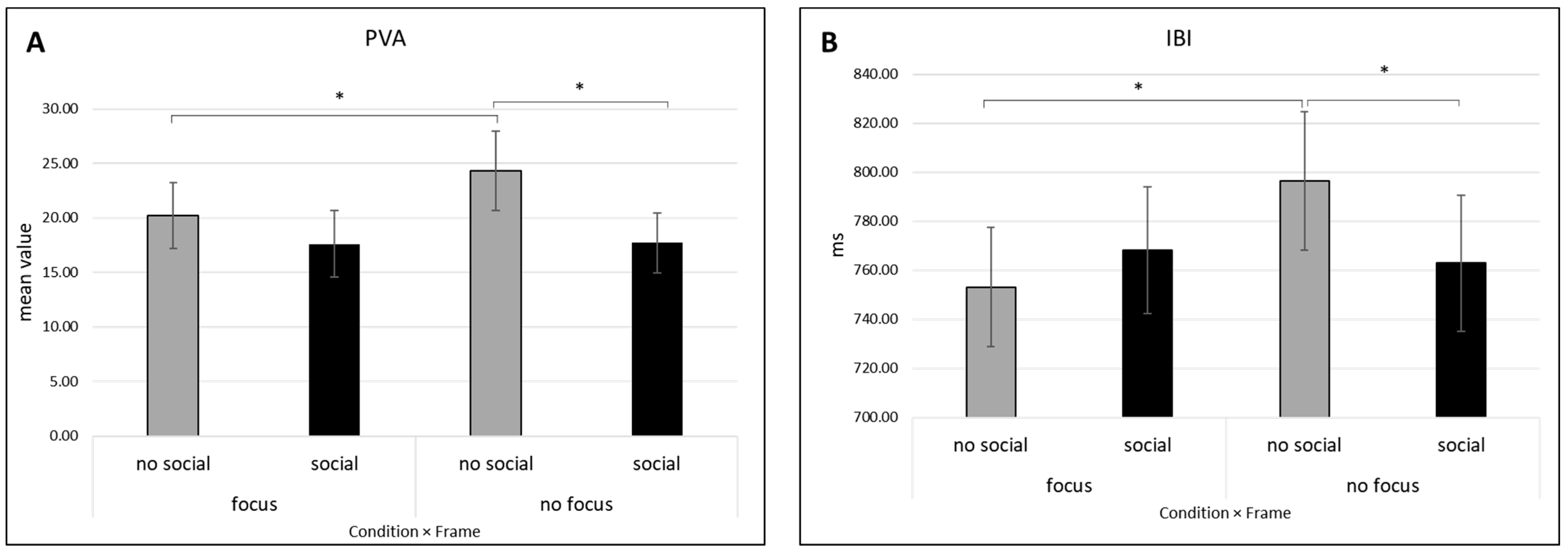
3.2.2. PVA
3.2.3. IBI
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Craig, A. How Do You Feel? Interoception: The Sense of the Physiological Condition of the Body. Nat. Neurosci. 2002, 3, 655–666. [Google Scholar] [CrossRef]
- Schandry, R. Heartbeat Detection and Emotional Experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- Tsakiris, M.; De Preester, H. The Interoceptive Mind: From Homeostasis to Awareness; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Ainley, V.; Brass, M.; Tsakiris, M. Heartfelt Imitation: High Interoceptive Awareness Is Linked to Greater Automatic Imitation. Neuropsychologia 2014, 60, 21–28. [Google Scholar] [CrossRef]
- Weng, H.Y.; Feldman, J.L.; Leggio, L.; Napadow, V.; Park, J.; Price, C.J. Interventions and Manipulations of Interoception. Trends Neurosci. 2021, 44, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Farb, N.A.S.; Segal, Z.V.; Anderson, A.K. Mindfulness Meditation Training Alters Cortical Representations of Interoceptive Attention. Soc. Cogn. Affect. Neurosci. 2013, 8, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Schulz, S.M. Neural Correlates of Heart-Focused Interoception: A Functional Magnetic Resonance Imaging Meta-Analysis. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20160018. [Google Scholar] [CrossRef] [PubMed]
- Angioletti, L.; Balconi, M. The Increasing Effect of Interoception on Brain Frontal Responsiveness During a Socially Framed Motor Synchronization Task. Front. Hum. Neurosci. 2022, 16, 834619. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Angioletti, L. Interoceptive Attentiveness Induces Significantly More PFC Activation during a Synchronized Linguistic Task Compared to a Motor Task as Revealed by Functional Near-Infrared Spectroscopy. Brain Sci. 2022, 12, 301. [Google Scholar] [CrossRef]
- Palmer, C.E.; Tsakiris, M. Going at the Heart of Social Cognition: Is There a Role for Interoception in Self-Other Distinction? Curr. Opin. Psychol. 2018, 24, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Ping, X.; Chen, W. Body Influences on Social Cognition through Interoception. Front. Psychol. 2019, 10, 2066. [Google Scholar] [CrossRef]
- Arnold, A.J.; Winkielman, P.; Dobkins, K. Interoception and Social Connection. Front. Psychol. 2019, 10, 2589. [Google Scholar] [CrossRef] [PubMed]
- Burleson, M.H.; Quigley, K.S. Social Interoception and Social Allostasis through Touch: Legacy of the Somatovisceral Afference Model of Emotion. Soc. Neurosci. 2021, 16, 92–102. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. EEG Brain Oscillations Are Modulated by Interoception in Response to a Synchronized Motor vs. Cognitive Task. Front. Neuroanat. 2022, 16, 991522. [Google Scholar] [CrossRef]
- Ferri, F.; Ardizzi, M.; Ambrosecchia, M.; Gallese, V. Closing the Gap between the Inside and the Outside: Interoceptive Sensitivity and Social Distances. PLoS ONE 2013, 8, e75758. [Google Scholar] [CrossRef]
- Berntson, G.G.; Thomas Bigger, J.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart Rate Variability: Origins Methods, and Interpretive Caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef]
- Grossman, P.; Taylor, E.W. Toward Understanding Respiratory Sinus Arrhythmia: Relations to Cardiac Vagal Tone, Evolution and Biobehavioral Functions. Biol. Psychol. 2007, 74, 263–285. [Google Scholar] [CrossRef]
- Porges, S.W. Social Engagement and Attachment: A Phylogenetic Perspective. Ann. N. Y. Acad. Sci. 2003, 1008, 31–47. [Google Scholar] [CrossRef]
- Graziano, P.A.; Keane, S.P.; Calkins, S.D. Cardiac Vagal Regulation and Early Peer Status. Child. Dev. 2007, 78, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Harden, E.; Lamb, D.; Van Hecke, A.V.; Denver, J.W.; Porges, S.W. Emotion Recognition in Children with Autism Spectrum Disorders: Relations to Eye Gaze and Autonomic State. J. Autism Dev. Disord. 2010, 40, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Patriquin, M.A.; Scarpa, A.; Friedman, B.H.; Porges, S.W. Respiratory Sinus Arrhythmia: A Marker for Positive Social Functioning and Receptive Language Skills in Children with Autism Spectrum Disorders. Dev. Psychobiol. 2013, 55, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W.; Doussard-Roosevelt, J.A.; Maiti, A.K. Vagal Tone and the Physiological Regulation of Emotion. Monogr. Soc. Res. Child Dev. 1994, 59, 167–186. [Google Scholar] [CrossRef] [PubMed]
- Ambrosecchia, M.; Ardizzi, M.; Russo, E.; Ditaranto, F.; Speciale, M.; Vinai, P.; Todisco, P.; Maestro, S.; Gallese, V. Interoception and Autonomic Correlates during Social Interactions. Implications for Anorexia. Front. Hum. Neurosci. 2017, 11, 219. [Google Scholar] [CrossRef]
- Angioletti, L.; Balconi, M. Interoceptive Attentiveness and Autonomic Reactivity in Pain Observation. Somatosens. Mot. Res. 2022, 39, 81–89. [Google Scholar] [CrossRef]
- Singleton, C.J.; Ashwin, C.; Brosnan, M. Physiological Responses to Social and Nonsocial Stimuli in Neurotypical Adults With High and Low Levels of Autistic Traits: Implications for Understanding Nonsocial Drive in Autism Spectrum Disorders. Autism Res. 2014, 7, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Balconi, M.; Venturella, I.; Fronda, G.; De Filippis, D.; Salati, E.; Vanutelli, M.E. To Rate or Not to Rate? Autonomic Response and Psychological Well-Being of Employees during Performance Review. Health Care Manag. 2019, 38, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Marci, C.D.; Ham, J.; Moran, E.; Orr, S.P. Physiologic Correlates of Perceived Therapist Empathy and Social-Emotional Process during Psychotherapy. J. Nerv. Ment. Dis. 2007, 195, 103–111. [Google Scholar] [CrossRef]
- Sequeira, H.; Hot, P.; Silvert, L.; Delplanque, S. Electrical Autonomic Correlates of Emotion. Int. J. Psychophysiol. 2009, 71, 50–56. [Google Scholar] [CrossRef]
- Sohn, J.H.; Sokhadze, E.; Watanuki, S. Electrodermal and Cardiovascular Manifestations of Emotions in Children. J. Physiol. Anthropol. Appl. Human. Sci. 2001, 20, 55–64. [Google Scholar] [CrossRef]
- Kelsen, B.A.; Sumich, A.; Kasabov, N.; Liang, S.H.Y.; Wang, G.Y. What Has Social Neuroscience Learned from Hyperscanning Studies of Spoken Communication? A Systematic Review. Neurosci. Biobehav. Rev. 2022, 132, 1249–1262. [Google Scholar] [CrossRef]
- Jobbágy, Á.; Harcos, P.; Karoly, R.; Fazekas, G. Analysis of Finger-Tapping Movement. J. Neurosci. Methods 2005, 141, 29–39. [Google Scholar] [CrossRef]
- Kawasaki, M.; Yamada, Y.; Ushiku, Y.; Miyauchi, E.; Yamaguchi, Y. Inter-Brain Synchronization during Coordination of Speech Rhythm in Human-to-Human Social Interaction. Sci. Rep. 2013, 3, 1692. [Google Scholar] [CrossRef]
- Lischke, A.; Lemke, D.; Neubert, J.; Hamm, A.O.; Lotze, M. Inter-Individual Differences in Heart Rate Variability Are Associated with Inter-Individual Differences in Mind-Reading. Sci. Rep. 2017, 7, 11557. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. J. Mater. Environ. Sci. 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Scholey, A.B. Glucose Administration, Heart Rate and Cognitive Performance: Effects of Increasing Mental Effort. Psychopharmacology 2000, 149, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, S.; Tomasello, M. Joint Drumming: Social Context Facilitates Synchronization in Preschool Children. J. Exp. Child. Psychol. 2009, 102, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart Rate Variability as an Index of Regulated Emotional Responding. Rev. General. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Mather, M.; Thayer, J.F. How Heart Rate Variability Affects Emotion Regulation Brain Networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lischke, A.; Pahnke, R.; Mau-Moeller, A.; Behrens, M.; Grabe, H.J.; Freyberger, H.J.; Hamm, A.O.; Weippert, M. Inter-Individual Differences in Heart Rate Variability Are Associated with Inter-Individual Differences in Empathy and Alexithymia. Front. Psychol. 2018, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Relationship between Heart Rate Variability and Cognitive Function during Threat of Shock. Anxiety Stress. Coping 2009, 22, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal Influence on Working Memory and Attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Handouzi, W.; Maaoui, C.; Pruski, A.; Moussaoui, A. Objective Model Assessment for Short-Term Anxiety Recognition from Blood Volume Pulse Signal. Biomed. Signal Process Control 2014, 14, 217–227. [Google Scholar] [CrossRef]
- Porges, S.W. The Polyvagal Theory: Phylogenetic Substrates of a Social Nervous System. Int. J. Psychophysiol. 2001, 42, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Gordon, I.; Gilboa, A.; Cohen, S.; Milstein, N.; Haimovich, N.; Pinhasi, S.; Siegman, S. Physiological and Behavioral Synchrony Predict Group Cohesion and Performance. Sci. Rep. 2020, 10, 8484. [Google Scholar] [CrossRef]
- Tschacher, W.; Meier, D. Physiological Synchrony in Psychotherapy Sessions. Psychother. Res. 2020, 30, 558–573. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balconi, M.; Angioletti, L. Social Interoception and Autonomic System Reactivity during Synchronization Behavior. Behav. Sci. 2024, 14, 149. https://doi.org/10.3390/bs14030149
Balconi M, Angioletti L. Social Interoception and Autonomic System Reactivity during Synchronization Behavior. Behavioral Sciences. 2024; 14(3):149. https://doi.org/10.3390/bs14030149
Chicago/Turabian StyleBalconi, Michela, and Laura Angioletti. 2024. "Social Interoception and Autonomic System Reactivity during Synchronization Behavior" Behavioral Sciences 14, no. 3: 149. https://doi.org/10.3390/bs14030149
APA StyleBalconi, M., & Angioletti, L. (2024). Social Interoception and Autonomic System Reactivity during Synchronization Behavior. Behavioral Sciences, 14(3), 149. https://doi.org/10.3390/bs14030149






