Neuroticism Mediates the Association between Autistic Traits and Choice Reaction Time among Young Adults
Abstract
1. Introduction
Current Study
2. Method
2.1. Participants
2.2. Materials and Tasks
2.3. Procedure
2.4. Data Reduction
2.5. Analytic Approach
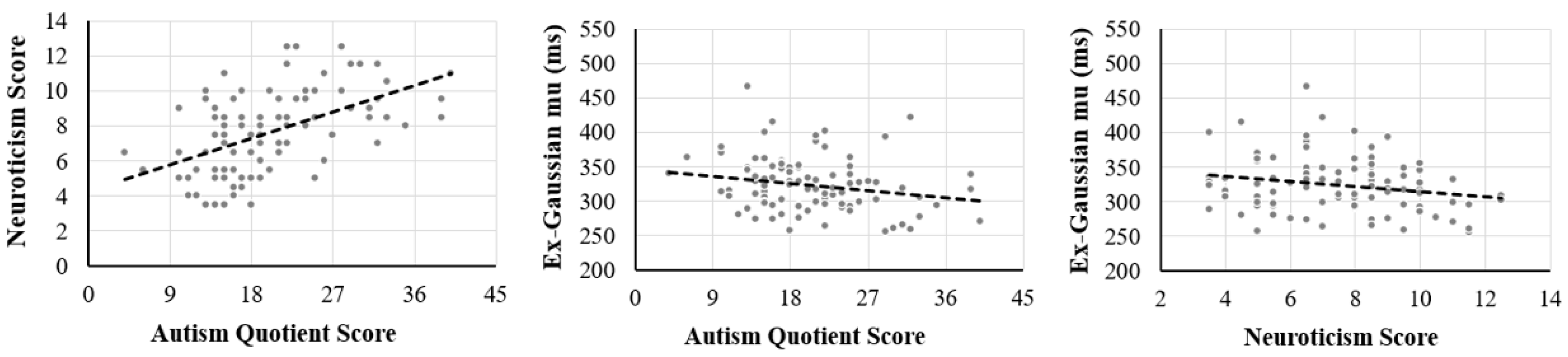
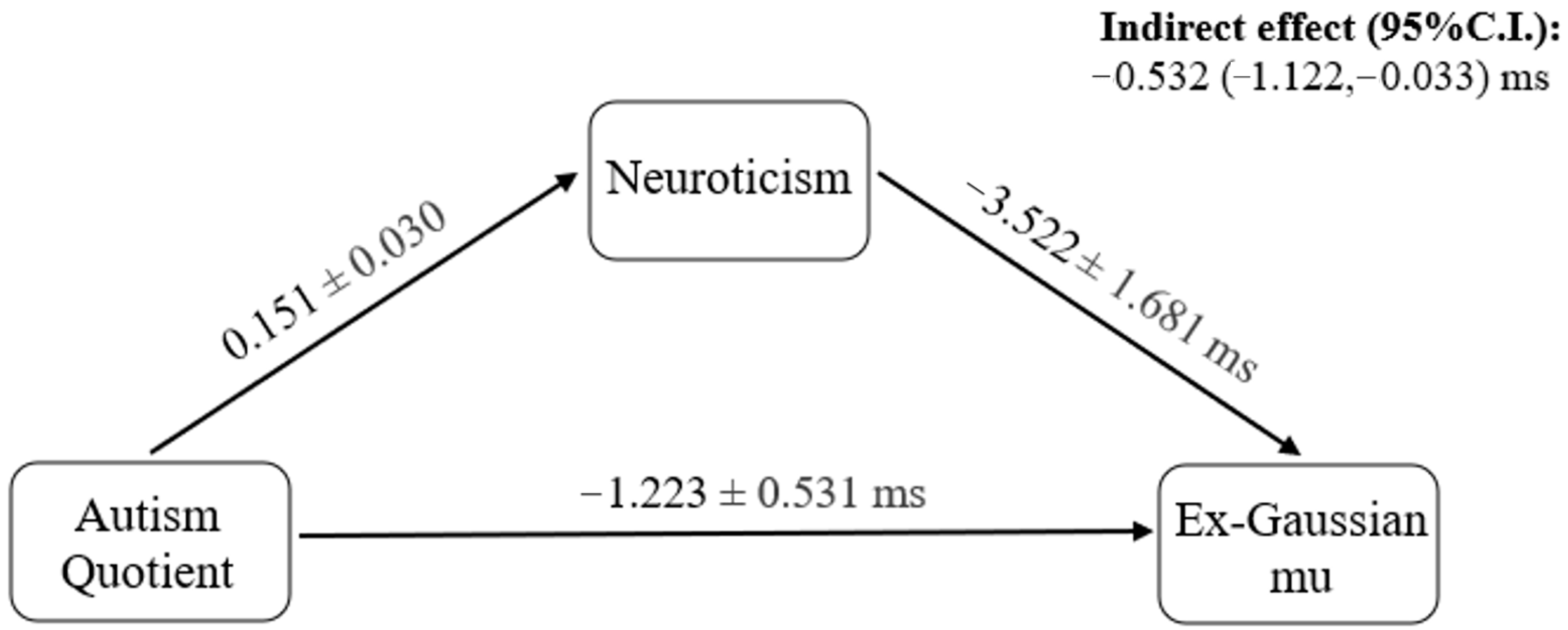
3. Results
4. Discussion
Implications, Limitations, and Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buescher, A.V.; Cidav, Z.; Knapp, M.; Mandell, D.S. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatrics 2014, 168, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.P.; Du, J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.J.; Brugha, T.S.; Erskine, H.E.; Scheurer, R.W.; Vos, T.; Scott, J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015, 45, 601–613. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2020. [Google Scholar]
- Kuehn, B.M. CDC: Autism spectrum disorders common. JAMA 2007, 297, 940. [Google Scholar] [CrossRef]
- Mandell, D.S.; Wiggins, L.D.; Carpenter, L.A.; Daniels, J.; DiGuiseppi, C.; Durkin, M.S.; Giarelli, E.; Morrier, M.J.; Nicholas, J.S.; Pinto-Martin, J.A.; et al. Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am. J. Public Health 2009, 99, 493–498. [Google Scholar] [CrossRef]
- Golson, M.E.; Ficklin, E.; Haverkamp, C.R.; McClain, M.B.; Harris, B. Cultural differences in social communication and interaction: A gap in autism research. Autism Res. 2022, 15, 208–214. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Ashwin, E.; Ashwin, C.; Tavassoli, T.; Chakrabarti, B. Talent in autism: Hyper-systemizing, hyper-attention to detail and sensory hypersensitivity. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Sasson, N.J.; Bottema-Beutel, K. Studies of autistic traits in the general population are not studies of autism. Autism 2021, 26, 1007–1008. [Google Scholar] [CrossRef]
- Santosh, P.J.; Mijovic, A. Social impairment in hyperkinetic disorder. Eur. Child Adolesc. Psychiatry 2004, 13, 141–150. [Google Scholar] [CrossRef]
- Bishop, D.; Baird, G. Parent and teacher report of Pragmatic Aspects of Communication: Use of the Children’s Communication Checklist in a clinical setting, erratum. Dev. Med. Child Neurol. 2005, 47, 287. [Google Scholar] [CrossRef]
- Gadow, K.D.; Devincent, C.J.; Pomeroy, J.; Azizian, A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism 2005, 9, 392–415. [Google Scholar] [CrossRef]
- Clark, T.; Feehan, C.; Tinline, C.; Vostanis, P. Autistic symptoms in children with attention deficit-hyperactivity disorder. Eur. Child Adolesc. Psychiatry 1999, 8, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.A.; Landa, R.J. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism 2014, 18, 272–280. [Google Scholar] [CrossRef] [PubMed]
- John, O.P.; Donahue, E.M.; Kentle, R.L. The Big Five Inventory-Versions 4a and 54; University of California, Berkeley, Institute of Personality and Social Research: Berkeley, CA, USA, 1991. [Google Scholar]
- Robinson, E.; Hull, L.; Petrides, K.V. Big Five model and trait emotional intelligence in camouflaging behaviours in autism. Personal. Individ. Differ. 2020, 152, 109565. [Google Scholar] [CrossRef]
- Lodi-Smith, J.; Rodgers, J.D.; Cunningham, S.A.; Lopata, C.; Thomeer, M.L. Meta-analysis of big five personality traits in autism spectrum disorder. Autism 2018, 23, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Arnold, S.R.; Foley, K.-R.; Trollor, J.N. Diagnosis of autism in adulthood: A scoping review. Autism 2020, 24, 1311–1327. [Google Scholar] [CrossRef]
- Wakabayashi, A.; Baron-Cohen, S.; Uchiyama, T.; Yoshida, Y.; Tojo, Y.; Kuroda, M.; Wheelwright, S. The autism-spectrum quotient (AQ) children’s version in Japan: A cross-cultural comparison. J. Autism Dev. Disord. 2007, 37, 491–500. [Google Scholar] [CrossRef]
- Stimpson, N.J.; Hull, L.; Mandy, W. The association between autistic traits and mental well-being. J. Happiness Stud. 2020, 22, 287–304. [Google Scholar] [CrossRef]
- Uzunova, G.; Pallanti, S.; Hollander, E. Excitatory/inhibitory imbalance in autism spectrum disorders: Implications for interventions and therapeutics. World J. Biol. Psychiatry 2015, 17, 174–186. [Google Scholar] [CrossRef]
- Geurts, H.M.; Grasman, R.P.P.P.; Verté, S.; Oosterlaan, J.; Roeyers, H.; van Kammen, S.M.; Sergeant, J.A. Intra-individual variability in ADHD, autism spectrum disorders and Tourette’s syndrome. Neuropsychologia 2008, 46, 3030–3041. [Google Scholar] [CrossRef]
- Schmitt, L.M.; White, S.P.; Cook, E.H.; Sweeney, J.A.; Mosconi, M.W. Cognitive mechanisms of inhibitory control deficits in autism spectrum disorder. J. Child Psychol. Psychiatry 2017, 59, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Ferraro, F.R. No evidence of reaction time slowing in autism spectrum disorder. Autism 2016, 20, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Geurts, H.M.; van den Bergh, S.F.; Ruzzano, L. Prepotent response inhibition and interference control in autism spectrum disorders: Two meta-analyses. Autism Res. 2014, 7, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Noterdaeme, M.; Amorosa, H.; Mildenberger, K.; Sitter, S.; Minow, F. Evaluation of attention problems in children with autism and children with a specific language disorder. Eur. Child Adolesc. Psychiatry 2001, 10, 58–66. [Google Scholar] [CrossRef]
- Henderson, H.; Schwartz, C.; Mundy, P.; Burnette, C.; Sutton, S.; Zahka, N.; Pradella, A. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006, 61, 96–109. [Google Scholar] [CrossRef]
- Yang, X.; Jennings, J.R.; Friedman, B.H. Exteroceptive stimuli override interoceptive state in reaction time control. Psychophysiology 2017, 54, 1940–1950. [Google Scholar] [CrossRef]
- Yang, X.; Spangler, D.P.; Jennings, J.R.; Friedman, B.H. Cardiac timing and threatening stimuli influence response inhibition and ex-Gaussian parameters of reaction time in a Go/No-go task. Psychophysiology 2023, 60, e14260. [Google Scholar] [CrossRef]
- Yang, X.; Herberlein, K.; Reid, A.; Jiao, D.; Fang, F. Mental workload modulates the effects of baroreceptor afferents on sensorimotor processing. J. Clin. Basic Psychosom. 2024, 2, 2248. [Google Scholar] [CrossRef]
- Heathcote, A.; Popiel, S.J.; Mewhort, D.J. Analysis of response time distributions: An example using the Stroop task. Psychol. Bull. 1991, 109, 340–347. [Google Scholar] [CrossRef]
- Spieler, D.H.; Balota, D.A.; Faust, M.E. Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. J. Exp. Psychol. Hum. Percept. Perform. 1996, 22, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Spangler, D.P.; Yang, X.; Weidler, B.J.; Thayer, J.F.; McGinley, J.J. Unraveling the cognitive correlates of heart rate variability with the drift diffusion model. Int. J. Psychophysiol. 2022, 181, 73–84. [Google Scholar] [CrossRef]
- Gosling, S.D.; Rentfrow, P.J.; Swann, W.B., Jr. A very brief measure of the Big-Five personality domains. J. Res. Personal. 2003, 37, 504–528. [Google Scholar] [CrossRef]
- Woodbury-Smith, M.R.; Clare, I.C.; Holland, A.J.; Kearns, A.; Staufenberg, E.; Watson, P. A case-control study of offenders with high functioning autistic spectrum disorders. J. Forensic Psychiatry Psychol. 2005, 16, 747–763. [Google Scholar] [CrossRef]
- Grijalva, E.; Newman, D.A.; Tay, L.; Donnellan, M.B.; Harms, P.D.; Robins, R.W.; Yan, T. Gender differences in narcissism: A meta-analytic review. Psychol. Bull. 2015, 141, 261–310. [Google Scholar] [CrossRef] [PubMed]
- Massidda, D.; Massidda, M.D. Package ‘Retimes’. 2013. Available online: https://CRAN.Rproject.org/package=retimes (accessed on 2 August 2024).
- Hayes, A.F.; Preacher, K.J. Statistical mediation analysis with a multicategorical independent variable. Br. J. Math. Stat. Psychol. 2014, 67, 451–470. [Google Scholar] [CrossRef]
- Yang, X.; Daches, S.; Yaroslavsky, I.; George, C.J.; Kovacs, M. Cardiac vagal control mediates the relation between past depression and blood pressure several years later among young adults. Psychophysiology 2020, 57, e13535. [Google Scholar] [CrossRef]
- Tofighi, D.; MacKinnon, D.; Yoon, M. Covariances between regression coefficient estimates in a single mediator model. Br. J. Math. Stat. Psychol. 2009, 62, 457–484. [Google Scholar] [CrossRef]
- Leekam, S.R.; Nieto, C.; Libby, S.J.; Wing, L.; Gould, J. Describing the sensory abnormalities of children and adults with autism. J. Autism Dev. Disord. 2007, 37, 894–910. [Google Scholar] [CrossRef]
- Goudriaan, A.E.; Oosterlaan, J.; de Beurs, E.; van den Brink, W. Decision making in pathological gambling: A comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cogn. Brain Res. 2005, 23, 137–151. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The autism-spectrum quotient (AQ): Evidence from asperger syndrome/high functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Rapee, R.M.; Kennedy, S.J.; Ingram, M.; Edwards, S.L.; Sweeney, L. Altering the trajectory of anxiety in at-risk young children. Am. J. Psychiatry 2010, 167, 1518–1525. [Google Scholar] [CrossRef] [PubMed]
- McRae, K.; Gross, J.J. Emotion regulation. Emotion 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Sheppes, G.; Scheibe, S.; Suri, G.; Gross, J.J. Emotion-regulation choice. Psychol. Sci. 2011, 22, 1391–1396. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Sauer-Zavala, S.; Bentley, K.H.; Wilner, J.G.; Barlow, D.H. Transdiagnostic treatment of borderline personality disorder and comorbid disorders: A clinical replication series. J. Personal. Disord. 2016, 30, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Segal, Z.; Teasdale, J.; Williams, M. Mindfulness-Based Cognitive Therapy for Depression; Guilford Press: New York, NY, USA, 2002. [Google Scholar]
- Liu, F.; Scheeren, A.M.; Grove, R.; Hoekstra, R.A.; Wang, K.; Guo, D.; Begeer, S. Exploring cultural differences in autistic traits: A factor analytic study of children with autism in China and the Netherlands. J. Autism Dev. Disord. 2022, 52, 4750–4762. [Google Scholar] [CrossRef]
- Matson, J.L.; Matheis, M.; Burns, C.O.; Esposito, G.; Venuti, P.; Pisula, E.; Goldin, R. Examining cross-cultural differences in autism spectrum disorder: A multinational comparison from Greece, Italy, Japan, Poland, and the United States. Eur. Psychiatry 2017, 42, 70–76. [Google Scholar] [CrossRef]
| Female (n = 69) | Male (n = 31) | Unadjusted F Statistics | |
|---|---|---|---|
| Age (M years; SD) | 20.29 (3.49) | 20.39 (4.16) | 0.02 |
| Autistic Quotient (M; SD) | 20.53 (7.54) | 19.06 (6.49) | 0.87 |
| Neuroticism (M; SD) | 8.06 (2.12) | 6.66 (2.50) | 8.36 ** |
| Mean reaction time (M ms; SD) | 397.27 (70.23) | 378.32 (61.30) | 1.63 |
| Ex-Gaussian mu (M ms; SD) | 322.34 (42.54) | 322.69 (38.01) | 0.01 |
| Ex-Gaussian sigma (M ms; SD) | 40.05 (14.35) | 33.29 (13.77) | 4.74 * |
| Ex-Gaussian tau (M ms; SD) | 74.80 (59.40) | 55.81 (30.49) | 2.74 |
| Autism Quotient | Neuroticism | Accuracy | mu | sigma | tau | |
|---|---|---|---|---|---|---|
| Neuroticism | 0.48 ** | |||||
| Accuracy | −0.11 | −0.05 | ||||
| Ex-Gaussian mu | −0.23 * | −0.20 | 0.32 ** | |||
| Ex-Gaussian sigma | −0.01 | 0.10 | 0.05 | 0.62 ** | ||
| Ex-Gaussian tau | −0.11 | −0.01 | −0.26 * | 0.14 | 0.27 ** | |
| Mean reaction time | −0.06 | −0.09 | 0.07 | 0.63 ** | 0.58 ** | 0.80 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Jesus Cintron, K.; Yang, X. Neuroticism Mediates the Association between Autistic Traits and Choice Reaction Time among Young Adults. Behav. Sci. 2024, 14, 903. https://doi.org/10.3390/bs14100903
De Jesus Cintron K, Yang X. Neuroticism Mediates the Association between Autistic Traits and Choice Reaction Time among Young Adults. Behavioral Sciences. 2024; 14(10):903. https://doi.org/10.3390/bs14100903
Chicago/Turabian StyleDe Jesus Cintron, Kassandra, and Xiao Yang. 2024. "Neuroticism Mediates the Association between Autistic Traits and Choice Reaction Time among Young Adults" Behavioral Sciences 14, no. 10: 903. https://doi.org/10.3390/bs14100903
APA StyleDe Jesus Cintron, K., & Yang, X. (2024). Neuroticism Mediates the Association between Autistic Traits and Choice Reaction Time among Young Adults. Behavioral Sciences, 14(10), 903. https://doi.org/10.3390/bs14100903






