Maternal Pregnancy and Pre-Pregnancy Weight and Behavioural Outcomes in Children
Abstract
1. Introduction
2. Methods
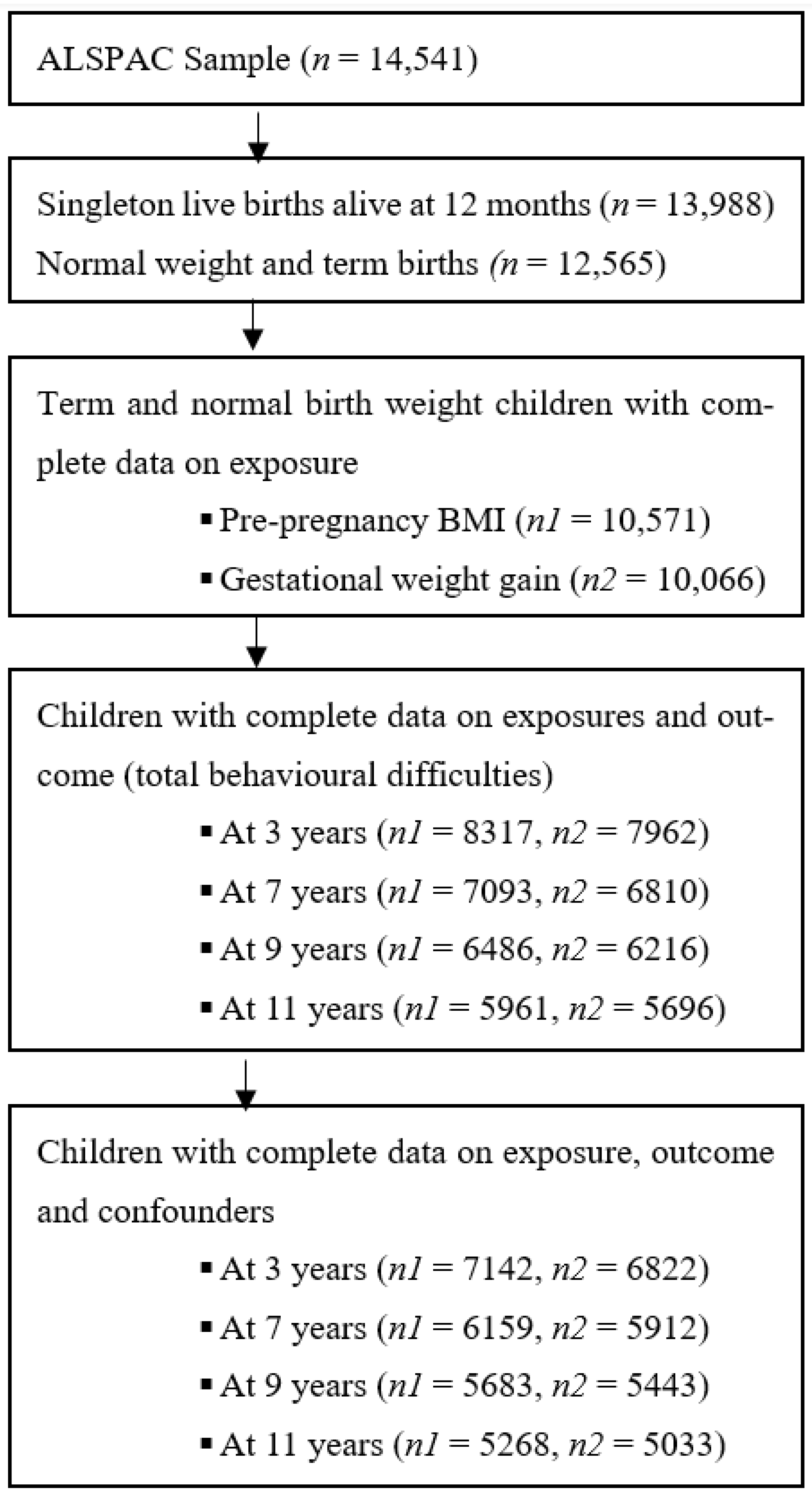
2.1. Study Participants
2.2. Exposure Measures
2.2.1. Pre-Pregnancy BMI
2.2.2. Gestational Weight Gain
2.3. Outcome Measures
2.4. Covariates
2.5. Statistical Analysis
3. Results
Maternal vs. Paternal BMI
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dachew, B.A.; Ayano, G.; Betts, K.; Alati, R. The impact of pre-pregnancy BMI on maternal depressive and anxiety symptoms during pregnancy and the postpartum period: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, L.; Wang, Y.; Zhang, Y.; Du, Y.; Sun, Y.; Wang, Z. Association between perinatal outcomes and maternal pre-pregnancy body mass index. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Adane, A.A.; Shepherd, C.C.J.; Lim, F.J.; White, S.W.; Farrant, B.M.; Bailey, H.D. The impact of pre-pregnancy body mass index and gestational weight gain on placental abruption risk: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2019, 300, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.Y.; Li, Y.J.; Ou, J.J.; Li, Y.M. Association between parental body mass index and autism spectrum disorder: A systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry 2019, 28, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Jenabi, E.; Bashirian, S.; Khazaei, S.; Basiri, Z. The maternal prepregnancy body mass index and the risk of attention deficit hyperactivity disorder among children and adolescents: A systematic review and meta-analysis. Korean J. Pediatr. 2019, 62, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain With Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef] [PubMed]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain With Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Nutritional Status During Pregnancy; Lactation. Nutrition during Pregnancy: Part I Weight Gain: Part II Nutrient Supplements; National Academies Press (US), National Academy of Sciences: Washington, DC, USA, 1990. [Google Scholar]
- Institute of Medicine; National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. In Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press (US), National Academy of Sciences: Washington, DC, USA, 2009. [Google Scholar]
- Hartley, E.; McPhie, S.; Skouteris, H.; Fuller-Tyszkiewicz, M.; Hill, B. Psychosocial risk factors for excessive gestational weight gain: A systematic review. Women Birth 2015, 28, e99–e109. [Google Scholar] [CrossRef]
- Dachew, B.A.; Ayano, G.; Alati, R. Does weight gain during pregnancy influence antenatal depressive symptoms? A systematic review and meta-analysis. J. Psychosom. Res. 2020, 138, 110255. [Google Scholar] [CrossRef]
- van der Burg, J.W.; Sen, S.; Chomitz, V.R.; Seidell, J.C.; Leviton, A.; Dammann, O. The role of systemic inflammation linking maternal BMI to neurodevelopment in children. Pediatr. Res. 2016, 79, 3–12. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Kurti, A.; Fair, D.A.; Fryer, J.D. Dietary intervention rescues maternal obesity induced behavior deficits and neuroinflammation in offspring. J. Neuroinflamm. 2014, 11, 156. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.H.; Thomsen, P.H.; Nohr, E.A.; Lemcke, S. Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. Eur. Child Adolesc. Psychiatry 2018, 27, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Denison, F.C.; Räikkönen, K.; Norman, J.E.; Reynolds, R.M. Prenatal exposure to maternal very severe obesity is associated with impaired neurodevelopment and executive functioning in children. Pediatr. Res. 2017, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Barry, C.; Sabhlok, A.; Russell, K.; Majors, A.; Kollins, S.H.; Fuemmeler, B.F. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: A meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2018, 19, 464–484. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, S.; Xu, S.; Weng, S.; Liu, Z. Maternal Body Mass Index and Risk of Autism Spectrum Disorders in Offspring: A Meta-analysis. Sci. Rep. 2016, 6, 34248. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.E.; Carlson, J.M.; Kebede, N.; Werler, M.M.; Janulewicz, P.A. Pre-pregnancy body mass index and parent and teacher-reported behavioral outcomes among offspring in childhood. Neurotoxicol. Teratol. 2022, 89, 107049. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Schieve, L.A.; Sharma, A.J.; Hinkle, S.N.; Li, R.; Lind, J.N. Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics 2015, 135, e1198–e1209. [Google Scholar] [CrossRef]
- Mikkelsen, S.H.; Hohwü, L.; Olsen, J.; Bech, B.H.; Liew, Z.; Obel, C. Parental Body Mass Index and Behavioral Problems in Their Offspring: A Danish National Birth Cohort Study. Am. J. Epidemiol. 2017, 186, 593–602. [Google Scholar] [CrossRef]
- Brion, M.J.; Zeegers, M.; Jaddoe, V.; Verhulst, F.; Tiemeier, H.; Lawlor, D.A.; Smith, G.D. Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics 2011, 127, e202–e211. [Google Scholar] [CrossRef]
- Menting, M.D.; van de Beek, C.; de Rooij, S.R.; Painter, R.C.; Vrijkotte, T.G.M.; Roseboom, T.J. The association between pre-pregnancy overweight/obesity and offspring’s behavioral problems and executive functioning. Early Hum. Dev. 2018, 122, 32–41. [Google Scholar] [CrossRef]
- Robinson, S.L.; Ghassabian, A.; Sundaram, R.; Trinh, M.H.; Lin, T.C.; Bell, E.M.; Yeung, E. Parental Weight Status and Offspring Behavioral Problems and Psychiatric Symptoms. J. Pediatr. 2020, 220, 227–236.e1. [Google Scholar] [CrossRef] [PubMed]
- Tore, E.C.; Antoniou, E.E.; de Groot, R.H.M.; Gielen, M.; Godschalk, R.W.L.; Roumeliotaki, T.; Smits, L.; Southwood, T.R.; Spaanderman, M.E.A.; Stratakis, N.; et al. Gestational Weight Gain by Maternal Pre-pregnancy BMI and Childhood Problem Behaviours in School-Age Years: A Pooled Analysis of Two European Birth Cohorts. Matern. Child Health J. 2020, 24, 1288–1298. [Google Scholar] [CrossRef]
- Pugh, S.J.; Hutcheon, J.A.; Richardson, G.A.; Brooks, M.M.; Himes, K.P.; Day, N.L.; Bodnar, L.M. Gestational weight gain, prepregnancy body mass index and offspring attention-deficit hyperactivity disorder symptoms and behaviour at age 10. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.; Macdonald-Wallis, C.; Tilling, K.; Boyd, A.; Golding, J.; Davey Smith, G.; Henderson, J.; Macleod, J.; Molloy, L.; Ness, A.; et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013, 42, 97–110. [Google Scholar] [CrossRef]
- Golding, J.; Pembrey, M.; Jones, R. ALSPAC—The Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr. Perinat. Epidemiol. 2001, 15, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Golding, J.; Macleod, J.; Lawlor, D.A.; Fraser, A.; Henderson, J.; Molloy, L.; Ness, A.; Ring, S.; Davey Smith, G. Cohort Profile: The ‘children of the 90s’--the index offspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013, 42, 111–127. [Google Scholar] [CrossRef]
- Avon Longitudinal Study of Parents and Children (ALSPAC). Reserach Ethics. Available online: http://www.bristol.ac.uk/alspac/researchers/research-ethics/ (accessed on 8 July 2023).
- World Health Organization. Body Mass Index—BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi (accessed on 13 April 2023).
- Goodman, R. Psychometric properties of the strengths and difficulties questionnaire. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 1337–1345. [Google Scholar] [CrossRef]
- Goodman, R. The Strengths and Difficulties Questionnaire: A research note. J. Child Psychol. Psychiatry Allied Discip. 1997, 38, 581–586. [Google Scholar] [CrossRef]
- Psychogiou, L.; Moberly, N.J.; Parry, E.; Nath, S.; Kallitsoglou, A.; Russell, G. Parental depressive symptoms, children’s emotional and behavioural problems, and parents’ expressed emotion-Critical and positive comments. PLoS ONE 2017, 12, e0183546. [Google Scholar] [CrossRef]
- Dachew, B.A.; Scott, J.G.; Mamun, A.; Fetene, D.M.; Alati, R. Maternal hypertensive disorders during pregnancy and the trajectories of offspring emotional and behavioral problems: The ALSPAC birth cohort study. Ann. Epidemiol. 2021, 53, 63–68.e1. [Google Scholar] [CrossRef] [PubMed]
- Reiss, F. Socioeconomic inequalities and mental health problems in children and adolescents: A systematic review. Soc. Sci. Med. 2013, 90, 24–31. [Google Scholar] [CrossRef]
- Roza, S.J.; Verhulst, F.C.; Jaddoe, V.W.V.; Steegers, E.A.P.; Mackenbach, J.P.; Hofman, A.; Tiemeier, H. Maternal smoking during pregnancy and child behaviour problems: The Generation R Study. Int. J. Epidemiol. 2009, 38, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.S.; Schwalberg, R.; Noonan, G.; Gabor, V. Factors influencing inadequate and excessive weight gain in pregnancy: Colorado, 2000–2002. Matern. Child Health J. 2006, 10, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Voerman, E.; Amiano, P.; Barros, H.; Beilin, L.J.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; Chrousos, G.P.; et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: An individual participant data meta-analysis of European, North American and Australian cohorts. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Dachew, B.A.; Scott, J.G.; Mamun, A.; Alati, R. Hypertensive disorders of pregnancy and emotional and behavioural problems in children: A longitudinal population-based study. Eur. Child Adolesc. Psychiatry 2020, 29, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; McKenzie-McHarg, K.; Shakespeare, J.; Price, J.; Gray, R. A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr. Scand. 2009, 119, 350–364. [Google Scholar] [CrossRef]
- Capron, L.E.; Glover, V.; Pearson, R.M.; Evans, J.; O’Connor, T.G.; Stein, A.; Murphy, S.E.; Ramchandani, P.G. Associations of maternal and paternal antenatal mood with offspring anxiety disorder at age 18 years. J. Affect. Disord. 2015, 187, 20–26. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Dow, C.; Galera, C.; Charles, M.A.; Heude, B. Maternal pre-pregnancy BMI and offspring hyperactivity-inattention trajectories from 3 to 8 years in the EDEN birth cohort study. Eur. Child Adolesc. Psychiatry 2023, 32, 2057–2065. [Google Scholar] [CrossRef]
- Van Lieshout, R.J.; Robinson, M.; Boyle, M.H. Maternal pre-pregnancy body mass index and internalizing and externalizing problems in offspring. Can. J. Psychiatry. Rev. Can. Psychiatr. 2013, 58, 151–159. [Google Scholar] [CrossRef]
- Dow, C.; Lorthe, E.; Galera, C.; Tafflet, M.; Marchand-Martin, L.; Ancel, P.-Y.; Charles, M.-A.; Heude, B. High maternal pre-pregnancy BMI is associated with increased offspring peer-relationship problems at 5 years. Front. Child Adolesc. Psychiatry 2022, 1, 971743. [Google Scholar] [CrossRef]
- Dow, C.; Lorthe, E.; Marchand-Martin, L.; Galera, C.; Tafflet, M.; Ancel, P.Y.; Charles, M.A.; Heude, B. Maternal pre-pregnancy obesity and offspring hyperactivity-inattention symptoms at 5 years in preterm and term children: A multi-cohort analysis. Sci. Rep. 2022, 12, 18190. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; Eaves, L.A.; Gaona, A.R.; Santos, H.P., Jr.; Smeester, L.; Bangma, J.T.; Rager, J.E.; O’Shea, T.M.; Fry, R.C. Pre-pregnancy BMI-associated miRNA and mRNA expression signatures in the placenta highlight a sexually-dimorphic response to maternal underweight status. Sci. Rep. 2021, 11, 15743. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Mulla, S.; Beyene, J.; Liao, G.; McDonald, S.D. Maternal underweight and the risk of preterm birth and low birth weight: A systematic review and meta-analyses. Int. J. Epidemiol. 2011, 40, 65–101. [Google Scholar] [CrossRef]
- Benton, D.; ILSI Europe, a.i.s.b.l. Micronutrient status, cognition and behavioral problems in childhood. Eur. J. Nutr. 2008, 47, 38–50. [Google Scholar] [CrossRef]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- de Kieviet, J.F.; Zoetebier, L.; van Elburg, R.M.; Vermeulen, R.J.; Oosterlaan, J. Brain development of very preterm and very low-birthweight children in childhood and adolescence: A meta-analysis. Dev. Med. Child Neurol. 2012, 54, 313–323. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Zeng, L.; Dang, S.; Zhou, J.; Pei, L.; Watson, V.; Chen, T.; Wang, D.; Yan, H. Effect of maternal pre-pregnancy underweight and average gestational weight gain on physical growth and intellectual development of early school-aged children. Sci. Rep. 2018, 8, 12014. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Zeng, L.; Dang, S.; Zhou, J.; Yan, H. Effect of prenatal and postnatal malnutrition on intellectual functioning in early school-aged children in rural western China. Medicine 2016, 95, e4161. [Google Scholar] [CrossRef]
- Hinkle, S.N.; Schieve, L.A.; Stein, A.D.; Swan, D.W.; Ramakrishnan, U.; Sharma, A.J. Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. Int. J. Obes. (Lond.) 2012, 36, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.A.R.; Gatica-Domínguez, G.; Santos, I.S.; Bertoldi, A.D.; Domingues, M.; Murray, J.; Silveira, M.F. Poor maternal nutritional status before and during pregnancy is associated with suspected child developmental delay in 2-year old Brazilian children. Sci. Rep. 2020, 10, 1851. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chen, Y.; Dai, Y.; Xiao, L.; Zhao, P.; Ben, X. Prepregnancy body mass index and gestational weight gain affect the offspring neurobehavioral development at one year of age. J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obs. 2022, 35, 6140–6149. [Google Scholar] [CrossRef] [PubMed]
- Brunner Huber, L.R. Validity of self-reported height and weight in women of reproductive age. Matern. Child Health J. 2007, 11, 137–144. [Google Scholar] [CrossRef]
- Wolke, D.; Waylen, A.; Samara, M.; Steer, C.; Goodman, R.; Ford, T.; Lamberts, K. Selective drop-out in longitudinal studies and non-biased prediction of behaviour disorders. Br. J. Psychiatry 2009, 195, 249–256. [Google Scholar] [CrossRef]
| Characteristics | n (%) $ or Mean (SD) | ||||
|---|---|---|---|---|---|
| Total (n = 10,571) | Under Weight (n = 520) | Normal Weight (n = 7855) | Overweight (n = 1607) | Obese (n = 589) | |
| Maternal age at delivery (mean, SD) | 28.0 (4.8) | 26.2 (5.3) | 28.1 (4.8) | 28.0 (4.7) | 27.7 (4.7) |
| Maternal education # | |||||
| Certificate of secondary education | 1320 (13.8) | 92 (20.6) | 859 (12.0) | 254 (17.7) | 115 (22.5) |
| Vocational | 961 (10.1) | 50 (11.2) | 682 (9.5) | 154 (10.8) | 75 (14.7) |
| O level | 3530 (37.0) | 154 (34.5) | 2620 (36.7) | 562 (39.3) | 194 (38.0) |
| A level | 2347 (24.6) | 100 (22.4) | 1818 (25.4) | 338 (23.6) | 91 (17.8) |
| Degree | 1381 (14.5) | 51 (11.4) | 1170 (16.4) | 124 (8.7) | 36 (7.0) |
| Marital status | |||||
| Married | 7977 (77.3) | 324 (64.9) | 5934 (77.3) | 265 (80.6) | 454 (78.7) |
| Never married | 1759 (17.0) | 146 (29.3) | 1292 (16.8) | 226 (14.4) | 95 (16.5) |
| Widowed/divorced/Separated | 585 (5.7) | 29 (5.8) | 449 (5.9) | 79 (5.0) | 28 (4.8) |
| Gestational weight gain, IOM category | |||||
| Inadequate (less than recommended) | 3176 (33.4) | 249 (54.6) | 2543 (36.1) | 255 (17.4) | 129 (23.7) |
| Adequate (recommended) | 3724 (39.1) | 162 (25.5) | 2951 (41.8) | 482 (33.0) | 129 (23.7) |
| Excessive (more than recommended) | 2615 (27.5) | 45 (9.9) | 1559 (22.1) | 725 (49.6) | 286 (52.6) |
| Parity | |||||
| Nullipara | 4489 (44.2) | 227 (46.4) | 3459 (45.7) | 614 (39.6) | 189 (33.6) |
| Multipara | 5674 (55.8) | 262 (53.6) | 4102 (54.3) | 936 (60.4) | 374 (66.4) |
| Alcohol drinking in pregnancy | |||||
| Yes | 1610 (15.7) | 67 (13.5) | 1236 (16.2) | 242 (15.4) | 65 (11.3) |
| No | 8667 (84.3) | 429 (86.5) | 6396 (83.8) | 1333 (84.6) | 509 (88.7) |
| Smoking during pregnancy | |||||
| Yes | 2374 (23.0) | 168 (33.50) | 1695 (22.1) | 376 (23.8) | 135 (23.4) |
| No | 7963 (77.0) | 334 (66.5) | 5986 (77.9) | 1202 (76.2) | 441 (76.6) |
| Maternal antenatal anxiety symptoms | |||||
| Yes | 2122 (22.1) | 134 (29.3) | 1548 (21.6) | 315 (21.7) | 125 (24.3) |
| No | 7469 (77.9) | 323 (70.7) | 5623 (78.4) | 1133 (78.3) | 390 (75.7) |
| Maternal antenatal depressive symptoms | |||||
| Yes | 1811 (18.4) | 122 (26.0) | 1277 (17.5) | 278 (18.5) | 134 (24.9) |
| No | 8016 (81.6) | 347 (74.0) | 6042 (82.6) | 1222 (81.5) | 405 (75.1) |
| Child sex | |||||
| Male | 5388 (50.9) | 252 (48.5) | 4013 (51.1) | 817 (50.8) | 306 (52.0) |
| Female | 5183 (49.1) | 268 (51.5) | 3842 (48.9) | 790 (49.2) | 283 (48.0) |
| Gestational age at delivery in weeks (mean, SD) | 39.8 (1.3) | 39.7 (1.30) | 39.8 (1.3) | 39.8 (1.3) | 39.9 (1.3) |
| Birth weight in kg (mean, SD) | 3.5 (0.45) | 3.3 (0.39) | 3.5 (0.44) | 3.6 (0.47) | 3.7 (0.51) |
| Emotional and Behavioural Problems | Sex | 3 Years (n = 9207) | 7 Years (n = 7748) | 9 Years (n = 7447) | 11 Years (n = 6811) | 16 Years (n = 5202) |
|---|---|---|---|---|---|---|
| Total behavioural difficulties (n, %) | Male | 1970 (41.5) | 489 (12.3) | 445 (11.9) | 404 (11.9) | 189 (7.6) |
| Female | 1611 (36.1) | 323 (8.6) | 298 (8.0) | 250 (7.3) | 247 (9.1) | |
| Total | 3581 (38.9) | 812 (10.5) | 743 (9.9) | 654 (9.6) | 436 (8.4) | |
| Hyperactivity/inattention problems (n, %) | Male | 393 (8.3) | 929 (23.4) | 674 (17.2) | 555 (16.4) | 310 (12.3) |
| Female | 255 (5.7) | 477 (12.6) | 342 (9.2) | 253 (7.4) | 199 (7.3) | |
| Total | 648 (7.0) | 1517 (18.5) | 989 (13.2) | 808 (11.9) | 509 (9.7) | |
| Conduct problems (n, %) | Male | 3127 (66.6) | 1027 (25.8) | 725 (19.3) | 596 (17.6) | 289 (11.5) |
| Female | 2717 (61.3) | 843 (22.2) | 598 (16.1) | 493 (14.4) | 370 (13.6) | |
| Total | 5844 (64.0) | 1870 (24.1) | 1323 (17.7) | 1089 (16.0) | 659 (12.6) | |
| Emotional symptoms (n, %) | Male | 1185 (25.0) | 475 (11.9) | 428 (11.4) | 377 (11.2) | 211 (8.4) |
| Female | 1178 (26.4) | 525 (13.9) | 575 (15.5) | 472 (13.8) | 510 (18.8) | |
| Total | 2363 (25.7) | 1000 (13.5) | 1003 (13.6) | 849 (12.5) | 721 (13.8) | |
| Peer relationship problems (n, %) | Male | --- | 647 (16.3) | 665 (17.7) | 598 (17.6) | 432 (17.2) |
| Female | --- | 463 (12.2) | 520 (14.0) | 472 (13.8) | 385 (14.1) | |
| Total | --- | 1110 (14.3) | 1185 (15.9) | 1070 (15.7) | 817 (15.6) | |
| Prosocial behaviour (n, %) | Male | --- | 522 (13.1) | 382 (10.2) | 329 (9.7) | 346 (13.8) |
| Female | --- | 229 (6.0) | 177 (4.8) | 172 (5.0) | 269 (9.9) | |
| Total | --- | 751 (9.7) | 559 (7.5) | 501 (7.3) | 615 (11.8) |
| Pre-Pregnancy BMI (Kg/m2) and Gestational Weight Gain | Crude OR (95% CI) | |||||
|---|---|---|---|---|---|---|
| Total Behavioural Difficulties | Hyperactivity/Inattention Problems | Conduct Problems | Emotional Symptoms | Peer Relationship Problems | Pro-Social Behaviours | |
| Pre-pregnancy BMI | ||||||
| <18.5 | 1.38 (1.16–1.64) | 1.27 (1.01–1.61) | 1.18 (1.02–1.35) | 1.53 (1.27–1.83) | 1.24 (0.98–1.58) | 1.30 (0.98–1.71) |
| 18.5–24.99 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–29.99 | 1.12 (1.01–1.24) | 1.12 (0.98–1.29) | 1.13 (1.05–1.23) | 1.03 (0.92–1.15) | 1.20 (1.04–1.37) | 0.93 (0.77–1.10) |
| ≥30 | 1.11 (0.94–1.32) | 1.06 (0.84–1.33) | 1.12 (0.99–1.28) | 1.17 (0.97–1.39) | 1.48 (1.20–1.82) | 0.62 (0.44–0.86) |
| Gestational weight gain | ||||||
| Inadequate | 1.05 (0.96–1.15) | 1.11 (0.98–1.25) | 1.01 (0.95–1.08) | 1.00 (0.91–1.11) | 1.00 (0.89–1.13) | 0.92 (0.80–1.07) |
| Adequate | 1 | 1 | 1 | 1 | 1 | 1 |
| Excessive | 1.03 (0.94–1.13) | 1.02 (0.90–1.17) | 0.95 (0.88–1.02) | 1.10 (0.99–1.22) | 1.12 (0.99–1.27) | 0.82 (0.70–0.96) |
| Adjusted OR (95% CI) # | ||||||
| Pre-pregnancy BMI | ||||||
| <18.5 | 1.22 (1.02–1.45) | 1.15 (0.91–1.47) | 1.09 (0.95–1.26) | 1.37 (1.14–1.64) | 1.14 (0.90–1.46) | 1.33 (1.01–1.75) |
| 18.5–24.99 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–29.99 | 1.08 (0.98–1.20) | 1.08 (0.94–1.25) | 1.11 (1.03–1.21) | 1.01 (0.90–1.14) | 1.18 (1.03–1.37) | 0.97 (0.81–1.15) |
| ≥30 | 1.03 (0.87–1.21) | 0.98 (0.77–1.23) | 1.07 (0.94–1.22) | 1.10 (0.92–1.32) | 1.43 (1.16–1.77) | 0.62 (0.44–0.86) |
| Gestational weight gain | ||||||
| Inadequate | 1.04 (0.96–1.14) | 1.09 (0.97–1.24) | 0.99 (0.92–1.06) | 1.00 (0.91–1.11) | 1.01 (0.89–1.14) | 0.91 (0.79–1.05) |
| Adequate | 1 | 1 | 1 | 1 | 1 | 1 |
| Excessive | 0.96 (0.87–1.05) | 0.96 (0.84–1.09) | 0.92 (0.85–0.99) | 1.03 (0.93–1.14) | 1.06 (0.94–1.20) | 0.82 (0.70–0.96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dachew, B.A.; Adane, A.A.; Alati, R. Maternal Pregnancy and Pre-Pregnancy Weight and Behavioural Outcomes in Children. Behav. Sci. 2024, 14, 49. https://doi.org/10.3390/bs14010049
Dachew BA, Adane AA, Alati R. Maternal Pregnancy and Pre-Pregnancy Weight and Behavioural Outcomes in Children. Behavioral Sciences. 2024; 14(1):49. https://doi.org/10.3390/bs14010049
Chicago/Turabian StyleDachew, Berihun A., Akilew A. Adane, and Rosa Alati. 2024. "Maternal Pregnancy and Pre-Pregnancy Weight and Behavioural Outcomes in Children" Behavioral Sciences 14, no. 1: 49. https://doi.org/10.3390/bs14010049
APA StyleDachew, B. A., Adane, A. A., & Alati, R. (2024). Maternal Pregnancy and Pre-Pregnancy Weight and Behavioural Outcomes in Children. Behavioral Sciences, 14(1), 49. https://doi.org/10.3390/bs14010049






