Abstract
We aimed to investigate depression, anxiety, stress, and PTSD symptoms and their relationship with disease severity in acutely ill hospitalized Coronavirus disease 2019 (COVID-19) patients. A single-center cross-sectional observational survey study screening for psychiatric symptoms using the Depression, Anxiety and Stress Scale—21 Items (DASS-21) and the Impact of Events Scale-Revised (IES-R) questionnaires was performed including a total of 169 acutely ill COVID-19 patients. All patients were adults and of white race and developed respiratory insufficiency during hospitalization. Demographic, clinical and laboratory data were evaluated as predictors of psychiatric symptoms. We hypothesized that higher intensity of COVID-19 symptoms and higher oxygen requirement would be associated with occurrence of depression, anxiety, stress, and PTSD symptoms. Depressive symptoms were absent in 29%, mild in 16%, moderate in 27.8%, severe in 10.7% and extremely severe in 16.6% patients. Anxiety symptoms were absent in 43.8%, mild in 6.5%, moderate in 17.2%, severe in 5.3% and extremely severe in 27.2% patients. Stress symptoms were absent in 78.7%, mild in 4.7%, moderate in 7.1%, severe in 7.7%, and extremely severe in 1.8% patients. A total of 60.9% patients had no PTSD symptoms, 16% had undiagnosed symptoms, and 23.1% met the criteria for a PTSD diagnosis. All psychiatric symptoms were more pronounced in female patients, depression and anxiety symptoms were associated with prior chronic obstructive pulmonary disease. Only depressive symptoms were significantly associated with higher intensity of COVID-19 symptoms and higher oxygen requirement. Acutely ill hospitalized COVID-19 patients presented a high prevalence of emergent psychiatric sequelae, especially in females, and more severe COVID-19 influenced mostly the severity of depressive symptoms.
1. Introduction
The cCoronavirus disease 2019 (COVID-19) pandemic substantially challenged stress resilience and mental health outcomes, especially among vulnerable populations such as patients with previous psychiatric disorders, severely ill COVID-19 patients, health care workers, children, and elderly [1]. Mental health consequences may occur in weeks, months or longer after the infection. Hospitalized COVID-19 patients suffering from more severe forms of COVID-19 may be particularly susceptible to adverse mental health conditions [2].
COVID-19 is a multisystemic disease affecting dominantly respiratory system. Although a majority of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infected patients experience no or only mild symptoms, prior to wide-spread availability of vaccination, a substantial proportion of patients (15–20%) developed acute respiratory insufficiency, requiring oxygen supplementation and thus needed to be hospitalized [3]. Subsequent vaccination program and occurrence of novel, less aggressive viral strains significantly reduced the number of patients presenting with severe and critical forms of the disease. Despite benefits of vaccination, even among those with breakthrough infections [4], vaccine hesitancy, inadequate immunization among elderly and immunosuppressed patients, as well as waning effects of vaccine-induced immunization remain substantial problems for global control of the COVID-19 pandemic [5,6].
Several putative mechanisms by which COVID-19 may induce psychological symptoms have been proposed [7,8]. Clinical, post-mortem, animal, in vitro, and cell culture studies demonstrated that coronaviruses are potentially neurotropic and can induce neuronal injuries [9]. Notwithstanding possible brain infiltration, “cytokines storm” involved in the immune response to coronaviruses may cause psychiatric symptoms by precipitating neuroinflammation [10].
Also, the brain is highly sensitive to the change in the arterial concentration of oxygen [11]; it ineluctably sustains the hypoxic stress. Decreased oxygen content might lead to neuronal injury in the brains of chronic obstructive pulmonary disease (COPD) patients, which is demonstrated by clinical symptoms such as mood disorders and neuropsychological deficits. Furthermore, the systemic inflammation is accompanied by a hypoxic injury that may exacerbate the neuronal injury. A large number of patients with intermittent hypoxia manifest a series of symptoms related to the injury to the nervous system, which is exhibited as a deficiency in memory, learning, and decision-making ability. In addition, the hypoxic damage in the brains of other patients is manifested as depression, anxiety, physical disabilities, and neuropsychological deficits [12]. Some studies demonstrate that hypoxia is associated with the changes in the brain structure, including volume atrophy and a decrease in the gray matter in the amygdala, hippocampus, anterior cingulate cortex, prefrontal cortex, and other regions [13].
Being hospitalized for a serious illness can negatively affect mental health and which may be further intensified when hospitalized with COVID-19 due to the exceptional circumstances within and outside the hospital during the pandemic [14]. Patients hospitalized with COVID-19 are isolated to avoid the virus spreading to other patients or health care staff. COVID-19 patients are thus limited in their access to social support, and their only in-person contact is with health care staff in full personal protective equipment. Studies have highlighted that isolation itself may have psychological implications [15]. Finally, the host immune response to SARS-CoV-2 infection and the persistent psychological stress before and during infection, as well as adverse effects of treatment, such as insomnia caused by corticosteroids, have also been suggested as possible mechanisms [16].
A number of studies have investigated psychiatric symptoms among COVID-19 patients and health care workers. Depression, anxiety, and stress symptoms seem to be highly prevalent among hospitalized COVID-19 patients [17,18,19], similar to previous severe acute respiratory syndrome (SARS) outbreaks [20]. Specific approaches like cognitive behavioral therapy might help in reducing the burden COVID-19 imposes on mental health of affected patients [19]. Pandemic also negatively affects psychological distress of community dwellers [21,22] and may increase rates of acute psychiatric admissions due to acute psychosis [23]. It also negatively affects mental health of the health care workers [24,25].
To better understand clinical context in which psychiatric symptoms may occur in COVID-19 patients and considering the lack of published data on depression, anxiety, stress, and PTSD symptoms in the acute setting, we decided to prospectively investigate this issue in a real-life cohort of acutely ill hospitalized COVID-19 patients with severe or critical clinical presentation. We hypothesized that higher intensity of COVID-19 symptoms and higher oxygen requirement would be associated with occurrence of depression, anxiety, stress, and PTSD symptoms.
2. Materials and Methods
2.1. Participants and Their Medical Evaluation
We performed a cross-sectional evaluation of 169 hospitalized COVID-19 patients hospitalized in the University hospital Dubrava, Zagreb, Croatia in period from November 2020 to May 2021. All patients were tested positive for COVID-19 by polymerase chain reaction or antigen test and had compatible clinical symptoms. All patients were adults and of white race. Patients were treated according to the contemporary guidelines, receiving oxygen supplementation, dexamethasone, and low molecular weight heparins in addition to their chronic and other acute therapies [26,27]. Demographic, clinical, and laboratory data were recorded at the time of hospital admission and were obtained through analysis of written and electronical medical records. Severity of COVID-19 at admission was classified using the World health organization recommendations into mild, moderate, severe, and critical [26]. Modified early warning score (MEWS) was used to assess intensity of COVID-19 symptoms [28]. Patient comorbidities were recorded as individual diseases and as a cumulative comorbidity burden quantified using the Charlson comorbidity index [29]. Functional status of patients at admission was classified using the Eastern cooperative oncology group (ECOG) scale [30]. Patients with disorders of consciousness, poor physical condition, patients currently requiring mechanical ventilation or in the intensive care unit, and who have severe communication difficulties (dementia, deafness, intellectual difficulties) were excluded from the study. All included patients developed severe or critical form of disease (respiratory insufficiency) and required oxygen supplementation. Patients with critical intensity of symptoms were allowed to participate upon stabilization of their clinical condition and transferred to medical wards.
2.2. Evalution of Psychiatric Symptoms
Written informed consent was obtained from all participants prior to entry into the study. After the introductory interview and informed consent, demographic data was obtained, and then, psychological questionnaires were administered. Study questionnaires included general questionnaire on demographic variables, psychiatric scales the Depression, Anxiety and Stress Scale—21 Items (DASS-21) Croatian adaptation [31], and the Impact of Events Scale-Revised (IES-R) [11].
DASS-21 is a short form of the DASS-42, a self-reported scale designed to measure negative emotional states of depression, anxiety, and stress. It has 21 items with seven questions for each condition, depression, anxiety, and stress, and each item having four possible answers scored from 0 to 3 points. The score for each item is multiplied by factor two to comply with original scale comprising 42 items. For depression, scores 10–13, 14–20, 21–27, and ≥28 points are considered to represent mild, moderate, severe, and extremely severe symptoms, respectively. For anxiety, scores 8–9, 10–14, 15–19, and ≥20 points are considered to represent mild, moderate, severe, and extremely severe symptoms, respectively. For stress, scores 15–18, 19–25, 26–33, and ≥34 points are considered to represent mild, moderate, severe, and extremely severe symptoms, respectively. The scale was shown to have good internal consistency and concurrent validity [32,33].
IES-R is a short self-reported scale designed to measure PTSD. It has 22 items, each scored from 0 to 4 points. The total IES-R score was divided into 0–23, 24–32, and ≥33 points considered to represent absence of PTSD symptoms, undiagnosed symptoms, and meeting the criteria for PTSD diagnosis, respectively. The scale was shown to have good internal consistency and concurrent validity [34,35].
Total DASS-21 score (Cronbach’s alpha 0.930), DASS-21 scores for individual scales (Cronbach’s alpha values of 0.788, 0.878, and 0.837 for depression, anxiety, and stress, respectively) and IES-R score (Cronbach’s alpha 0.918) showed good to excellent internal consistency in our sample as well, implying the reliability of scales.
The median duration of the disease until filling out of the questionnaire was 15 days. The questionnaires were administered to patients with investigators using personal protective equipment.
2.3. Statistical Methods
The normality of the distribution of numerical variables was tested with the Kolmogorov–Smirnov test. Numerical variables were non-normally distributed and presented as median and interquartile range (IQR) and were compared between groups using the Mann–Whitney U test and the Kruskal–Wallis ANOVA test. Spearman correlation was used to compare two numerical variables. Categorical variables were compared using the chi square test. Multivariate analyses were performed using the logistic regression models, with presence of individual psychiatric symptoms being dependent variables for each model, and intensity of COVID-19 symptoms and required oxygen supplementation, age, sex, Charlson comorbidity index, and prior psychiatric treatment being independent variables. p-values < 0.05 were considered statistically significant. All analyses were performed using the MedCalc statistical software version 22.007 (MedCalc Software Ltd., Ostend, Belgium).
2.4. Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University hospital Dubrava (Nm. 2020/1012-05).
2.5. Patients’ Characteristics
A total of 169 patients were analyzed. There were 105 (62.1%) male and 64 (37.9%) female patients. Median age was 65 years, IQR (57–71). Median Charlson comorbidity index was 3 points, IQR (2–4). At the time of hospital admission 134 (79.3%) patients had severe and 17 (10.1%) patients had critical severity of COVID-19 symptoms. Median MEWS score was 3 points, IQR (1–4). Median C reactive protein (CRP) was 72.75 mg/L, IQR (31.4–141.4).
3. Results
3.1. Depression
The median score for depressive symptoms was 14 points, IQR (8–22). Depressive symptoms were absent in 49 (29%), mild in 27 (16%), moderate in 47 (27.8%), severe in 18 (10.7%), and extremely severe in 28 (16.6%) patients. Relationship of presence of depressive symptoms with clinical characteristics are shown in Table 1.

Table 1.
Relationship of presence of depressive symptoms with clinical characteristics.
Patients with the presence of depressive symptoms at a statistically significant level were more often of female sex (Figure 1), had chronic obstructive pulmonary disease, had a more pronounced intensity of symptoms of COVID-19 at the time of hospital admission, required oxygen supplementation at higher flows, less frequently had deep vein thrombosis, and had lower red blood cell distribution width (RDW), lower D-dimers, and lower procalcitonin. They also more frequently felt that their lives were in greater danger due to COVID-19 (p < 0.05 for all analyses). Neither age, CRP, nor other investigated parameters were significantly associated with the presence or severity of depressive symptoms during hospitalization.
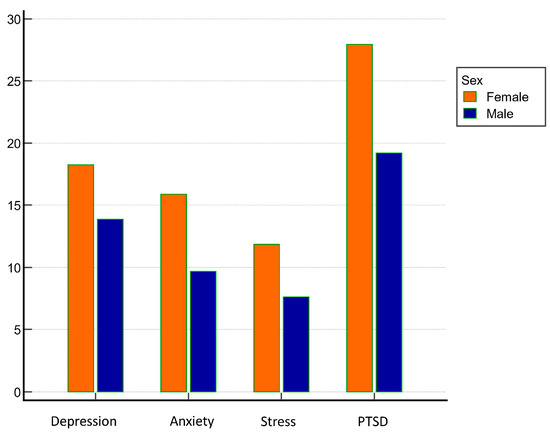
Figure 1.
Sex differences in assessed psychiatric symptoms’ measurements.
3.2. Anxiety
The median score for anxiety symptoms was 8 points, IQR (2–20). Anxiety symptoms were absent in 74 (43.8%), mild in 11 (6.5%), moderate in 29 (17.2%), severe in 9 (5.3%), and extremely severe in 46 (27.2%) patients. Relationship of presence of anxiety symptoms with clinical characteristics are shown in Table 2.

Table 2.
Relationship of presence of anxiety symptoms with clinical characteristics.
Patients with the presence of anxiety symptoms at a statistically significant level were more often female (Figure 1), felt more threatened by COVID-19, more often had COPD, more often had bacteremia during the stay, and lower D-dimers on admission (p < 0.05 for all analyses). There was no statistically significant association of the presence or severity of anxiety symptoms during hospitalization with the severity of COVID-19 at admission, with CRP or with other examined characteristics.
3.3. Stress
The median score for stress symptoms was 6 points, IQR (2–12.5). Stress symptoms were absent in 133 (78.7%), mild in 8 (4.7%), moderate in 12 (7.1%), severe in 13 (7.7%), and extremely severe in 3 (1.8%) patients. Relationship of presence of stress symptoms with clinical characteristics are shown in Table 3.

Table 3.
Relationship of presence of stress symptoms with clinical characteristics.
Patients with the presence stress symptoms at a statistically significant level were more often female (Figure 1), felt more threatened by COVID-19, were more likely to have been previously psychiatrically treated, and had lower D-dimer values and lower procalcitonin values at admission (p < 0.05 for all analyzes).
3.4. PTSD
The median IES-R score was 20 points, IQR (11–31.25). A total of 103 (60.9%) patients had no PTSD symptoms, 27 (16%) had undiagnosed symptoms, and 39 (23.1%) met the criteria for a PTSD diagnosis. Relationship of presence of PTSD symptoms with clinical characteristics are shown in Table 4.

Table 4.
Relationship of presence of PTSD symptoms with clinical characteristics.
Patients with PTSD symptoms present at a statistically significant level were more often female (Figure 1), felt that their lives were more at risk due to COVID-19, had lower values of RDW, procalcitonin, and creatinine during hospital admission, and were previously psychiatrically treated (p < 0.05 for all analyses).
3.5. Multivariate Analyses
We performed a series of multivariate logistic regression models investigating relationship of presence of individual psychiatric symptoms with severity of COVID-19 symptoms, required intensity of oxygen supplementation, age, sex, Charlson comorbidity index, and previous psychiatric treatment. Results are presented in Table 5. Presence of depressive symptoms was significantly associated with higher intensity of COVID-19 symptoms and was nearly significant for female sex, whereas presence of anxiety, stress, and PTSD symptoms remained significantly associated with female sex in the context of aforementioned clinically relevant adjustments.

Table 5.
Multivariate logistic regression models evaluating independent contribution of COVID-19 severity, age, sex, Charlson comorbidity index, and previous psychiatric treatment to individual psychiatric symptoms.
4. Discussion
We performed a cross-sectional study evaluating prevalence of depression, anxiety, stress, and PTSD symptoms among acutely ill, hospitalized COVID-19 patients, using the personal protective equipment as patients were considered infectious and required mandatory isolation. As we report, psychopathology is prevalent in this patient population and demographic and clinical characteristics affect mostly the severity of depressive symptoms. Depressive symptoms were the only symptoms significantly associated with the intensity of COVID-19 symptoms. Also, female patients might require special consideration since all investigated psychiatric symptoms were more prevalent among female than male patients, even after adjustments for clinically relevant variables.
In order of prevalence, depressive symptoms were the most frequent psychiatric disorder affecting 71% of patients, followed by anxiety symptoms affecting 56.2% patients, and PTSD symptoms with 23.1% patients meeting the criteria for its diagnosis, and additional 16% having undiagnosed symptoms, followed by stress symptoms present in 21.3% patients. One should bear in mind that we investigated a cohort of real-life patients with high prevalence of severe or critical COVID-19 symptoms upon their clinical stabilization. Our results are not readily comparable or translatable to other clinical settings, as other studies evaluated more heterogenous patient population with milder forms of COVID-19 in different stages of development.
Despite male patients experiencing higher severity of COVID-19, especially among elderly [36], females might be more vulnerable regarding mental health as shown in the current, as well as in multiple previous studies [37,38,39]. This is most likely due to a number of biological, psychological, and cultural factors associated with female sex [39]. Females generally tend to assume a caregiving role in their families, which is very demanding to balance with work and household tasks, making them vulnerable and predisposing them to mental health issues in situations of overload [40].
Similar to acute setting, in the post-illness stage, depressed mood, insomnia, anxiety, irritability, memory impairment, fatigue, and traumatic memories were frequently reported in the literature as well [41]. The social situation to which COVID-19 survivors return is completely different from that of SARS and Middle East respiratory syndrome (MERS) (diseases caused by coronaviruses) survivors. These differences are relevant for the prevalence of psychiatric disorders in both acute and post-illness stages. In the COVID-19 era, unlike the previous SARS and MERS outbreaks, fear for shortage of medical facilities such as ventilators can further increase stress. Staying at the intensive care unit (ICU) is a risk factor for developing psychiatric disorders by itself. In 2018, a large study among almost 5000 ICU survivors showed that prevalence of post-traumatic stress disorder was 46%, that of anxiety was 40%, and that of depression was 22% [42].
Depression among hospitalized patients is often unrecognized, undiagnosed, and therefore untreated. Feasibility of screening for depression during hospitalization, or whether depression is associated with poorer outcomes, longer hospital stays, and higher readmission rates are still insufficiently explored. The prevalence of depression ranged from 5% to 60%, with a median of 33%, among hospitalized patients prior to COVID-19 [43].
The association between COPD and psychiatric disorders, in particular generalized anxiety, panic disorder, and depression, has been acknowledged for many years. As the findings of the current study show, having prior COPD was associated mostly with presence of anxiety, whereas no significant association was determined regarding other psychiatric symptoms. In recent years, the role of the immune system in the pathogenesis of chronic disease has been extensively studied. This trend is also prominent in psychiatry and systemic inflammation as well as localized modulation of microglial cell activity in the central nervous system (CNS) have been associated with most psychiatric conditions. Depression in particular may be associated with systemic inflammation and higher serum concentrations of inflammatory biomarkers such as interleukin (IL)-1 and tumor necrosis factor-alpha (TNF-a), though the nature of the association has yet to be clearly elucidated. A recent study [44] found elevated levels of IL-2, IL-6, and interferon-gamma (IFN-γ) in patients suffering from co-morbid COPD and depression compared to healthy controls, though the implications of these findings remain uncertain. On the opposite, results obtained from the cohort of the ECLIPSE study [45] indicate that there is no significant association between inflammatory biomarkers and symptoms of depression in individuals suffering from COPD. Similarly, we could not identify higher inflammatory burden measured through laboratory biomarkers of inflammation to be associated with presence of particular psychiatric symptoms.
Patients with the presence of depressive symptoms during hospitalization in our study, at a statistically significant level, had a more pronounced intensity of symptoms of COVID-19 at the time of hospital admission and required oxygen supplementation at higher flows. Hypoxia in the brain leads to oxidative stress, alterations of white matter, and alterations of endothelial cells, which can ultimately result in permanent cognitive defects and dementia. It modifies neuronal functions by altering the synthesis of neurotransmitters. High levels of carbon dioxide may activate the respiratory center of the brain stem, triggering anxiety in attempt to alert the body of possible suffocation. Interestingly, the onset of depression has also been demonstrated in COPD patients experiencing hypoxemia. In addition, hypoxemia can enhance systemic inflammation, and it has been demonstrated to induce nuclear factor κB, the master regulator of cellular inflammatory responses resulting in systemic inflammation [46]. Andelid and colleagues found hypoxemia after COPD exacerbations to be associated with systemic neutrophilic activity [47]. Moreover, it is hypothesized that hypoxia of adipose tissue in obese COPD patients might be an important source of systemic inflammation [48]. Hypoxemia-associated oxidative stress is a potential organic mechanism for the onset of depressive symptoms [49]. Supporting this, Forlenza and colleagues found increased serum levels of a biomarker for oxidative damage, 8-hydroxy-2′-deoxyguanosine, in depressed patients [50]. Translating this phenomenon to COPD, increased levels of oxidative stress have also been found in COPD patients [51]. Oxidative stress regulates inflammation in COPD, and decreased levels of the antioxidant glutathione are found after severe and very severe exacerbations (89.2% and 52.3%, respectively) relative to stable COPD. Taken together, hypoxemia and oxidative stress, directly and/or indirectly through associated systemic inflammation, might be involved in the onset of brain-associated comorbidities in COPD patients.
CNS viral infections [52], inflammatory process, and cerebral hypoxia [53] have substantial impact on cognitive functions producing transient or permanent cognitive impairment. Limbic and associated brain structures such as the hippocampus and basal ganglia contain more enzymes that are involved in inflammatory responses than other areas. Therefore, there is an increased risk of developing deficits in neurocognitive processes like memory, attention, and emotion [54]. Research demonstrates that poorer cognitive function is associated with increased risk of depression, social withdrawal, and dependence [55] and can contribute to decreased quality of life.
Main limitations of our study are being a single center study and limited number of included patients, thus reflecting the statistical power of some of the presented analyses. Our findings are representative of a high volume tertiary referral center for treatment of most severe COVID-19 patients. The study was performed during the alpha SARS-CoV-2 viral strain dominated wave of the disease. Thus we were unable to estimate how potential vaccination could affect our measurements, as well as how occurrence and clinical correlations of investigated psychiatric symptoms might be related to infection by other viral strains. Inclusion of patients of only white race may also be considered as a limitation regarding generalizability of our findings. Due to cross-sectional study design, no causal relationship between investigated variables can be inferred.
5. Conclusions
Acutely ill hospitalized COVID-19 patients presented a high prevalence of emergent psychiatric sequelae, and clinical and demographic characteristics influenced mostly the severity of depressive symptoms. Female patients might require special considerations since all investigated psychiatric symptoms were more prevalent among female than male patients, even after adjustments for clinically relevant variables. Higher than average incidence of major depression, anxiety, and stress, all high-burden non-communicable conditions associated with years of life lived with disability, is expected in survivors. These findings motivate further follow-up studies of mental health among patients recovering from COVID-19 and other serious infections and impose the need for heightened clinical surveillance of those recovering from a severe illness.
Author Contributions
Conceptualization, D.L., A.M.P. and N.P.Z.; Methodology, D.L., M.L. and M.K.; software, M.L.; validation, L.M.M.; formal analysis, M.L.; investigation, D.L. and M.K.; resources, D.L.; data curation, D.L. and M.L.; writing—original draft preparation, D.L.; writing—review and editing, A.M.P., N.P.Z., M.L., M.K. and L.M.M.; supervision, A.M.P., N.P.Z. and L.M.M.; project administration, D.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University hospital Dubrava (Nm. 2020/1012-05).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available per reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Manchia, M.; Gathier, A.W.; Yapici-Eser, H.; Schmidt, M.V.; de Quervain, D.; van Amelsvoort, T.; Bisson, J.I.; Cryan, J.F.; Howes, O.D.; Pinto, L.; et al. The impact of the prolonged COVID-19 pandemic on stress resilience and mental health: A critical review across waves. Eur. Neuropsychopharmacol. 2022, 55, 22–83. [Google Scholar] [CrossRef]
- Kahl, K.G.; Correll, C.U. Management of patients with severe mental illness during the coronavirus disease 2019 pandemic. JAMA Psychiatry 2020, 77, 977–978. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Busic, N.; Lucijanic, T.; Barsic, B.; Luksic, I.; Busic, I.; Kurdija, G.; Barbic, L.; Kunstek, S.; Jelic, T.; Lucijanic, M. Vaccination provides protection from respiratory deterioration and death among hospitalized COVID-19 patients: Differences between vector and mRNA vaccines. J. Med. Virol. 2022, 94, 2849–2854. [Google Scholar] [CrossRef] [PubMed]
- Lucijanic, M.; Marelic, D.; Stojic, J.; Markovic, I.; Sedlic, F.; Kralj, I.; Rucevic, D.; Busic, N.; Javor, P.; Lucijanic, T.; et al. Predictors of prolonged hospitalization of COVID-19 patients. Eur. Geriatr. Med. 2023, 14, 511–516. [Google Scholar] [CrossRef]
- Sertić, Z.; Lucijanić, M.; Bašić-Kinda, S.; Serventi Seiwerth, R.; Periša, V.; Sertić, D.; Coha, B.; Pulanić, D.; Perić, Z.; Desnica, L.; et al. Non-Myelofibrosis Chronic Myeloproliferative Neoplasm Patients Show Better Seroconversion Rates after SARS-CoV-2 Vaccination Compared to Other Hematologic Diseases: A Multicentric Prospective Study of KroHem. Biomedicines 2022, 10, 2892. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, K.; Liu, J.N.; Yuan, L.J.; Zhang, J.J.; Gao, L. Associations between brain gene expression perturbations implicated by COVID-19 and psychiatric disorders. J. Psychiatr. Res. 2023, 162, 79–87. [Google Scholar] [CrossRef]
- Thomasson, M.; Voruz, P.; Cionca, A.; Jacot de Alcântara, I.; Nuber-Champier, A.; Allali, G.; Benzakour, L.; Lalive, P.H.; Lövblad, K.O.; Braillard, O.; et al. Markers of limbic system damage following SARS-CoV-2 infection. Brain Commun. 2023, 5, fcad177. [Google Scholar] [CrossRef] [PubMed]
- Desforges, M.; Le Coupanec, A.; Dubeau, P.; Bourgouin, A.; Lajoie, L.; Dubé, M.; Talbot, P.J. Human Coronaviruses and Other Respiratory Viruses: Underestimated Opportunistic Pathogens of the Central Nervous System? Viruses 2019, 12, 14. [Google Scholar] [CrossRef]
- Dantzer, R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol. Rev. 2018, 98, 477–504. [Google Scholar] [CrossRef]
- Weiss, D.S. The Impact of Event Scale: Revised. In Cross-Cultural Assessment of Psychological Trauma and PTSD; Wilson, J.P., Tang, C.S.-K., Eds.; Springer: Boston, MA, USA, 2007; pp. 219–238. [Google Scholar]
- Incalzi, R.A.; Gemma, A.; Marra, C.; Muuolon, R. Chronic Obstrudive Pulmonary Disease. Am. Rev. Respir. Dis. 1993, 148, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Rao, B.S.; Raju, T.; Meti, B. Increased numerical density of synapses in CA3 region of hippocampus and molecular layer of motor cortex after self-stimulation rewarding experience. Neuroscience 1999, 91, 799–803. [Google Scholar] [PubMed]
- Vlake, J.H.; Wesselius, S.; van Genderen, M.E.; van Bommel, J.; Boxma-de Klerk, B.; Wils, E.J. Psychological distress and health-related quality of life in patients after hospitalization during the COVID-19 pandemic: A single-center, observational study. PLoS ONE 2021, 16, e0255774. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Wu, D.; Lin, R.; Wang, Z.; Pan, L. Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19. Complement. Ther. Clin. Pract. 2020, 39, 101132. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.T.; Yang, Y.; Li, W.; Zhang, L.; Zhang, Q.; Cheung, T.; Ng, C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry 2020, 7, 228–229. [Google Scholar] [CrossRef] [PubMed]
- Zandifar, A.; Badrfam, R.; Yazdani, S.; Arzaghi, S.M.; Rahimi, F.; Ghasemi, S.; Khamisabadi, S.; Mohammadian Khonsari, N.; Qorbani, M. Prevalence and severity of depression, anxiety, stress and perceived stress in hospitalized patients with COVID-19. J. Diabetes Metab. Disord. 2020, 19, 1431–1438. [Google Scholar] [CrossRef]
- Moayed, M.S.; Vahedian-Azimi, A.; Mirmomeni, G.; Rahimi-Bashar, F.; Goharimoghadam, K.; Pourhoseingholi, M.A.; Abbasi-Farajzadeh, M.; Hekmat, M.; Sathyapalan, T.; Guest, P.C.; et al. Depression, Anxiety, and Stress Among Patients with COVID-19: A Cross-Sectional Study. Adv. Exp. Med. Biol. 2021, 1321, 229–236. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Jiang, J.; Xu, X.; Wu, J.; Xu, Y.; Lin, X.; Hall, J.; Xu, H.; Xu, J.; et al. The Effect of Cognitive Behavioral Therapy on Depression, Anxiety, and Stress in Patients With COVID-19: A Randomized Controlled Trial. Front. Psychiatry 2020, 11, 580827. [Google Scholar] [CrossRef]
- Chua, S.E.; Cheung, V.; McAlonan, G.M.; Cheung, C.; Wong, J.W.; Cheung, E.P.; Chan, M.T.; Wong, T.K.; Choy, K.M.; Chu, C.M.; et al. Stress and psychological impact on SARS patients during the outbreak. Can. J. Psychiatry 2004, 49, 385–390. [Google Scholar] [CrossRef]
- Moayed, M.S.; Vahedian-Azimi, A.; Mirmomeni, G.; Rahimi-Bashar, F.; Goharimoghadam, K.; Pourhoseingholi, M.A.; Abbasi-Farajzadeh, M.; Babaei, M.; Sathyapalan, T.; Guest, P.C.; et al. A Survey of Psychological Distress Among the Community in the COVID-19 Epidemic: A Cross-Sectional Study. Adv. Exp. Med. Biol. 2021, 1321, 253–260. [Google Scholar] [CrossRef]
- Ausserhofer, D.; Mahlknecht, A.; Engl, A.; Piccoliori, G.; Pfitscher, G.; Silbernagl, P.; Giacomoni, F.; Pycha, R.; Lombardo, S.; Gärtner, T.; et al. Relationship between depression, anxiety, stress, and SARS-CoV-2 infection: A longitudinal study. Front. Psychol. 2023, 14, 1116566. [Google Scholar] [CrossRef] [PubMed]
- Kelbrick, M.; da Silva, K.; Griffiths, C.; Ansari, S.; Paduret, G.; Tanner, J.; Mann, N.; Johnson, S. The impact of COVID-19 on acute psychiatric admissions for first and repeated episode psychosis. Int. J. Soc. Psychiatry 2023, 207640231188031. [Google Scholar] [CrossRef]
- Portillo-Van Diest, A.; Vilagut, G.; Alayo, I.; Ferrer, M.; Amigo, F.; Amann, B.L.; Aragón-Peña, A.; Aragonès, E.; Asúnsolo Del Barco, Á.; Campos, M.; et al. Traumatic stress symptoms among Spanish healthcare workers during the COVID-19 pandemic: A prospective study. Epidemiol. Psychiatr. Sci. 2023, 32, e50. [Google Scholar] [CrossRef] [PubMed]
- Moayed, M.S.; Vahedian-Azimi, A.; Mirmomeni, G.; Rahimi-Bashar, F.; Goharimoghadam, K.; Pourhoseingholi, M.A.; Abbasi-Farajzadeh, M.; Hekmat, M.; Sathyapalan, T.; Guest, P.C.; et al. Survey of Immediate Psychological Distress Levels Among Healthcare Workers in the COVID-19 Epidemic: A Cross-Sectional Study. Adv. Exp. Med. Biol. 2021, 1321, 237–243. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Geneva: World Health Organization. Contract No.: WHO/2019-nCoV/clinical/2020.5. 2020. Available online: https://reliefweb.int/report/world/clinical-management-covid-19-interim-guidance-may-2020?gclid=Cj0KCQjw9MCnBhCYARIsAB1WQVVv_QTTGpS4vnC91c5zWQTrRSg0LdpiIFjP86_TIrYv04O35PUs34QaAozCEALw_wcB (accessed on 1 August 2023).
- Ministarstvo Zdravstva Republike Hrvatske. Smjernice za Liječenje Oboljelih od COVID-19, Verzija 1 od 8. Rujna 2020. 2020. Available online: https://www.hzjz.hr/wp-content/uploads/2021/11/Smjernice-za-lijecenje-oboljelih-od-koronavirusne-bolesti-2019-COVID-19-verzija-8.pdf (accessed on 1 August 2023).
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. Qjm 2001, 94, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Oken, M.M.; Creech, R.H.; Tormey, D.C.; Horton, J.; Davis, T.E.; McFadden, E.T.; Carbone, P.P. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am. J. Clin. Oncol. 1982, 5, 649–655. [Google Scholar] [CrossRef]
- Jakšić, N.; Ivezić, E.; Jokic-Begic, N.; Surányi, Z. Validation of the Croatian adaptation of the Depression, Anxiety, Stress Scales-21 (DASS-21) in a clinical sample. In Proceedings of the 18th Psychology Days in Zadar, Zadar, Croatia, 24–26 May 2012. [Google Scholar]
- Gloster, A.T.; Rhoades, H.M.; Novy, D.; Klotsche, J.; Senior, A.; Kunik, M.; Wilson, N.; Stanley, M.A. Psychometric properties of the Depression Anxiety and Stress Scale-21 in older primary care patients. J. Affect. Disord. 2008, 110, 248–259. [Google Scholar] [CrossRef]
- Henry, J.D.; Crawford, J.R. The short-form version of the Depression Anxiety Stress Scales (DASS-21): Construct validity and normative data in a large non-clinical sample. Br. J. Clin. Psychol. 2005, 44, 227–239. [Google Scholar] [CrossRef]
- Abas, M.A.; Müller, M.; Gibson, L.J.; Derveeuw, S.; Dissanayake, N.; Smith, P.; Verhey, R.; Danese, A.; Chibanda, D. Prevalence of post-traumatic stress disorder and validity of the Impact of Events Scale—Revised in primary care in Zimbabwe, a non-war-affected African country. BJPsych Open 2023, 9, e37. [Google Scholar] [CrossRef]
- Weigl, T.; Beck-Hiestermann, F.M.L.; Stenzel, N.M.; Benson, S.; Schedlowski, M.; Garthus-Niegel, S. Assessment of Childbirth-Related PTSD: Psychometric Properties of the German Version of the City Birth Trauma Scale. Front. Psychiatry 2021, 12, 731537. [Google Scholar] [CrossRef] [PubMed]
- Piskač Živković, N.; Lucijanić, M.; Bušić, N.; Jurin, I.; Atić, A.; Andrilović, A.; Penović, T.; Domić, I.; Gnjidić, J.; Demaria, M.; et al. The associations of age, sex, and comorbidities with survival of hospitalized patients with coronavirus disease 2019: Data from 4014 patients from a tertiary-center registry. Croat. Med. J. 2022, 63, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, R.P. COVID-19 and mental health: A review of the existing literature. Asian J. Psychiatr. 2020, 52, 102066. [Google Scholar] [CrossRef] [PubMed]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav. Immun. 2020, 89, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Del Río-Casanova, L.; Sánchez-Martín, M.; García-Dantas, A.; González-Vázquez, A.; Justo, A. Psychological Responses According to Gender during the Early Stage of COVID-19 in Spain. Int. J. Environ. Res. Public Health 2021, 18, 3731. [Google Scholar] [CrossRef]
- González-Sanguino, C.; Ausín, B.; Castellanos, M.; Saiz, J.; López-Gómez, A.; Ugidos, C.; Muñoz, M. Mental health consequences during the initial stage of the 2020 Coronavirus pandemic (COVID-19) in Spain. Brain Behav. Immun. 2020, 87, 172–176. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Hatch, R.; Young, D.; Barber, V.; Griffiths, J.; Harrison, D.A.; Watkinson, P. Anxiety, Depression and Post Traumatic Stress Disorder after critical illness: A UK-wide prospective cohort study. Crit. Care 2018, 22, 310. [Google Scholar] [CrossRef]
- IsHak, W.W.; Collison, K.; Danovitch, I.; Shek, L.; Kharazi, P.; Kim, T.; Jaffer, K.Y.; Naghdechi, L.; Lopez, E.; Nuckols, T. Screening for depression in hospitalized medical patients. J. Hosp. Med. 2017, 12, 118–125. [Google Scholar] [CrossRef]
- Rybka, J.; Korte, S.M.; Czajkowska-Malinowska, M.; Wiese, M.; Kędziora-Kornatowska, K.; Kędziora, J. The links between chronic obstructive pulmonary disease and comorbid depressive symptoms: Role of IL-2 and IFN-γ. Clin. Exp. Med. 2016, 16, 493–502. [Google Scholar] [CrossRef]
- Janssen, D.J.; Müllerova, H.; Agusti, A.; Yates, J.C.; Tal-Singer, R.; Rennard, S.I.; Vestbo, J.; Wouters, E.F. Persistent systemic inflammation and symptoms of depression among patients with COPD in the ECLIPSE cohort. Respir. Med. 2014, 108, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Kent, B.D.; Mitchell, P.D.; McNicholas, W.T. Hypoxemia in patients with COPD: Cause, effects, and disease progression. Int. J. Chronic Obstruct. Pulmon. Dis. 2011, 6, 199–208. [Google Scholar] [CrossRef]
- Andelid, K.; Glader, P.; Yoshihara, S.; Andersson, A.; Ekberg-Jansson, A.; Lindén, A. Hypoxia associated with increased systemic concentrations of MPO and NE during exacerbations of COPD. Eur. Respir. J. 2015, 46, PA873. [Google Scholar] [CrossRef]
- Trayhurn, P.; Wang, B.; Wood, I.S. Hypoxia in adipose tissue: A basis for the dysregulation of tissue function in obesity? Br. J. Nutr. 2008, 100, 227–235. [Google Scholar] [CrossRef]
- Rose, M.; Sharafkhaneh, A. Overview of Psychological Considerations in the Management of Patients with Chronic Respiratory Conditions. In Depression and Anxiety in Patients with Chronic Respiratory Diseases; Sharafkhaneh, A., Yohannes, A.M., Hanania, N.A., Kunik, M.E., Eds.; Springer: New York, NY, USA, 2017; pp. 1–9. [Google Scholar]
- Forlenza, M.J.; Miller, G.E. Increased Serum Levels of 8-Hydroxy-2′-Deoxyguanosine in Clinical Depression. Psychosom. Med. 2006, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Drost, E.M.; Skwarski, K.M.; Sauleda, J.; Soler, N.; Roca, J.; Agusti, A.; MacNee, W. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax 2005, 60, 293–300. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Forbes, H.J.; Williamson, E.; Breuer, J.; Hayward, A.C.; Mavrodaris, A.; Ridha, B.H.; Rossor, M.N.; Thomas, S.L.; Smeeth, L. Human herpesvirus infections and dementia or mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 4743. [Google Scholar] [CrossRef]
- McMorris, T.; Hale, B.J.; Barwood, M.; Costello, J.; Corbett, J. Effect of acute hypoxia on cognition: A systematic review and meta-regression analysis. Neurosci. Biobehav. Rev. 2017, 74, 225–232. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The impact of inflammation on cognitive function in older adults: Implications for healthcare practice and research. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef]
- Black, S.A.; Rush, R.D. Cognitive and functional decline in adults aged 75 and older. J. Am. Geriatr. Soc. 2002, 50, 1978–1986. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).