Clinical Profiles in Multiple Sclerosis: Cognitive Reserve and Motor Impairment along Disease Duration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Assessment and Materials
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Oreja-Guevara, C.; Ayuso Blanco, T.; Brieva Ruiz, L.; Hernández Pérez, M.Á.; Meca-Lallana, V.; Ramió-Torrentà, L. Cognitive Dysfunctions and Assessments in Multiple Sclerosis. Front. Neurol. 2019, 10, 581. [Google Scholar] [PubMed]
- Sumowski, J.F.; Benedict, R.; Enzinger, C.; Filippi, M.; Geurts, J.J.; Hamalainen, P.; Hulst, H.; Inglese, M.; Leavitt, V.M.; Rocca, M.A.; et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018, 90, 278–288. [Google Scholar] [CrossRef]
- Chiaravalloti, N.D.; DeLuca, J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008, 7, 1139–1151. [Google Scholar]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [PubMed]
- Grzegorski, T.; Losy, J. Cognitive impairment in multiple sclerosis—A review of current knowledge and recent research. Rev. Neurosci. 2017, 28, 845–860. [Google Scholar]
- Rocca, M.A.; Amato, M.P.; De Stefano, N.; Enzinger, C.; Geurts, J.J.; Penner, I.K.; Rovira, A.; Sumowski, J.F.; Valsasina, P.; Filippi, M.; et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015, 14, 302–317. [Google Scholar] [PubMed]
- Ghaffar, O.; Fiati, M.; Feinstein, A. Occupational attainment as a marker of cognitive reserve in multiple sclerosis. PloS ONE 2012, 7, e47206. [Google Scholar] [CrossRef]
- Benedict, R.H.; Zivadinov, R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat. Rev. Neurol. 2011, 7, 332–342. [Google Scholar]
- Brochet, B.; Ruet, A. Cognitive Impairment in Multiple Sclerosis with Regards to Disease Duration and Clinical Phenotypes. Front. Neurol. 2019, 10, 261. [Google Scholar]
- Tremblay, A.; Charest, K.; Brando, E.; Roger, E.; Duquette, P.; Rouleau, I. The effects of aging and disease duration on cognition in multiple sclerosis. Brain Cogn. 2020, 146, 105650. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Yigit, P.; Acikgoz, A.; Mehdiyev, Z.; Dayi, A.; Ozakbas, S. The relationship between cognition, depression, fatigue, and disability in patients with multiple sclerosis. Ir. J. Med. Sci. 2021, 190, 1129–1136. [Google Scholar] [PubMed]
- Heled, E.; Aloni, R.; Achiron, A. Cognitive functions and disability progression in relapsing-remitting multiple sclerosis: A longitudinal study. Appl. Neuropsychol. Adult 2021, 28, 210–219. [Google Scholar] [CrossRef]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Iudice, A.; Della Pina, D.; Malentacchi, G.; Sabatini, S.; Annunziata, P.; Falcini, M.; et al. Relevance of cognitive deterioration in early relapsing-remitting MS: A 3-year follow-up study. Mult. Scler. J. 2010, 16, 1474–1482. [Google Scholar]
- Martins Da Silva, A.; Cavaco, S.; Moreira, I.; Bettencourt, A.; Santos, E.; Pinto, C.; Gonçalves, A.; Coutinho, E.; Samões, R.; Dias, C.C.; et al. Cognitive reserve in multiple sclerosis: Protective effects of education. Mult. Scler. J. 2015, 21, 1312–1321. [Google Scholar]
- Amato, M.P.; Razzolini, L.; Goretti, B.; Stromillo, M.L.; Rossi, F.; Giorgio, A.; Hakiki, B.; Giannini, M.; Pastò, L.; Portaccio, E.; et al. Cognitive reserve and cortical atrophy in multiple sclerosis: A longitudinal study. Neurology 2015, 80, 1728–1733. [Google Scholar] [CrossRef]
- Mondini, S.; Pucci, V.; Montemurro, S.; Rumiati, R.I. Protective factors for subjective cognitive decline individuals: Trajectories and changes in a longitudinal study with Italian elderly. Eur. J. Neurol. 2022, 29, 691–697. [Google Scholar]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. J. Alzheimer’s Assoc. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Pettigrew, C.; Soldan, A. Defining Cognitive Reserve and Implications for Cognitive Aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1. [Google Scholar]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [PubMed]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsycol. Soc. 2002, 8, 448–460. [Google Scholar]
- Grotz, C.; Seron, X.; Van Wissen, M.; Adam, S. How should proxies of cognitive reserve be evaluated in a population of healthy older adults? Int. Psychogeriatr. 2017, 29, 123–136. [Google Scholar] [PubMed]
- Harrison, S.L.; Sajjad, A.; Bramer, W.M.; Ikram, M.A.; Tiemeier, H.; Stephan, B.C. Exploring strategies to operationalize cognitive reserve: A systematic review of reviews. J. Clin. Exp. Neuropsychol. 2015, 37, 253–264. [Google Scholar] [PubMed]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index questionnaire (CRIq): A new instrument for measuring cognitive reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [PubMed]
- Luerding, R.; Gebel, S.; Gebel, E.M.; Schwab-Malek, S.; Weissert, R. Influence of Formal Education on Cognitive Reserve in Patients with Multiple Sclerosis. Front. Neurol. 2016, 7, 46. [Google Scholar]
- Sumowski, J.F.; Rocca, M.A.; Leavitt, V.M.; Riccitelli, G.; Meani, A.; Comi, G.; Filippi, M. Reading, writing, and reserve: Literacy activities are linked to hippocampal volume and memory in multiple sclerosis. Mult. Scler. J. 2016, 22, 1621–1625. [Google Scholar]
- Santangelo, G.; Altieri, M.; Enzinger, C.; Gallo, A.; Trojano, L. Cognitive reserve and neuropsychological performance in multiple sclerosis: A meta-analysis. Neuropsychology 2019, 33, 379–390. [Google Scholar] [CrossRef]
- Ifantopoulou, P.; Artemiadis, A.K.; Bakirtzis, C.; Zekiou, K.; Papadopoulos, T.S.; Diakogiannis, I.; Hadjigeorgiou, G.; Grigoriadis, N.; Orologas, A. Cognitive and brain reserve in multiple sclerosis—A cross-sectional study. Mult. Scler. Relat. Disord. 2019, 35, 128–134. [Google Scholar]
- Sumowski, J.F.; Leavitt, V.M. Cognitive reserve in multiple sclerosis. Mult. Scler. J. 2013, 19, 1122–1127. [Google Scholar] [CrossRef]
- Amato, M.P.; Prestipino, E.; Bellinvia, A.; Niccolai, C.; Razzolini, L.; Pastò, L.; Fratangelo, R.; Tudisco, L.; Fonderico, M.; Mattiolo, P.L.; et al. Cognitive impairment in multiple sclerosis: An exploratory analysis of environmental and lifestyle risk factors. PloS ONE 2019, 14, e0222929. [Google Scholar]
- Nunnari, D.; De Cola, M.C.; Costa, A.; Rifici, C.; Bramanti, P.; Marino, S. Exploring cognitive reserve in multiple sclerosis: New findings from a cross-sectional study. J. Clin. Exp. Neuropsychol. 2016, 38, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Rimkus, C.M.; Avolio, I.M.B.; Miotto, E.C.; Pereira, S.A.; Mendes, M.F.; Callegaro, D.; Leite, C.D.C. The protective effects of high-education levels on cognition in different stages of multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 22, 41–48. [Google Scholar] [PubMed]
- Artemiadis, A.; Bakirtzis, C.; Ifantopoulou, P.; Zis, P.; Bargiotas, P.; Grigoriadis, N.; Hadjigeorgiou, G. The role of cognitive reserve in multiple sclerosis: A cross-sectional study in 526 patients. Mult. Scler. Relat. Disord. 2020, 41, 102047. [Google Scholar] [CrossRef]
- Trembley, A.; Charest, K.; Brando, E.; Roger, E.; Duquette, P.; Rouleau, I. Cognitive reserve as a moderating factor between EDSS and cognition in multiple sclerosis. Mult. Scler. Relat. Disord. 2023, 70, 104482. [Google Scholar]
- Wood, S.N. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Montemurro, S.; Mondini, S.; Pucci, V.; Durante, G.; Riccardi, A.; Maffezzini, S.; Scialpi, G.; Signorini, M.; Arcara, G. Tele-Global Examination of Mental State (Tele-GEMS): An open tool for the remote neuropsychological screening. Neurol. Sci. 2023, 1–10. [Google Scholar]
- CIR Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 14 August 2023).
- Burham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Symonds, M.R.E.; Moussalli, A. A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike’s information criterion. Behav. Ecol. Sociobiol. 2011, 65, 13–21. [Google Scholar]
- Opdebeeck, C.; Martyr, A.; Clare, L. Cognitive reserve and cognitive function in healthy older people: A meta-analysis. Aging Neuropsychol. Cogn. 2016, 23, 40–60. [Google Scholar]
- Nelson, M.E.; Jester, D.J.; Petkus, A.J.; Andel, R. Cognitive Reserve, Alzheimer’s Neuropathology, and Risk of Dementia: A Systematic Review and Meta-Analysis. Neuropsychol. Rev. 2021, 31, 233–250. [Google Scholar]

| Mean | SD | Median | Min | Max | Kurtosis | Skewness | Q1 | Q3 | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 50.89 | 9.25 | 51 | 30 | 74 | 0.09 | 0.04 | 46 | 56 |
| Education | 12.53 | 3.53 | 13 | 5 | 21 | −0.65 | 0.04 | 8 | 13 |
| Disease Duration | 14.82 | 9.28 | 13 | 1 | 37 | −0.75 | 0.45 | 7 | 21 |
| EDSS | 3.04 | 1.90 | 2.50 | 1 | 8 | −0.31 | 0.86 | 1.5 | 4 |
| CRI | 105.67 | 13.58 | 104 | 78 | 142 | −0.29 | 0.33 | 96 | 115 |
| Tele-GEMS | 76.74 | 15.65 | 81.8 | 19.5 | 98.3 | 1.78 | −1.31 | 60.08 | 87.73 |
| Variable | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1. Age | - | ||||
| 2. Disease Duration | 0.34 | - | |||
| 3. EDSS | 0.30 | 0.36 | - | ||
| 4. CRI | 0.13 | 0.05 | −0.36 | - | |
| 5. Tele-GEMS | −0.29 | −0.23 | −0.44 | 0.50 | - |
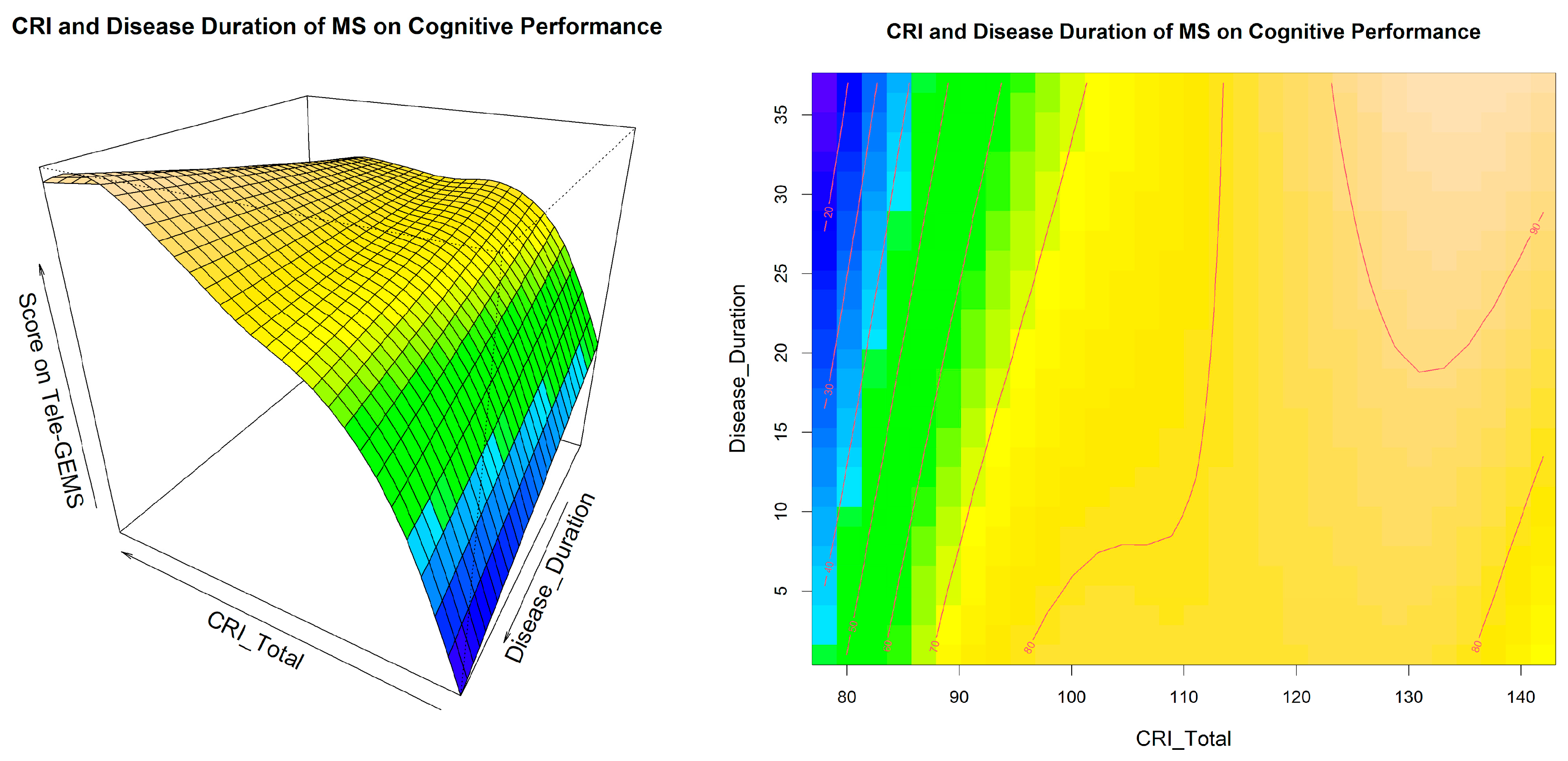
| Generalised Additive Models: Interaction between Cognitive Reserve, Disease Duration, and EDSS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tele-GEMS = Dependent Variable | |||||||||||
| Model | Intercept | Smooth Terms | Model Fit | ||||||||
| Estimate (Standard Error) | t | p | Terms | Edf | F | p | R2 | Dev | AIC | Model Weights | |
| 1 | 76.74 (1.19) | 64.71 | <0.001 | s(Age) | 1 | 3.49 | 0.065 | 0.43 | 46.50% | 787.96 | 0.1 |
| s(CRI) | 3.87 | 8.56 | <0.001 | ||||||||
| s(DD) | 1 | 1.54 | 0.217 | ||||||||
| s(EDSS) | 1 | 1.82 | 0.18 | ||||||||
| 2 | 76.67 (1.18) | 64.71 | <0.001 | s(Age) | 1 | 2.71 | 0.103 | 0.44 | 49.50% | 786.86 | 0.183 |
| s(CRI) | 3.94 | 7.67 | <0.001 | ||||||||
| s(DD) | 1 | 1.64 | 0.203 | ||||||||
| s(EDSS) | 1.29 | 0.74 | 0.337 | ||||||||
| ti(CRI,DD) | 1.93 | 1.41 | 0.257 | ||||||||
| 3 | 76.13 (1.32) | 57.59 | <0.001 | s(Age) | 1 | 3.71 | 0.567 | 0.43 | 47.60% | 788.43 | 0.079 |
| s(CRI) | 4.13 | 8.13 | <0.001 | ||||||||
| s(DD) | 1 | 1.94 | 0.167 | ||||||||
| s(EDSS) | 1 | 2.54 | 0.114 | ||||||||
| ti(CRI,EDSS) | 1 | 1.05 | 0.307 | ||||||||
| 4 | 75.05 (1.36) | 55.03 | <0.001 | s(Age) | 1 | 3.37 | 0.07 | 0.46 | 50.90% | 784.26 | 0.638 |
| s(CRI) | 4.27 | 8.55 | <0.001 | ||||||||
| s(DD) | 1 | 2.47 | 0.12 | ||||||||
| s(EDSS) | 1 | 2.79 | 0.098 | ||||||||
| ti(CRI,DD) | 1 | 5.7 | 0.019 | ||||||||
| ti(CRI,EDSS) | 1 | 4.98 | 0.028 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffezzini, S.; Pucci, V.; Riccardi, A.; Montemurro, S.; Puthenparampil, M.; Perini, P.; Rinaldi, F.; Gallo, P.; Arcara, G.; Mondini, S. Clinical Profiles in Multiple Sclerosis: Cognitive Reserve and Motor Impairment along Disease Duration. Behav. Sci. 2023, 13, 708. https://doi.org/10.3390/bs13090708
Maffezzini S, Pucci V, Riccardi A, Montemurro S, Puthenparampil M, Perini P, Rinaldi F, Gallo P, Arcara G, Mondini S. Clinical Profiles in Multiple Sclerosis: Cognitive Reserve and Motor Impairment along Disease Duration. Behavioral Sciences. 2023; 13(9):708. https://doi.org/10.3390/bs13090708
Chicago/Turabian StyleMaffezzini, Sabrina, Veronica Pucci, Alice Riccardi, Sonia Montemurro, Marco Puthenparampil, Paola Perini, Francesca Rinaldi, Paolo Gallo, Giorgio Arcara, and Sara Mondini. 2023. "Clinical Profiles in Multiple Sclerosis: Cognitive Reserve and Motor Impairment along Disease Duration" Behavioral Sciences 13, no. 9: 708. https://doi.org/10.3390/bs13090708
APA StyleMaffezzini, S., Pucci, V., Riccardi, A., Montemurro, S., Puthenparampil, M., Perini, P., Rinaldi, F., Gallo, P., Arcara, G., & Mondini, S. (2023). Clinical Profiles in Multiple Sclerosis: Cognitive Reserve and Motor Impairment along Disease Duration. Behavioral Sciences, 13(9), 708. https://doi.org/10.3390/bs13090708





