Spatial Configuration Effects on the Dissociation between Active and Latent States in Visual Working Memory
Abstract
1. Introduction
2. Experiment 1
2.1. Materials and Methods
2.1.1. Participants
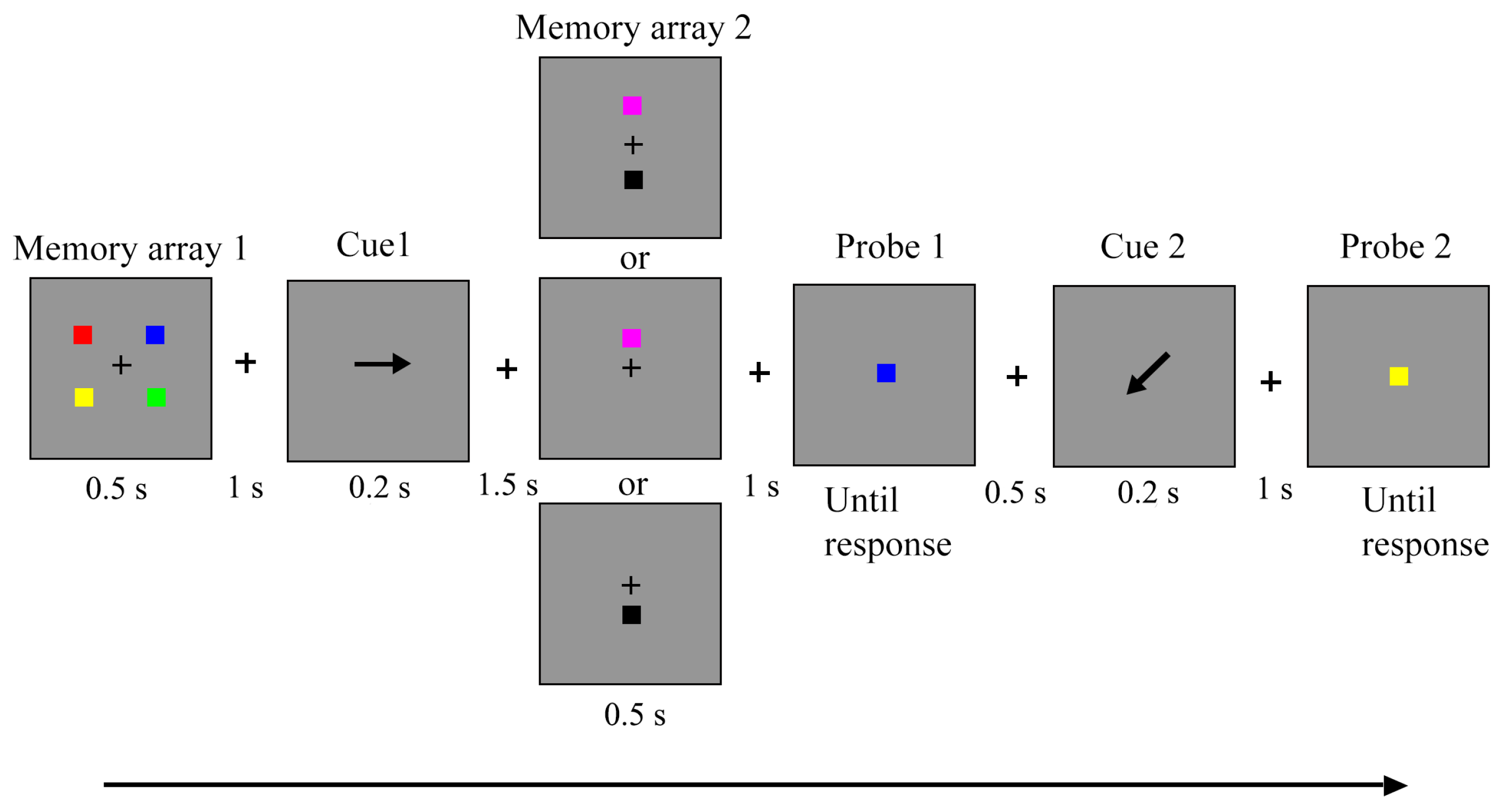
2.1.2. Stimuli and Procedure
2.2. Data Analysis
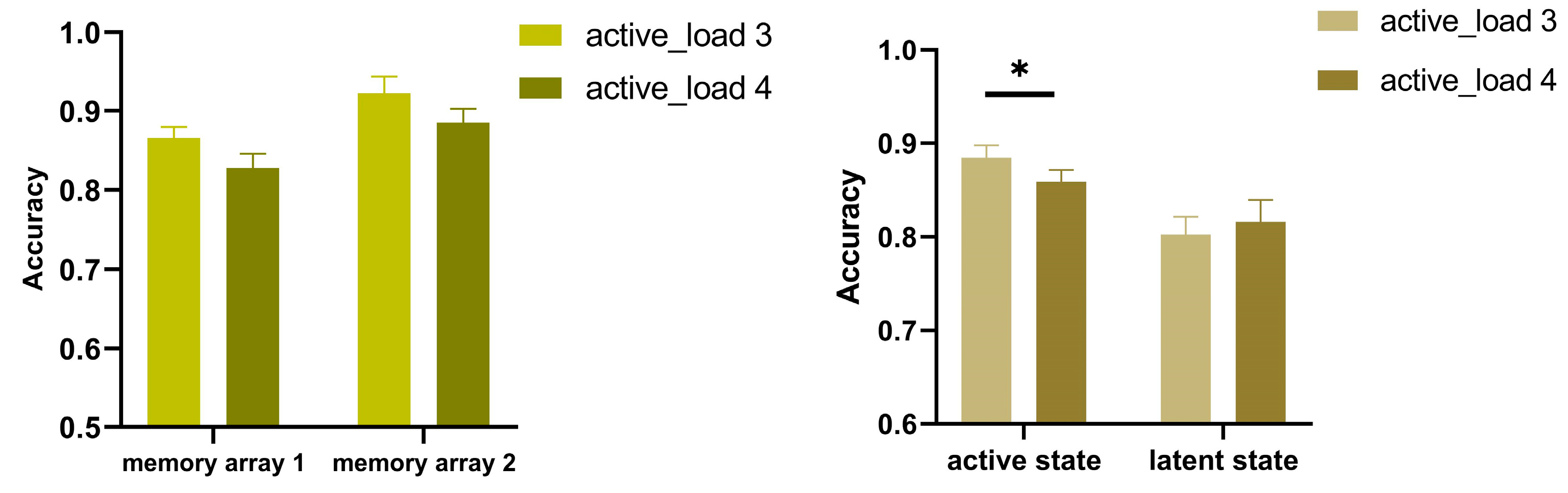
2.3. Results
3. Experiment 2
3.1. Materials and Methods
3.1.1. Participants
3.1.2. Stimuli and Procedure
3.2. Data Analysis
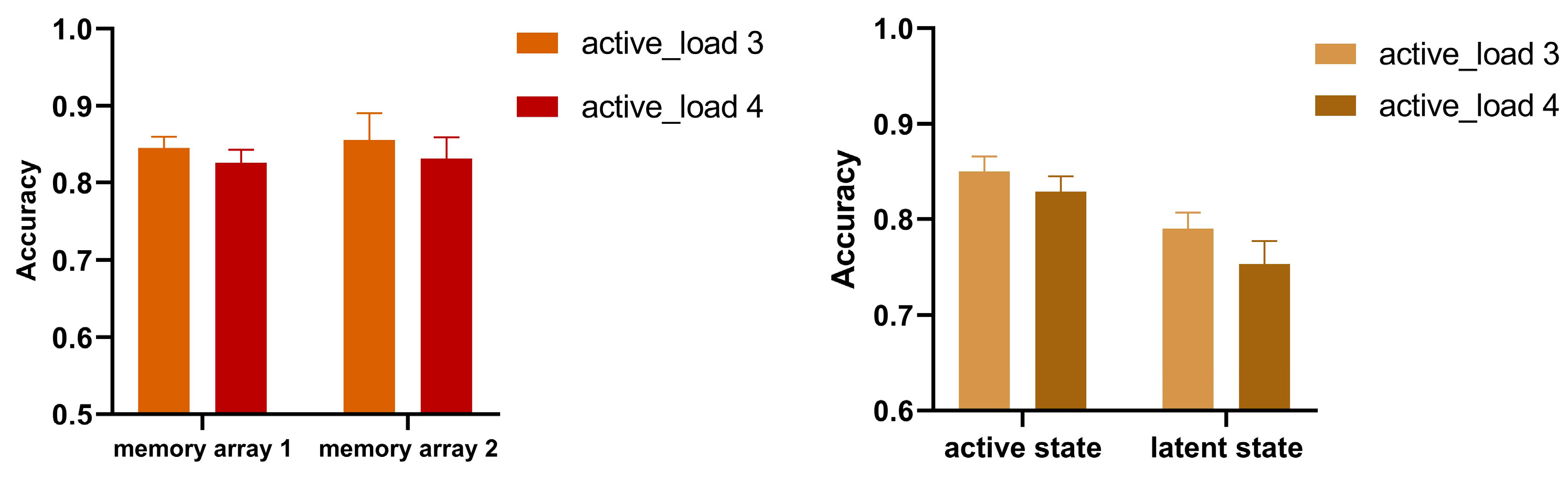
3.3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddeley, A. Working memory. Science 1992, 255, 556–559. [Google Scholar] [CrossRef]
- Baddeley, A. The episodic buffer: A new component of working memory? Trends Cogn. Sci. 2000, 4, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.K.; Lundqvist, M.; Bastos, A.M. Working Memory 2.0. Neuron 2018, 100, 463–475. [Google Scholar] [CrossRef]
- Rose, N.S.; Buchsbaum, B.R.; Craik, F.I.M. Short-term retention of a single word relies on retrieval from long-term memory when both rehearsal and refreshing are disrupted. Mem. Cogn. 2014, 42, 689–700. [Google Scholar] [CrossRef]
- Engle, R.W.; Laughlin, J.E.; Tuholski, S.W.; Conway, A.R.A. Working memory, short-term memory, and general fluid intelligence: A latent-variable approach. J. Exp. Psychol. Gen. 1999, 128, 309–331. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A. Working memory and conscious awareness. In Theories of Memory; Taylor & Francis: Abingdon, UK, 2019; pp. 11–28. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Cellular basis of working memory. Neuron 1995, 14, 477–485. [Google Scholar] [CrossRef]
- Durstewitz, D.; Seamans, J.K.; Sejnowski, T.J. Neurocomputational Models of Working Memory. Nat. Neurosci. 2000, 3, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Masse, N.Y.; Rosen, M.C.; Freedman, D.J. Reevaluating the Role of Persistent Neural Activity in Short-Term Memory. Trends Cogn. Sci. 2020, 24, 242–258. [Google Scholar] [CrossRef]
- Chota, S.; Van der Stigchel, S. Dynamic and flexible transformation and reallocation of visual working memory representations. Vis. Cogn. 2021, 29, 409–415. [Google Scholar] [CrossRef]
- Stokes, M.G.; Muhle-Karbe, P.S.; Myers, N.E. Theoretical distinction between functional states in working memory and their corresponding neural states. Vis. Cogn. 2020, 28, 420–432. [Google Scholar] [CrossRef]
- LaRocque, J.J.; Lewis-Peacock, J.A.; Postle, B.R. Multiple neural states of representation in short-term memory? It’s a matter of attention. Front. Hum. Neurosci. 2014, 8, 1–14. [Google Scholar] [CrossRef]
- Watanabe, K.; Funahashi, S. Prefrontal delay-period activity reflects the decision process of a saccade direction during a free-choice ODR task. Cerebral Cortex. 2007, 17 (Suppl. S1), i88–i100. [Google Scholar] [CrossRef]
- Stokes, M.G. “Activity-silent” working memory in prefrontal cortex: A dynamic coding framework. Trends Cogn. Sci. 2015, 19, 394–405. [Google Scholar] [CrossRef]
- Mongillo, G.; Barak, O.; Tsodyks, M. SynaptiC Theory of Working Memory. Science 2008, 319, 1543–1546. [Google Scholar] [CrossRef]
- Rose, N.S.; LaRocque, J.J.; Riggall, A.C.; Gosseries, O.; Starrett, M.J.; Meyering, E.E.; Postle, B.R. Reactivation of latent working memories with transcranial magnetic stimulation. Science 2016, 354, 1136–1139. [Google Scholar] [CrossRef]
- Wolff, M.J.; Jochim, J.; Akyürek, E.G.; Stokes, M.G. Dynamic hidden states underlying working memory guided behaviour. Nat. Neurosci. 2017, 20, 864–871. [Google Scholar] [CrossRef]
- Wolff, M.J.; Ding, J.; Myers, N.E.; Stokes, M.G. Revealing hidden states in visual working memory using electroencephalography. Front. Syst. Neurosci. 2015, 9, 1–12. [Google Scholar] [CrossRef]
- Kozachkov, L.; Tauber, J.; Lundqvist, M.; Brincat, S.L.; Slotine, J.J.; Miller, E.K. Robust and brain-like working memory through short-term synaptic plasticity. PLoS Comput. Biol. 2022, 18, e1010776. [Google Scholar] [CrossRef] [PubMed]
- Nee, D.E.; Jonides, J. Trisecting representational states in short-term memory. Front. Hum. Neurosci. 2013, 7, 796. [Google Scholar] [CrossRef]
- Kamiński, J.; Rutishauser, U. Between persistently active and activity-silent frameworks: Novel vistas on the cellular basis of working memory. Ann. N. Y. Acad. Sci. 2020, 1464, 64–75. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Liang, T.; Ye, C.; Liu, Q. Interval between two sequential arrays determines their storage state in visual working memory. Sci. Rep. 2020, 10, 7706. [Google Scholar] [CrossRef]
- LaRocque, J.J.; Lewis-Peacock, J.A.; Drysdale, A.T.; Oberauer, K.; Postle, B.R. Decoding Attended Information in Short-term Memory: An EEG Study. J. Cogn. Neurosci. 2013, 25, 127–142. [Google Scholar] [CrossRef]
- Buschman, T.J.; Miller, E.K. Working Memory Is Complex and Dynamic, Like Your Thoughts. J. Cogn. Neurosci. 2022, 35, 17–23. [Google Scholar] [CrossRef]
- Li, Z.; Liang, T.; Liu, Q. The storage resources of the active and passive states are independent in visual working memory. Cognition 2021, 217, 104911. [Google Scholar] [CrossRef]
- Oberauer, K. Access to Information in Working Memory: Exploring the Focus of Attention. J. Exp. Psychol. Learn. Mem. Cogn. 2002, 28, 411–421. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, C.; Sun, H.-J.; Zhou, J.; Liang, T.; Li, Y.; Liu, Q. The passive state: A protective mechanism for information in working memory tasks. J. Exp. Psychol. Learn. Mem. Cogn. 2022, 48, 1235–1248. [Google Scholar] [CrossRef]
- Huang, L. Supplemental Material for Unit of Visual Working Memory: A Boolean Map Provides a Better Account Than an Object Does. J. Exp. Psychol. Gen. 2020, 149, 1–30. [Google Scholar] [CrossRef]
- Treisman, A.; Zhang, W. Location and binding in visual working memory. Mem. Cogn. 2006, 34, 1704–1719. [Google Scholar] [CrossRef]
- Jiang, Y.; Olson, I.R.; Chun, M.M. Organization of Visual Short-Term Memory. J. Exp. Psychol. Learn. Mem. Cogn. 2000, 26, 683–702. [Google Scholar] [CrossRef]
- Pertzov, Y.; Husain, M. The privileged role of location in visual working memory. Atten. Percept. Psychophys. 2014, 76, 1914–1924. [Google Scholar] [CrossRef]
- Toh, Y.N.; Sisk, C.A.; Jiang, Y.V. Effects of changing object identity on location working memory. Atten. Percept. Psychophys. 2020, 82, 294–311. [Google Scholar] [CrossRef] [PubMed]
- Woodman, G.F.; Vecera, S.P.; Luck, S.J. Perceptual organization influences visual working memory. Psychon. Bull. Rev. 2003, 10, 80–87. [Google Scholar] [CrossRef]
- LaRocque, J.J.; Eichenbaum, A.S.; Starrett, M.J.; Rose, N.S.; Emrich, S.M.; Postle, B.R. The short- and long-term fates of memory items retained outside the focus of attention. Mem. Cognit. 2015, 43, 453–468. [Google Scholar] [CrossRef]
- Shaffer, W.; Shiffrin, R.M. Rehearsal and storage of visual information. J. Exp. Psychol. 1972, 92, 292–296. [Google Scholar] [CrossRef]
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.; Ly, A.; Gronau, Q.F.; Smíra, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Wood, J.N. When do spatial and visual working memory interact? Atten. Percept. Psychophys. 2011, 73, 420–439. [Google Scholar] [CrossRef]
- Oberauer, K. Binding and inhibition in working memory: Individual and age differences in short-term recognition. J. Exp. Psychol. Gen. 2005, 134, 368–387. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.W.H.; Ravizza, S.M.; Liu, T. Attention induces surround suppression in visual working memory. Psychon. Bull. Rev. 2019, 26, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yi, D.J. Out of Mind, Out of Sight: Perceptual Consequences of Memory Suppression. Psychol. Sci. 2013, 24, 569–574. [Google Scholar] [CrossRef]
- Myers, N.E.; Chekroud, S.R.; Stokes, M.G.; Nobre, A.C. Benefits of flexible prioritization in working memory can arise without costs. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 398–411. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Liu, Q. Spatial Configuration Effects on the Dissociation between Active and Latent States in Visual Working Memory. Behav. Sci. 2023, 13, 636. https://doi.org/10.3390/bs13080636
Li Z, Liu Q. Spatial Configuration Effects on the Dissociation between Active and Latent States in Visual Working Memory. Behavioral Sciences. 2023; 13(8):636. https://doi.org/10.3390/bs13080636
Chicago/Turabian StyleLi, Ziyuan, and Qiang Liu. 2023. "Spatial Configuration Effects on the Dissociation between Active and Latent States in Visual Working Memory" Behavioral Sciences 13, no. 8: 636. https://doi.org/10.3390/bs13080636
APA StyleLi, Z., & Liu, Q. (2023). Spatial Configuration Effects on the Dissociation between Active and Latent States in Visual Working Memory. Behavioral Sciences, 13(8), 636. https://doi.org/10.3390/bs13080636




