Reward Behavior Disengagement, a Neuroeconomic Model-Based Objective Measure of Reward Pathology in Depression: Findings from the EMBARC Trial
Abstract
1. Introduction
1.1. Conceptualization of Reward Behavior Disengagement (RBD)
1.2. Measuring RBD
- Does RBD in block 2 differ between HC and MDD participants?
- Among MDD participants, is there a level at which RBD is high enough to be considered objectively disengaged when compared to HC participants?
- Do reward task engaged and reward task disengaged MDD participants differ in sociodemographic or clinical features?
- Do reward task engaged and reward task disengaged MDD participants respond differently to sertraline versus placebo?
2. Materials and Methods
2.1. Design and Participants
2.2. Procedures
2.2.1. Probabilistic Reward Task (PRT)
2.2.2. RBD Subgrouping of MDD Participants
2.3. Statistical Analyses
3. Results
3.1. Does RBD in Block 2 Differ between HC and MDD Participants?
3.2. Among MDD Participants, Is There a Level at Which RBD Is High Enough to Be Considered Objectively Impaired (or “Disengaged”) When Compared to HC Participants?
3.3. Do Reward Task Engaged and Reward Task Disengaged MDD Participants Differ in Sociodemographic or Clinical Features?
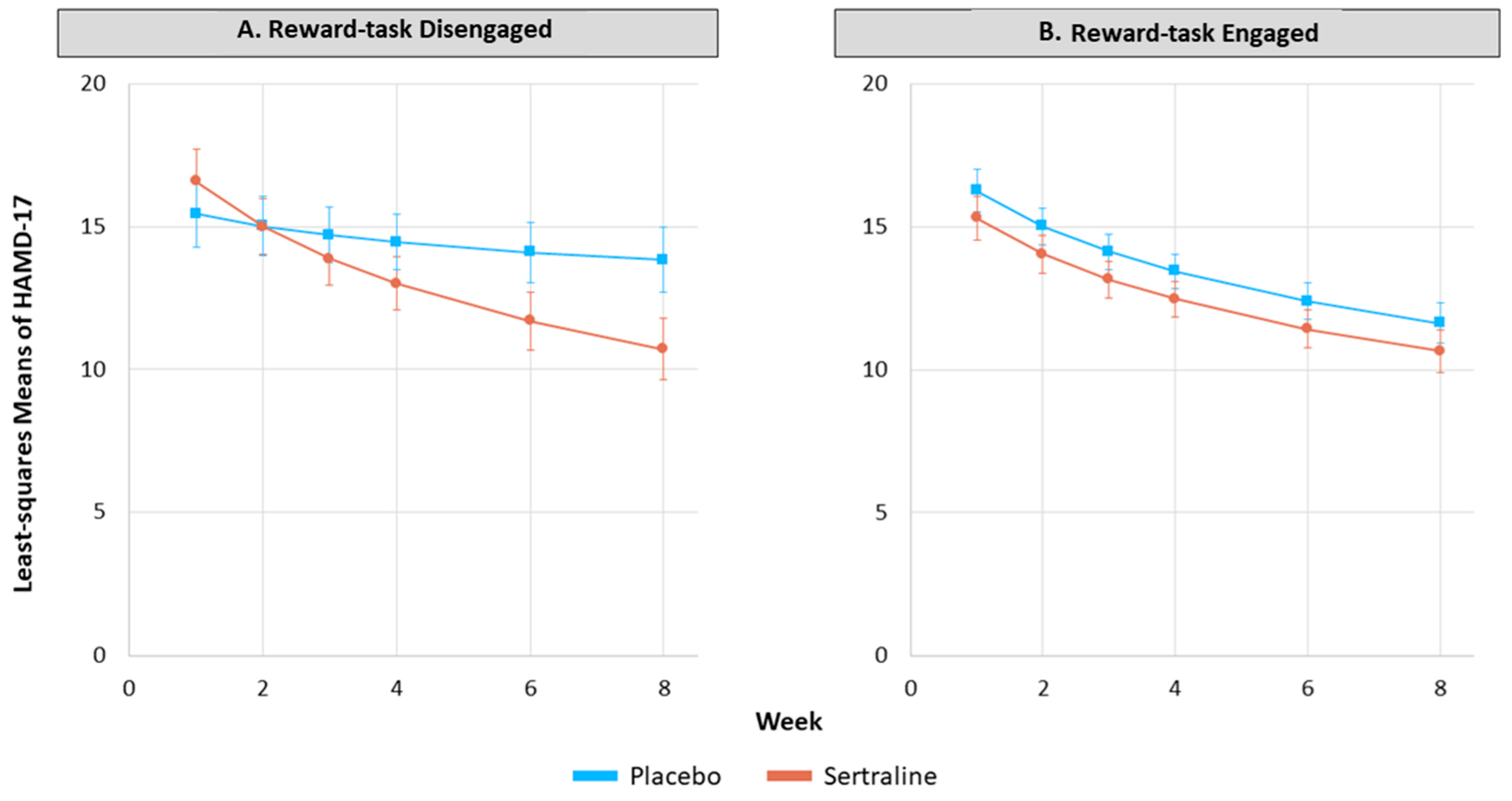
3.4. Do Reward Task Engaged and Reward Task Disengaged MDD Participants Respond Differently to Sertraline versus Placebo?
4. Discussion
Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.H.; Chan, R.C.; Wang, L.Z.; Huang, J.; Cheung, E.F.; Gong, Q.Y.; Gollan, J.K. Deficits in sustaining reward responses in subsyndromal and syndromal major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1045–1052. [Google Scholar] [CrossRef]
- Vrieze, E.; Pizzagalli, D.A.; Demyttenaere, K.; Hompes, T.; Sienaert, P.; de Boer, P.; Schmidt, M.; Claes, S. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry 2013, 73, 639–645. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Iosifescu, D.; Hallett, L.A.; Ratner, K.G.; Fava, M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J. Psychiatr. Res. 2008, 43, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Luking, K.R.; Neiman, J.S.; Luby, J.L.; Barch, D.M. Reduced Hedonic Capacity/Approach Motivation Relates to Blunted Responsivity to Gain and Loss Feedback in Children. J. Clin. Child Adolesc. Psychol. 2017, 46, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.H.; Bylsma, L.M.; Yaroslavsky, I.; Kovacs, M.; Rottenberg, J. Reward learning in pediatric depression and anxiety: Preliminary findings in a high-risk sample. Depress. Anxiety 2015, 32, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Lieblich, S.M.; Castle, D.J.; Pantelis, C.; Hopwood, M.; Young, A.H.; Everall, I.P. High heterogeneity and low reliability in the diagnosis of major depression will impair the development of new drugs. BJPsych Open 2015, 1, e5–e7. [Google Scholar] [CrossRef]
- Szekely, A.; Silton, R.L.; Heller, W.; Miller, G.A.; Mohanty, A. Differential functional connectivity of rostral anterior cingulate cortex during emotional interference. Soc. Cogn. Affect. Neurosci. 2017, 12, 476–486. [Google Scholar] [CrossRef]
- Treadway, M.T.; Zald, D.H. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011, 35, 537–555. [Google Scholar] [CrossRef]
- Bossaerts, P.; Murawski, C. From behavioural economics to neuroeconomics to decision neuroscience: The ascent of biology in research on human decision making. Curr. Opin. Behav. Sci. 2015, 5, 37–42. [Google Scholar] [CrossRef]
- Hamilton, M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Trivedi, M.H.; McGrath, P.J.; Fava, M.; Parsey, R.V.; Kurian, B.T.; Phillips, M.L.; Oquendo, M.A.; Bruder, G.; Pizzagalli, D.; Toups, M.; et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J. Psychiatr. Res. 2016, 78, 11–23. [Google Scholar] [CrossRef]
- First, M.B.; Spitzer, R.L.; Miriam, G.; Williams, J.B.W. Structured Clinical Interview for DSM-IV; New York State Psychiatric Institute, Biometrics Research: New York, NY, USA, 2002. [Google Scholar]
- Rush, A.J.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P.; Hamilton, M.; Morley, S.; Humayan, A.; Hargreaves, D.; Trigwell, P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry 1995, 167, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Tripp, G.; Alsop, B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J. Clin. Child Psychol. 1999, 28, 366–375. [Google Scholar] [CrossRef]
- Liu, W.H.; Roiser, J.P.; Wang, L.Z.; Zhu, Y.H.; Huang, J.; Neumann, D.L.; Shum, D.H.K.; Cheung, E.F.C.; Chan, R.C.K. Anhedonia is associated with blunted reward sensitivity in first-degree relatives of patients with major depression. J. Affect. Disord. 2016, 190, 640–648. [Google Scholar] [CrossRef]
- Luking, K.R.; Pagliaccio, D.; Luby, J.L.; Barch, D.M. Child Gain Approach and Loss Avoidance Behavior: Relationships With Depression Risk, Negative Mood, and Anhedonia. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Ryback, R.S.; Eckardt, M.J.; Rawlings, R.R.; Rosenthal, L.S. Quadratic Discriminant-Analysis as an Aid to Interpretive Reporting of Clinical Laboratory Tests. JAMA J. Am. Med. Assoc. 1982, 248, 2342–2345. [Google Scholar] [CrossRef]
- Jha, M.K.; Teer, R.B.; Minhajuddin, A.; Greer, T.L.; Rush, A.J.; Trivedi, M.H. Daily activity level improvement with antidepressant medications predicts long-term clinical outcomes in outpatients with major depressive disorder. Neuropsychiatr. Dis. Treat. 2017, 13, 803–813. [Google Scholar] [CrossRef]
- Lawlor, V.M.; Webb, C.A.; Wiecki, T.V.; Frank, M.J.; Trivedi, M.; Pizzagalli, D.A.; Dillon, D.G. Dissecting the impact of depression on decision-making. Psychol. Med. 2019, 50, 1613–1622. [Google Scholar] [CrossRef]
- Rutherford, B.R.; Roose, S.P. A model of placebo response in antidepressant clinical trials. Am. J. Psychiatry 2013, 170, 723–733. [Google Scholar] [CrossRef]
- Lynn, S.K.; Wormwood, J.B.; Barrett, L.F.; Quigley, K.S. Decision making from economic and signal detection perspectives: Development of an integrated framework. Front. Psychol. 2015, 6, 952. [Google Scholar] [CrossRef] [PubMed]
- Machina, M.J. Choice under Uncertainty—Problems Solved and Unsolved—Responses. J. Econ. Perspect. 1988, 2, 181–183. [Google Scholar]
- Hansson, H.; Lagerkvist, C.J. Decision making for animal health and welfare: Integrating risk-benefit analysis with prospect theory. Risk Anal. 2014, 34, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A. A quantitative and qualitative test of the Allais paradox using health outcomes. J. Econ. Psychol. 2003, 24, 35–48. [Google Scholar] [CrossRef]
- Tversky, A.; Kahneman, D. Advances in Prospect-Theory—Cumulative Representation of Uncertainty. J. Risk Uncertain. 1992, 5, 297–323. [Google Scholar] [CrossRef]
| Features | Healthy Controls | MDD Sample |
|---|---|---|
| No. of Participants | 40 | 196 * |
| Age, mean years (SD) | 37.6 (14.9) | 37.2 (13.1) |
| Female (%) | 25 (62.5) | 129 (66.2) |
| Race/Ethnicity | ||
| Caucasian (%) | 26 (65) | 124 (63.6) |
| African American (%) | 9 (22.5) | 45 (23.1) |
| Asian (%) | 3 (7.5) | 14 (7.18) |
| Native American/Alaskan (%) | 0 (0) | 1 (0.51) |
| Hawaiian/Pacific Islander (%) | 0 (0) | 0 (0) |
| Other (%) | 2 (5.0) | 11 (5.64) |
| Year of Education, Mean (SD) | 15.2 (2.3) | 14.9 (2.4) |
| Number of MDD Episodes (SD) | 0 (0) | 11.1 ** (20.4) |
| Age of Onset (SD) | ~ | 16.1 (6.01) |
| Category | Reward Task Disengaged n (%) | Reward Task Engaged n (%) |
|---|---|---|
| Sex ( = 0.62, p = 0.43) | ||
| Male | 22 (37.9) | 44 (32.1) |
| Female | 36 (62.1) | 93 (67.9) |
| Race ( = 3.02, p = 0.22) | ||
| Caucasian | 39 (67.2) | 85 (62.0) |
| African American | 15 (25.9) | 30 (21.9) |
| Other | 4 (6.9) | 22 (16.1) |
| Employment Status ( = 3.65, p = 0.16) | ||
| Full-time | 11 (19.0) | 42 (31.3) |
| Part-time | 14 (24.1) | 33 (24.6) |
| Unemployed | 33 (56.9) | 59 (44.0) |
| Length of Current MDE ( = 0.60, p = 0.74) | ||
| 0–6 months | 19 (32.8) | 49 (35.8) |
| 7–24 months | 14 (24.1) | 37 (27.0) |
| >24 months | 25 (43.1) | 51 (37.2) |
| Number of Lifetime MDEs ( = 0.24, p = 0.89) | ||
| <3 | 15 (27.8) | 28 (25.2) |
| 3–5 | 11 (20.4) | 21 (18.9) |
| >5 | 28 (51.9) | 62 (55.9) |
| Monthly Income in USD ( = 4.77, p = 0.09) | ||
| <2000 | 29 (63) | 51 (44.7) |
| 2000–4000 | 11 (23.9) | 35 (30.7) |
| >4000 | 6 (13.0) | 28 (24.6) |
| Marriage Status ( = 0.61, p = 0.43) | ||
| Married or partnered | 10 (17.2) | 30 (22.2) |
| Single, divorced, separated, or widowed | 48 (82.8) | 105 (77.8) |
| Education status ( = 1.80, p = 0.62) | ||
| Did not graduate high school | 2 (3.4) | 3 (2.1) |
| High school graduate or equivalent | 13 (22.4) | 37 (25.9) |
| Some college | 17 (29.3) | 52 (36.4) |
| College or advanced degree | 26 (44.8) | 51 (35.7) |
| Medical Comorbidities ( = 1.88, p = 0.60) | ||
| None | 20 (37.7) | 61 (47.7) |
| 1 | 8 (15.1) | 13 (10.2) |
| 2 | 9 (17.0) | 18 (14.1) |
| 3 or more | 16 (30.2) | 36 (28.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giles, M.A.; Cooper, C.M.; Jha, M.K.; Chin Fatt, C.R.; Pizzagalli, D.A.; Mayes, T.L.; Webb, C.A.; Greer, T.L.; Etkin, A.; Trombello, J.M.; et al. Reward Behavior Disengagement, a Neuroeconomic Model-Based Objective Measure of Reward Pathology in Depression: Findings from the EMBARC Trial. Behav. Sci. 2023, 13, 619. https://doi.org/10.3390/bs13080619
Giles MA, Cooper CM, Jha MK, Chin Fatt CR, Pizzagalli DA, Mayes TL, Webb CA, Greer TL, Etkin A, Trombello JM, et al. Reward Behavior Disengagement, a Neuroeconomic Model-Based Objective Measure of Reward Pathology in Depression: Findings from the EMBARC Trial. Behavioral Sciences. 2023; 13(8):619. https://doi.org/10.3390/bs13080619
Chicago/Turabian StyleGiles, Michael A., Crystal M. Cooper, Manish K. Jha, Cherise R. Chin Fatt, Diego A. Pizzagalli, Taryn L. Mayes, Christian A. Webb, Tracy L. Greer, Amit Etkin, Joseph M. Trombello, and et al. 2023. "Reward Behavior Disengagement, a Neuroeconomic Model-Based Objective Measure of Reward Pathology in Depression: Findings from the EMBARC Trial" Behavioral Sciences 13, no. 8: 619. https://doi.org/10.3390/bs13080619
APA StyleGiles, M. A., Cooper, C. M., Jha, M. K., Chin Fatt, C. R., Pizzagalli, D. A., Mayes, T. L., Webb, C. A., Greer, T. L., Etkin, A., Trombello, J. M., Chase, H. W., Phillips, M. L., McInnis, M. G., Carmody, T., Adams, P., Parsey, R. V., McGrath, P. J., Weissman, M., Kurian, B. T., ... Trivedi, M. H. (2023). Reward Behavior Disengagement, a Neuroeconomic Model-Based Objective Measure of Reward Pathology in Depression: Findings from the EMBARC Trial. Behavioral Sciences, 13(8), 619. https://doi.org/10.3390/bs13080619






