Music Listening as Coping Behavior: From Reactive Response to Sense-Making
Abstract
1. Introduction
2. Music as Sound Environment: Natural and Man-Made Sounds
3. Music Listening as Coping Behavior
4. Coping and Sense-Making
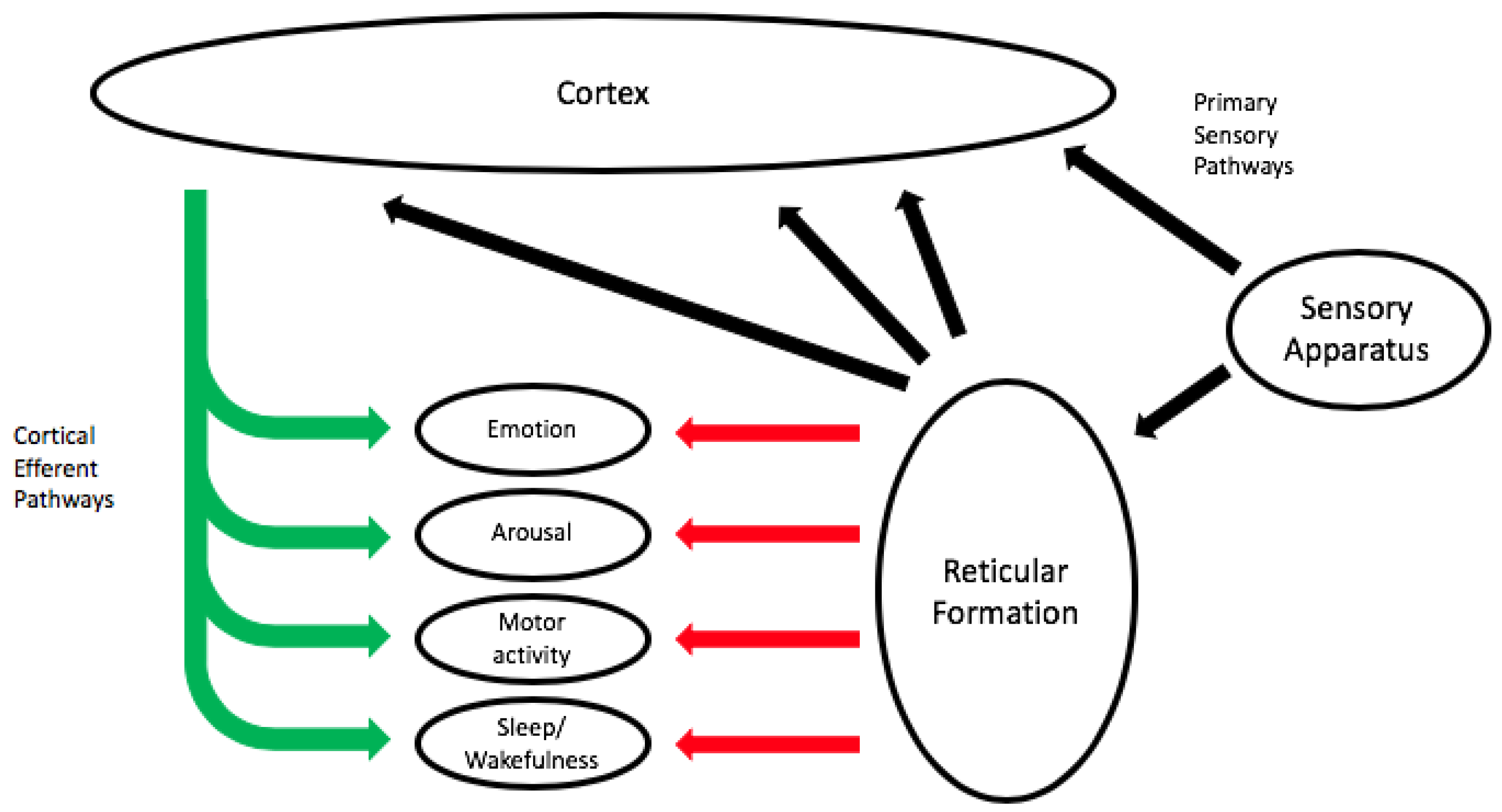
5. Neural Correlates of Coping with Sounds: Reactive Behavior and Phylogenetic Constraints
6. Musical Aesthetics and Coping Behavior: The Role of Adaptive Listening
6.1. Exploration of the Sound Environment
6.2. From Coping to Reward: Music and Arousal Regulation
6.3. Addiction and Maladaptive Listening
7. Conclusions: From Reactive Behavior to Sense-Making
Author Contributions
Funding
Conflicts of Interest
References
- Lazarus, R.S.; Folkman, S. Stress, Appraisal, and Coping; Springer: New York, NY, USA, 1984. [Google Scholar]
- Reybrouck, M. Music as environment: An ecological and biosemiotic approach. Behav. Sci. 2014, 5, 1–26. [Google Scholar] [CrossRef]
- Reybrouck, M. Musical Sense-Making: Enaction, Experience and Computation; Routledge: New York, NY, USA, 2020. [Google Scholar]
- von Georgi, R.; Göbel, M.; Gebhardt, S. Emotion modulation by means of music and coping behaviour. In Music that Works: Contributions of Biology, Neurophysiology, Psychology, Sociology, Medicine and Musicology; Haas, R., Brandes, V., Eds.; Springer: Vienna, Austria, 2009; pp. 301–309. [Google Scholar]
- Labbé, E.; Schmidt, N.; Babin, J.; Pharr, M. Coping with stress: The effectiveness of different types of music. Appl. Psychophysiol. Biofeedback 2007, 32, 163–168. [Google Scholar] [CrossRef]
- Miranda, D.; Claes, M. Music listening, coping, peer affiliation and depression in adolescence. Psychol. Music. 2009, 37, 215–233. [Google Scholar] [CrossRef]
- Stewart, J.; Garrido, S.; Hense, C.; McFerran, K. Music use for mood regulation: Self-awareness and conscious listening choices in young people with tendencies to depression. Front. Psychol. 2019, 10, 1199. [Google Scholar]
- Bernard, C. La Science Expérimentale; J.-B. Baillière & Fils: Paris, France, 1878. [Google Scholar]
- Gross, C.G. Claude bernard and the constancy of the internal environment. Neuroscientist 1998, 4, 380–385. [Google Scholar] [CrossRef]
- Maschke, C.; Rupp, T.; Hecht, T. The influence of stressors on biochemical reactions—A review of present scientific findings with noise. Int. J. Hyg. Environ. Health 2000, 203, 45–53. [Google Scholar] [CrossRef]
- Orians, G.H.; Heerwagen, J.H. Evolved responses to landscape. In The Adapted Mind; Barkow, J.H., Cosmides, L., Tooby, J., Eds.; Oxford University Press: Oxford, UK, 1992; pp. 555–579. [Google Scholar]
- Horowitz, S.S. The Universal Sense: How Hearing Shapes the Mind; Bloomsbury: New York, NY, USA, 2012. [Google Scholar]
- Reybrouck, M. Music as environment: Biological and ecological constraints on coping with the sounds. RSSI 2019, 38–39, 19–35. [Google Scholar]
- Fischer, W.; Schäfer, J. Direction-dependent amplification of the human outer ear. Br. J. Audiol. 1991, 25, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ashmore, J.; Avan, P.; Brownell, W.; Dallos, P.; Dierkes, K.; Fettiplace, R.; Grosh, K.; Hackney, C.; Hudspeth, A.; Julicher, F.; et al. The remarkable cochlear amplifier. Hear. Res. 2010, 266, 1–17. [Google Scholar] [CrossRef]
- Rabbitt, R.D.; Brownell, W. Efferent modulation of hair cell function. Curr. Opin. Otolaryngol. Head Neck Surg. 2011, 19, 376–381. [Google Scholar] [CrossRef]
- Basner, M.; Babisch, W.; Davis, A.; Brink, M.; Clark, C.; Janssen, S.; Stansfeld, S. Auditory and non-auditory effects of noise on health. Lancet 2014, 383, 1325–1332. [Google Scholar] [CrossRef]
- Lemaitre, G.; Rocchesso, D. On the effectiveness of vocal imitations and verbal descriptions of sounds. J. Acoust. Soc. Am. 2014, 135, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, N.H.; Rossing, T.D. The Physics of Musical Instruments; Springer Science and Business Media LLC: Berlin, Germany, 1998. [Google Scholar]
- Hansen, U.J. Materials in musical instruments. J. Acoust. Soc. Am. 2011, 129, 2518. [Google Scholar] [CrossRef]
- Zappas, K.R. The science of sound: Examining the role of materials in musical instruments. JOM 2007, 59, 13–17. Available online: https://www.tms.org/pubs/journals/jom/0708/roncone-0708.html (accessed on 19 July 2020).
- Bryant, G. Animal signals and emotion in music: Coordinating affect across groups. Front. Psychol. 2013, 4, 990. [Google Scholar] [CrossRef] [PubMed]
- Belin, P.; Fecteau, S.; Charest, I.; Nicastro, N.; Hauser, M.D.; Armony, J.L. Human cerebral response to animal affective vocalizations. Proc. R. Soc. B: Biol. Sci. 2007, 275, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Reybrouck, M.; Podlipniak, P. Preconceptual spectral and temporal cues as a source of meaning in speech and music. Brain Sci. 2019, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Fitch, W.T.; Neubauer, J.; Herzel, H. Calls out of chaos: The adaptive significance of nonlinear phenomena in mammalian vocal communication. Anim. Behav. 2002, 63, 407–418. [Google Scholar] [CrossRef]
- Lazarus, R.S. Psychological Stress and the Coping Process; McGraw-Hill: New York, NY, USA, 1966. [Google Scholar]
- Lambert, W.W.; Lazarus, R.S. Psychological stress and the coping process. Am. J. Psychol. 1970, 83, 634. [Google Scholar] [CrossRef]
- Folkman, S. Stress: Appraisal and Coping; Springer Science and Business Media LLC: Berlin, Germany, 2013. [Google Scholar]
- Boxer, P.; Sloan-Power, E.; Schappell, I. Coping with stress, coping with violence: Links to mental health outcomes among at-risk youth. J. Psychopathol. Behav. Assess. 2012, 34, 405–414. [Google Scholar] [CrossRef]
- Liu, W.; Li, Z.; Ling, Y.; Cai, T. Core self-evaluations and coping styles as mediators between social support and well-being. Pers. Individ. Differ. 2016, 88, 35–39. [Google Scholar] [CrossRef]
- Schwartz, R.M. The internal dialogue: On the asymmetry between positive and negative coping thoughts. Cogn. Res. 1986, 10, 591–605. [Google Scholar] [CrossRef]
- Zeidner, M.; Saklofske, D. Adaptive maladaptive coping. In Handbook of Coping: Theory, Research, Applications; Zeidner, M., Endler, N., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1996; pp. 505–531. [Google Scholar]
- Barrett, E. Investigation of the principle of helicy: The relationship of human field motion power. In Exploration on Martha Rogers Science of Unitary Human Beings; Malinski, V.M., Ed.; Appleton-Century-Crofts: Norwalk, CT, USA, 1986; pp. 173–188. [Google Scholar]
- Barrett, E. A measure of power as knowing participation in change. In Measurement of Nursing Outcomes; Self care and coping; Strickland, O., Dilorio, C., Eds.; Springer: New York, NY, USA, 2003; Volume 3, pp. 21–39. [Google Scholar]
- Barrett, E.A.M. Power as knowing participation in change: What’s new and what’s next. Nurs. Sci. Q. 2009, 23, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.; Caroselli, C. Methodological ponderings related to the power as knowing participation in change tool. Nurs. Sci. Q. 1998, 11, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Landalv, D.; Malmstrom, L.; Widen, S.E. Adolescents reported hearing symptoms and attitudes toward loud music. Noise Health 2013, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Mercier, V.; Hohmann, B.W. Is electronically amplified music too loud? What do young people think? Noise Health 2002, 4, 47–55. Available online: http://www.noiseandhealth.org/text.asp?2002/4/16/47/31828 (accessed on 19 July 2020).
- Reybrouck, M.; Podlipniak, P.; Welch, D. Music and noise: Same or different? What our body tells us. Front. Psychol. 2019, 10, 1153. [Google Scholar] [CrossRef]
- Welch, D.; Fremaux, G. Understanding why people enjoy loud sound. Semin Hear. 2017, 38, 348–358. [Google Scholar] [CrossRef]
- Welch, D.; Fremaux, G. Why do people like loud sound? A qualitative study. Int. J. Environ. Res. Pub. Health 2017, 14, 908. [Google Scholar] [CrossRef]
- Caroselli, C.; Barrett, E.A.M. A review of the power as knowing participation in change literature. Nurs. Sci. Q. 1998, 11, 9–16. [Google Scholar] [CrossRef]
- Reybrouck, M. Experience as cognition: Musical sense-making and the ‘in-time/outside-of-time’ dichotomy. ISM 2019, 19, 53–80. [Google Scholar] [CrossRef]
- Gamez, D. Conscious. Sensation, Conscious. Perception and Sensorimotor Theories of Consciousness; Springer Science and Business Media LLC: Berlin, Germany, 2014; Volume 15, pp. 159–174. [Google Scholar]
- Reybrouck, M. Perceptual immediacy in music listening: Multimodality and the “in time/outside of time” dichotomy. Versus 2017, 124, 89–104. [Google Scholar]
- Reybrouck, M. Music shaped in time: Musical sense-making between perceptual immediacy and symbolic representation. Rech. Sémiotiques 2018, 36, 99–120. [Google Scholar] [CrossRef][Green Version]
- Reybrouck, M. From sound to music: Real-time musical experience and the ‘in time/outside of time’ dichotomy. In Musical Analysis. Historia, Theoria, Praxis; Granat, J., Ed.; Karol Lipiński Academy of Music: Wroclaw, Poland, 2019; pp. 31–44. [Google Scholar]
- von Neumann, J. The general and logical theory of automata. In John von Neumann, Collected Works. Volume V. Design of Computers, Theory of Automata and Numerical Analysis; Taub, A., Ed.; Pergamon: Oxford, UK, 1961; pp. 288–328. [Google Scholar]
- Pattee, H.H. Artificial life needs a real epistemology. In Advances in Artificial Life; Moran, F., Moreno, A., Morelo, J., Chacon, P., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 1995; Volume 929, pp. 21–38. [Google Scholar]
- Gaver, W.W. How do we hear in the world? Explorations of ecological acoustics. Ecol. Psychol. 1993, 5, 285–313. [Google Scholar] [CrossRef]
- Gaver, W.W. What in the world do we hear? An ecological approach to auditory event perception. Ecol. Psychol. 1993, 5, 1–29. [Google Scholar] [CrossRef]
- Reybrouck, M. A biosemiotic and ecological approach to music cognition: Event perception between auditory listening and cognitive economy. Axiomathes 2005, 15, 229–266. [Google Scholar] [CrossRef]
- Reybrouck, M. Musical sense-making and the concept of affordance: An ecosemiotic and experiential approach. Biosemiotics 2012, 5, 391–409. [Google Scholar] [CrossRef]
- Balzano, G. Music perception as detection of pitch-time constraints. In Event Cognition: An Ecological Perspective; McCabe, V., Balzano Hillsdale, G., Eds.; Lawrence Erlbaum: London, UK, 1986; pp. 217–233. [Google Scholar]
- Lombardo, T.J. The reciprocity of perceiver and environment. Reciprocity Perceiver Environ. 2017. [Google Scholar] [CrossRef]
- Michaels, C.; Carello, C. Direct Perception; Prentice-Hall: Englewood Cliffs, NJ, USA, 1981. [Google Scholar]
- Imhof, J. Feeling the Music. A Neurological, Biological, and Linguistic Basis for An Embodied Explanation of What Happens When We Experience Music; ISME: Upper Montclair, NJ, USA, 2002. [Google Scholar]
- Damasio, A.R. The Feeling of What Happens: Body and Emotion in the Making of Consciousness, 1st ed.; Harcourt Brace: New York, NY, USA, 1999. [Google Scholar]
- Cassirer, E. Symbol, Myth, and Culture: Essays and Lectures of Ernst Cassirer, 1935–1945; Verene, D.F., Ed.; Yale University Press: New Haven, CN, USA, 1979. [Google Scholar]
- Tagg, P. Music’s Meanings. A Modern Musicology for Non-Musos; The Mass Media Music Scholars’s Press: New York, NY, USA, 2013. [Google Scholar]
- Chien, J.-P. Of animals and men: A study of umwelt in uexküll, cassirer, and heidegger. Conc. Lit. Cult. Stud. 2006, 32, 57–79. [Google Scholar]
- Dor, D.; Jablonka, E. How language changed the genes: Toward an explicit account of the evolution of language. In New Essays on the Origin of Language; Trabant, J., Ed.; Mouton: Berlin, Germany, 2011; pp. 149–175. [Google Scholar]
- Bernstein, A.S. The orienting response and direction of stimulus change. Psychon. Sci. 1968, 12, 127–128. [Google Scholar] [CrossRef]
- Le Poire, B.A. Orientation and defensive reactions as alternatives to arousal in theories of nonverbal reactions to changes in immediacy. South. Commun. J. 1991, 56, 138–146. [Google Scholar] [CrossRef]
- Le Poire, B.A.; Burgoon, J.K. Usefulness of differentiating arousal responses within communication theories: Orienting response or defensive arousal within nonverbal theories of expectancy violation. Commun. Monogr. 1996, 63, 208–230. [Google Scholar] [CrossRef]
- Bernstein, A.S. The orienting response as novelty and significance detector: Reply to O’Gorman. Psychophysiology 1979, 16, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Sokolov, E. Perception and the Conditioned Reflex; MacMillan: New York, NY, USA, 1963. [Google Scholar]
- Błaszczyk, J.W. Startle response to short acoustic stimuli in rats. Acta Neurobiol. Exp. 2003, 63, 25–30. [Google Scholar]
- Davis, M. The mammalian startle response. In Neural Mechanisms of Startle Behavior; Springer Science and Business Media LLC: Berlin, Germany, 1984; pp. 287–351. [Google Scholar]
- Koch, M. The neurobiology of startle. Prog. Neurobiol. 1999, 59, 107–128. [Google Scholar] [CrossRef]
- Koch, M.; Schnitzler, H.-U. The acoustic startle response in rats—Circuits mediating evocation, inhibition and potentiation. Behav. Brain Res. 1997, 89, 35–49. [Google Scholar] [CrossRef]
- Salloum, R.; Yurosko, C.; Santiago, L.; Sandridge, S.; Kaltenbach, J. Induction of enhanced acoustic startle response by noise exposure: Dependence on exposure conditions and testing parameters and possible relevance to hyperacusis. PLoS ONE 2014, 9, e111747. [Google Scholar] [CrossRef]
- Colebatch, J.G. Assessing saccular (otolith) function in man. J. Acoust. Soc. Am. 2006, 119, 3432. [Google Scholar] [CrossRef]
- Colebatch, J.; Halmagyi, G.; Skuse, N. Myogenic potentials genereated by a click-evoked vestibulocollic reflex. J. Neurol. Neurosurg. Psychiatry 1994, 57, 190–197. [Google Scholar] [CrossRef]
- Todd, N.P.; Cody, F.W.; Banks, J.R. A saccular origin of frequency tuning in myogenic vestibular evoked potentials? Implications for human responses to loud sounds. Hear. Res. 2000, 141, 180–188. [Google Scholar] [CrossRef]
- Yates, B.J. Vestibular influences on the autonomic nervous system. Ann. N. Y. Acad. Sci. 1996, 781, 458–473. [Google Scholar] [CrossRef]
- Katafuchi, T.; Puthuraya, K.P.; Yoshimatsu, H.; Oomura, Y. Responses of rat lateral hypothalamic neuron activity to vestibular nuclei stimulation. Brain Res. 1987, 400, 62–69. [Google Scholar] [CrossRef]
- Ginsburg, S.; Jablonka, E. The transition to experiencing: I. limited learning and limited experiencing. Boil. Theor. 2007, 2, 218–230. [Google Scholar] [CrossRef]
- Fettiplace, R.; Kim, K.X. The physiology of mechanoelectrical transduction channels in hearing. Physiol. Rev. 2014, 94, 951–986. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, C.J.; Stanley, G.B. Rapid sensory adaptation REDUX: A circuit perspective. Neuron 2016, 92, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Beach, E.F.; Williams, W.; Gilliver, M. Estimating young australian adults’ risk of hearing damage from selected leisure activities. Ear Hear. 2013, 34, 75–82. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087. [Google Scholar] [CrossRef]
- Siegel, A.; Sapru, N. Essential Neuroscience; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Orians, G. An ecological and evolutionary approach to landscape aesthetics. In Landscape Meaning and Values; Penning-Rowsell, E.C., Lowenthal, D., Eds.; Allen & Unwin: London, UK, 1986; pp. 3–25. [Google Scholar]
- Alberti, P. The anatomy and physiology of the ear and hearing. In Occupational Exposure to Noise: Evaluation, Prevention, and Control; Goelzer, B., Hansen, C., Sehrdt, G., Eds.; World Health Organization: Geneva, Switzerland, 2001; pp. 53–62. [Google Scholar]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Williams, W.; Beach, E.F.; Gilliver, M. Clubbing: The cumulative effect of noise exposure from attendance at dance clubs and night clubs on whole-of-life noise exposure. Noise Health 2010, 12, 155–158. [Google Scholar] [CrossRef]
- Gould van Praag, C.D.; Garfinkel, S.N.; Sparasci, O.; Mees, A.; Philippides, A.O.; Ware, M.; Ottaviani, C.; Critchley, H.D. Mind-wandering and alterations to default mode network connectivity when listening to naturalistic versus artificial sounds. Sci. Rep. 2017, 7, 45273. [Google Scholar] [CrossRef]
- Schafer, R.M. The Soundscape: Our Sonic Environment and the Tuning of the World; Destiny Books: Rochester, NY, USA, 1977. [Google Scholar]
- Davies, W.J.; Murphy, J.E. Reproducibility of soundscape dimensions. Paper presented at the Internoise Conference, New York, NY, USA, 19–22 August 2012. [Google Scholar]
- Botteldooren, D.; De Coensel, B.; De Muer, T. The temporal structure of urban soundscapes. J. Sound Vib. 2006, 292, 105–123. [Google Scholar] [CrossRef]
- Andringa, T.C.; Lanser, J.J.L. How pleasant sounds promote and annoying sounds impede health: A cognitive approach. Int. J. Environ. Res. Public Heal. 2013, 10, 1439–1461. [Google Scholar] [CrossRef]
- Van den Bosch, K.; Welch, D.; Andringa, T. The evolution of soundscape appraisal through enactive cognition. Front. Psychol. 2018, 9, 1129. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A. A circumplex model of affect. J. Pers. Soc. Psychol. 1980, 39, 1161–1178. [Google Scholar] [CrossRef]
- Welch, D.; Shepherd, D.; Dirks, K.; Tan, M.Y.; Coad, G. Use of creative writing to develop a semantic differential tool for assessing soundscapes. Front. Psychol. 2018, 9, 2698. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.; Lindgren, F. Pop music and hearing. Ear Hear. 1981, 2, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, M.; Saarikallio, S. Strategies and mechanisms in musical affect self-regulation: A new model. Music. Sci. 2017, 23, 177–195. [Google Scholar] [CrossRef]
- Van den Tol, A.; Edwards, J. Exploring a rationale for choosing to listen when feeling sad. Psychol. Music. 2011, 41, 440–465. [Google Scholar] [CrossRef]
- Van den Tol, A.; Edwards, J. Listening to sad music in adverse situations: How music selection strategies relate to self-regulatory goals, listening effects, and mood enhancement. Psychol. Music 2015, 43, 473–494. [Google Scholar] [CrossRef]
- Van Goethem, A.; Sloboda, J. The functions of music for affect regulation. Music Sci. 2011, 15, 208–228. [Google Scholar] [CrossRef]
- Lonsdale, A.J.; North, A. Why do we listen to music? A uses and gratifications analysis. Br. J. Psychol. 2011, 102, 108–134. [Google Scholar] [CrossRef]
- Saarikallio, S.H. Music in mood regulation: Initial scale development. Music. Sci. 2008, 12, 291–309. [Google Scholar] [CrossRef]
- Berlyne, D.E. Aesthetics and Psychobiology; Appleton Century Crofts: New York, NY, USA, 1971. [Google Scholar]
- Di Giulio, G.; Esposito, E.; Santolini, C.; Scalise, L. Experimental vibrational analysis of drum cymbals. In Proceedings of the XIX IMAC, Kessimmee, FL, USA, 5–9 February 2001; pp. 724–730. [Google Scholar]
- Hanser, W.; ter Bogt, T.; Van den Tol, A.; Mark, R.; Vingerhoets, A. Consolation through music: A survey study. Mus. Sci. 2016, 20, 122–137. [Google Scholar] [CrossRef]
- Gjestland, T.; Tronstad, T.V. The efficacy of sound regulations on the listening levels of pop concerts. J. Occup. Environ. Hyg. 2016, 14, 17–22. [Google Scholar] [CrossRef] [PubMed]
- North, A.; Hargreaves, D.J. Liking, arousal potential, and the emotions expressed by music. Scand. J. Psychol. 1997, 38, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, M.; Westerink, J.; van den Broek, E. Emotional and psychophysiological responses to tempo, mode, and percussiveness. Music Sci. 2011, 15, 250–269. [Google Scholar] [CrossRef]
- Saarikallio, S.; Erkkilä, J. The role of music in adolescents’ mood regulation. Psychol. Music 2007, 35, 88–109. [Google Scholar] [CrossRef]
- Van den Tol, A.; Edwards, J.; Heflick, N. Sad music as a means for acceptance-based coping. Mus. Sci. 2016, 20, 68–83. [Google Scholar] [CrossRef]
- Selye, H. The Stress of Life; McGraw-Hill: New York, NY, USA, 1956. [Google Scholar]
- Selye, H. Stress without Distress; Springer Science and Business Media LLC: Berlin, Germany, 1976. [Google Scholar]
- Selye, H. The stress syndrome. Am. J. Nurs. 1965, 3, 97–99. [Google Scholar]
- Perrez, M. Eustress. In The Oxford Companian to Emotion and the Affective Sciences; Sander, D., Scherer, K., Eds.; Oxford University Press: New York, NY, USA, 2009. [Google Scholar]
- Billings, B.P. Feeling the Music Can Be Dangerous to Your Health. A Comprehensive Review. 2000. Available online: http://www.omnisonic.com/bbillings.html (accessed on 19 July 2020).
- Glasser, W. Positive Addiction; Harper & Row: New York, NY, USA, 1976. [Google Scholar]
- Blood, A.J.; Zatorre, R.J.; Bermudez, P.; Evans, A.C. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat. Neurosci. 1999, 2, 382–387. [Google Scholar] [CrossRef]
- Carlson, E.; Saarikallio, S.; Toiviainen, P.; Bogert, B.; Kliuchko, M.; Brattico, E. Maladaptive and adaptive emotion regulation through music: A behavioral and neuroimaging study of males and females. Front. Hum. Neurosci. 2015, 9, 466. [Google Scholar] [CrossRef]
- Donovan, D. Assessment of addictive behaviors: Implications of an emerging biopsychosocial model. In Assessment of Addictive Behaviors; Donovan, D., Marlatt, G., Eds.; Guilford Press: New York, NY, USA, 1988; pp. 3–48. [Google Scholar]
- Florentine, M.; Hunter, W.; Robinson, M.; Ballou, M.; Buus, S. On the behavioral characteristics of loud-music listening. Ear Hear. 1998, 19, 420–428. [Google Scholar] [CrossRef]
- Gruber, J.; Mauss, I.B.; Tamir, M. A dark side of happiness? How, when, and why happiness is not always good. Perspect. Psychol. Sci. 2011, 6, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. Jaro 2002, 3, 248–268. [Google Scholar] [CrossRef] [PubMed]
- Salimpoor, V.N.; Benovoy, M.; Larcher, K.; Dagher, A.; Zatorre, R.J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011, 14, 257–262. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reybrouck, M.; Podlipniak, P.; Welch, D. Music Listening as Coping Behavior: From Reactive Response to Sense-Making. Behav. Sci. 2020, 10, 119. https://doi.org/10.3390/bs10070119
Reybrouck M, Podlipniak P, Welch D. Music Listening as Coping Behavior: From Reactive Response to Sense-Making. Behavioral Sciences. 2020; 10(7):119. https://doi.org/10.3390/bs10070119
Chicago/Turabian StyleReybrouck, Mark, Piotr Podlipniak, and David Welch. 2020. "Music Listening as Coping Behavior: From Reactive Response to Sense-Making" Behavioral Sciences 10, no. 7: 119. https://doi.org/10.3390/bs10070119
APA StyleReybrouck, M., Podlipniak, P., & Welch, D. (2020). Music Listening as Coping Behavior: From Reactive Response to Sense-Making. Behavioral Sciences, 10(7), 119. https://doi.org/10.3390/bs10070119





