Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women
Abstract
1. Introduction
2. Methods
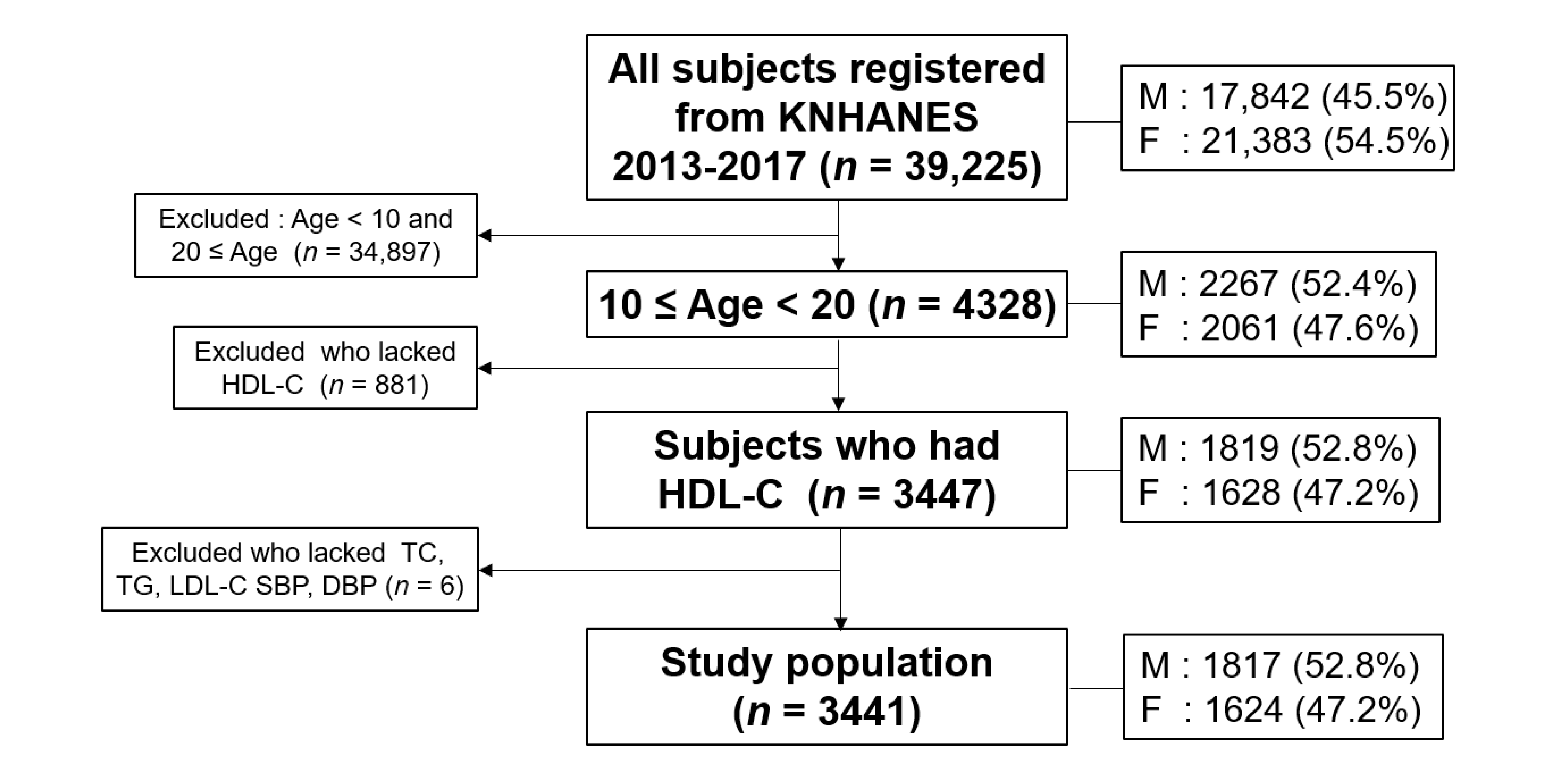
2.1. Study Population
2.2. Statistical Analysis
2.3. Ethics Statement
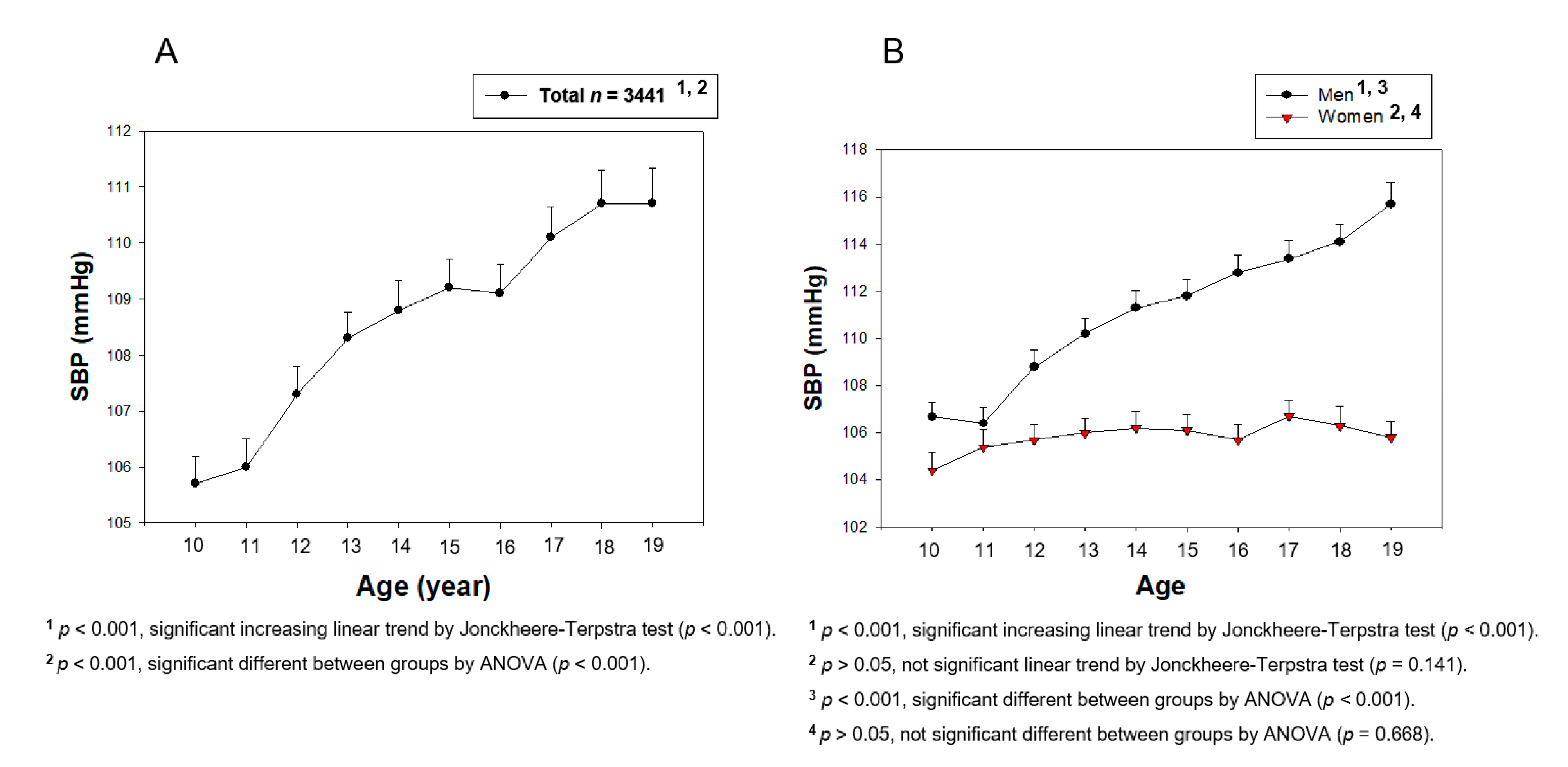
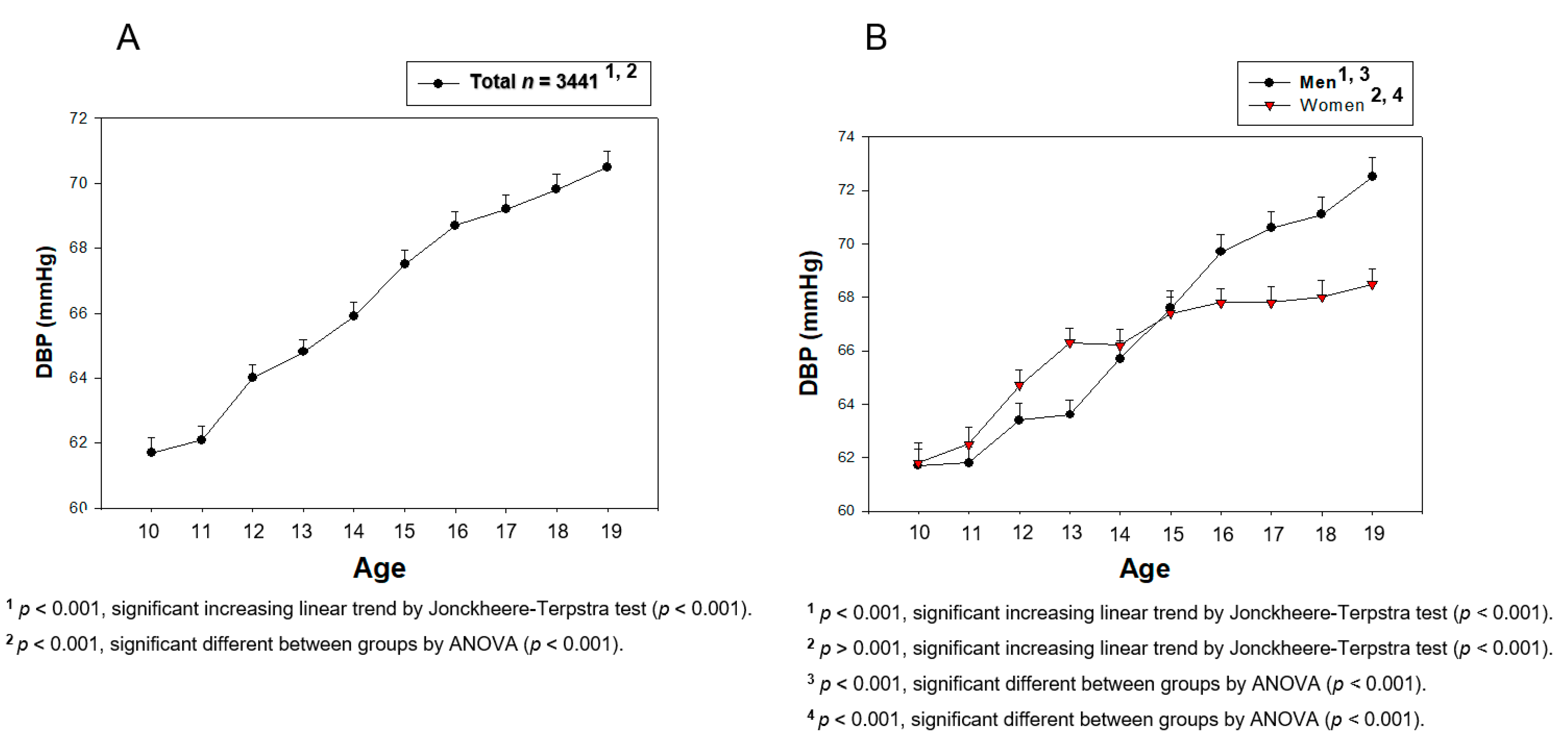
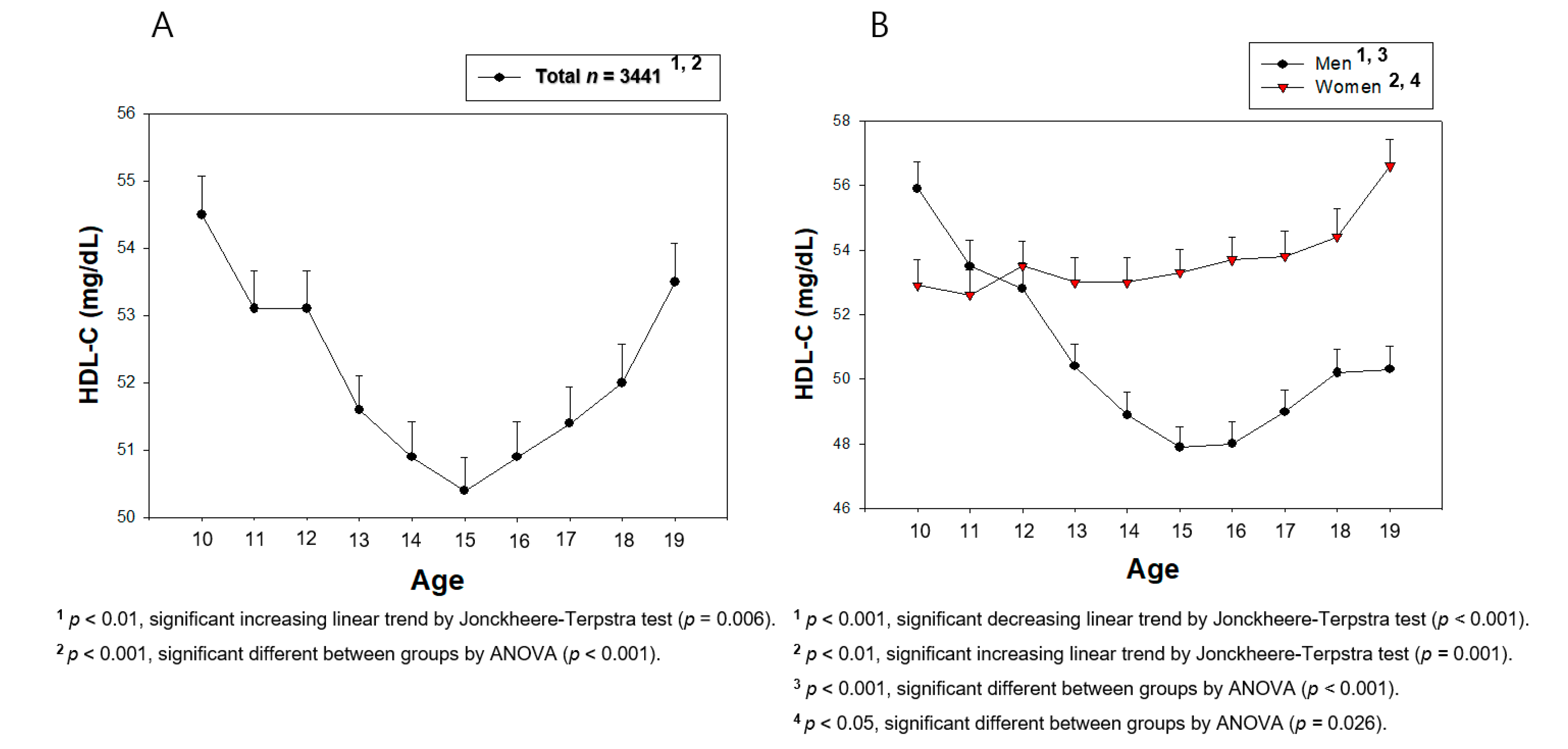
3. Results
3.1. Change in BP during Teenage
3.2. Change in the HDL-C Level during the Teenage Period
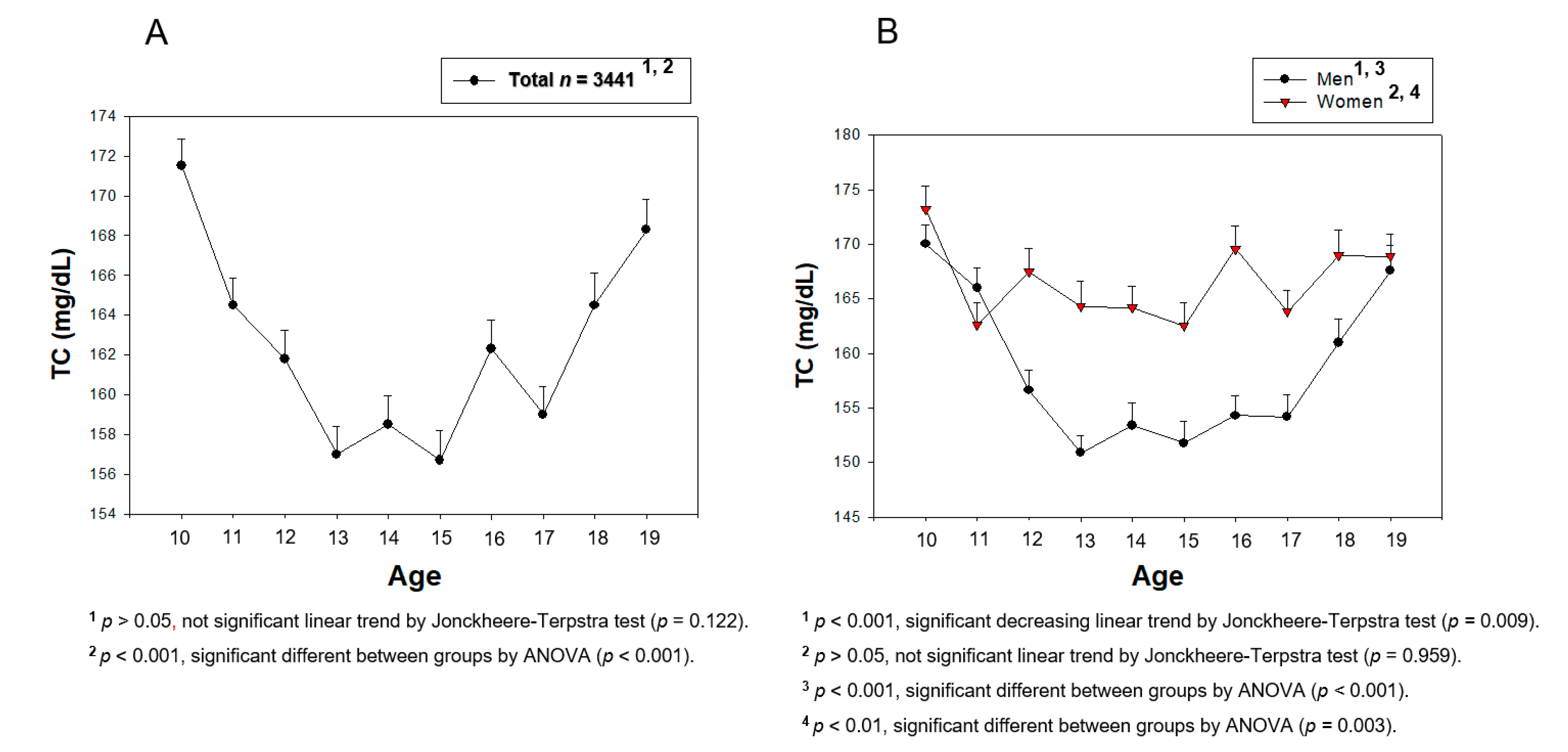
3.3. Change in the Serum TC during the Teenage Period
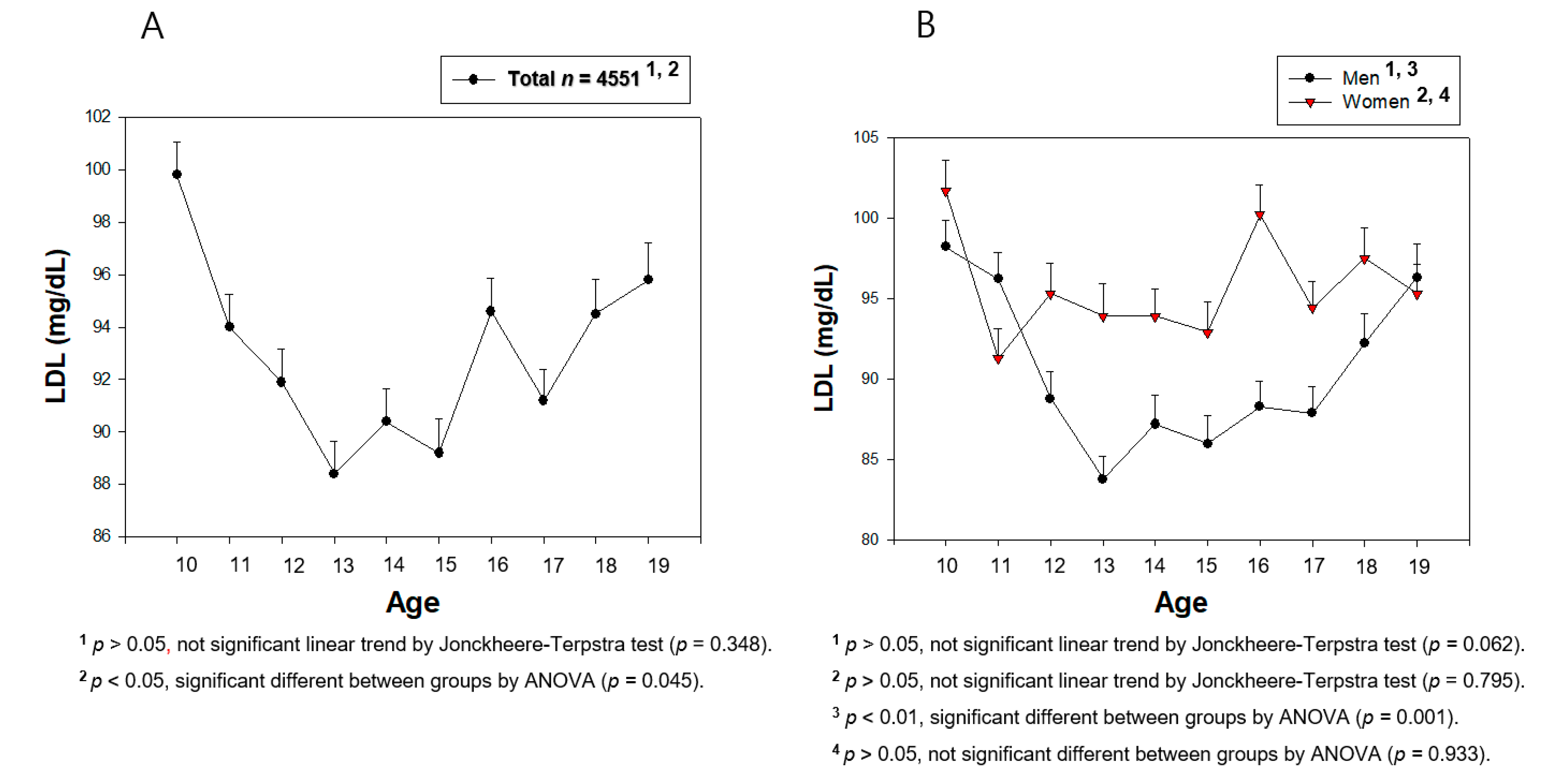
3.4. Change in the Serum LDL-C during Teenage
3.5. Change in the Serum TG during the Teenage Years
3.6. Correlation Analysis between the HDL-C Level and BP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bo, S.; Cavallo-Perin, P.; Gentile, L.; Repetti, E.; Pagano, G. Low HDL-cholesterol: A component of the metabolic syndrome only in the presence of fasting hypertriglyceridemia in type 2 diabetic patients. Diabetes Metab. 2001, 27, 31–35. [Google Scholar] [PubMed]
- Butto, E.B.; Robert, J.; Caffrey, T.M.; Fan, J.; Zhao, W.; Wellington, C.L. HDL from an Alzheimer’s disease perspective. Curr. Opin. Lipidol. 2019, 30, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Deng, W.; Ding, D.; Zhao, Q.; Liang, X.; Wang, F.; Luo, J.; Zheng, L.; Guo, Q.; Hong, Z. High low-density lipoprotein cholesterol inversely relates to dementia in community-dwelling older adults: The Shanghai aging study. Front. Neurol. 2018, 9, 952. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Dawber, T.R.; Friedman, G.D.; Glennon, W.E.; McNamara, P.M. Risk factors in coronary heart disease: An evaluation of several serum lipids as predictors of coronary heart disease; The Framingham Study. Ann. Intern. Med. 1964, 61, 888–899. [Google Scholar] [CrossRef]
- Brunner, F.J.; Waldeyer, C.; Ojeda, F.; Salomaa, V.; Kee, F.; Sans, S.; Thorand, B.; Giampaoli, S.; Brambilla, P.; Tunstall-Pedoe, H.; et al. Application of non-HDL cholesterol for population-based cardiovascular risk stratification: Results from the Multinational Cardiovascular Risk Consortium. Lancet 2019, 394, 2173–2183. [Google Scholar] [CrossRef]
- Rye, K.A.; Bursill, C.A.; Lambert, G.; Tabet, F.; Barter, P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid. Res. 2009, 50, S195–S200. [Google Scholar] [CrossRef] [PubMed]
- Riggs, K.A.; Rohatgi, A. HDL and reverse cholesterol transport biomarkers. Methodist Debakey Cardiovasc. J. 2019, 15, 39–46. [Google Scholar]
- Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [CrossRef]
- Kim, H.J.; Park, H.A.; Cho, Y.G.; Kang, J.H.; Kim, K.W.; Kang, J.H.; Kim, N.R.; Chung, W.C.; Kim, C.H.; Whang, D.H.; et al. Gender difference in the level of HDL cholesterol in korean adults. Korean J. Fam. Med. 2011, 32, 173–181. [Google Scholar] [CrossRef]
- Gorman, B.K.; Read, J.G. Why men die younger than women. Geriatrics Aging 2007, 10, 179–181. [Google Scholar]
- Cho, K.H.; Park, H.J.; Kim, S.J.; Kim, J.R. Decrease in HDL-C is associated with age and household income in adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation analysis of low HDL-C and poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef]
- Lewis, C.E.; Lewis, M.A. The potential impact of sexual equality on health. N. Engl. J. Med. 1977, 297, 863–869. [Google Scholar] [CrossRef]
- Min, K.J.; Lee, C.K.; Park, H.N. The lifespan of Korean eunuchs. Curr. Biol. 2012, 22, R792–R793. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.B.; Mestler, G.E. Mortality and survival: Comparison of eunuchs with intact men and women in a mentally retarded population. J. Gerontol. 1969, 24, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Kunutsor, S.K.; Kieneker, L.M.; Bakker, S.J.; James, R.W.; Dullaart, R.P. The inverse association of HDL-cholesterol with future risk of hypertension is not modified by its antioxidant constituent, paraoxonase-1: The PREVEND prospective cohort study. Atherosclerosis 2017, 263, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Terpstra, T.J. The asymptotic normality and consistency of Kendall’s test against trend, when ties are present in one ranking. Indagations Math. 1952, 14, 327–333. [Google Scholar] [CrossRef]
- Jonckheere, A.R. A distribution-free k-sample test against ordered alternatives. Biometrika 1954, 41, 133–145. [Google Scholar] [CrossRef]
- Fofana, M.; Maboundou, J.C.; Bocquet, J.; Le Goff, D. Transfer of cholesterol between high density lipoproteins and cultured rat Sertoli cells. Biochem. Cell Biol. 1996, 74, 681–686. [Google Scholar] [CrossRef]
- Vitali, C.; Wellington, C.L.; Calabresi, L. HDL and cholesterol handling in the brain. Cardiovasc. Res. 2014, 103, 405–413. [Google Scholar] [CrossRef]
- Shi, J.F.; Li, Y.K.; Ren, K.; Xie, Y.J.; Yin, W.D.; Mo, Z.C. Characterization of cholesterol metabolism in Sertoli cells and spermatogenesis (Review). Mol. Med. Rep. 2018, 17, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Fofana, M.; Travert, C.; Carreau, S.; Le Goff, D. Evaluation of cholesteryl ester transfer in the seminiferous tubule cells of immature rats in vivo and in vitro. J. Reprod. Fertil. 2000, 118, 79–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, E.Y.; Cho, K.H. High-doseconsumption of NaCl resulted in severe degradation of lipoproteins associated with hyperlipidemia, hyperglycemia, and infertility via impairment of testicular spermatogenesis. Toxicol. Res. 2016, 5, 557–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eissa, M.A.; Mihalopoulos, N.L.; Holubkov, R.; Dai, S.; Labarthe, D.R. Changes in Fasting Lipids during Puberty. J. Pediatr. 2016, 170, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Farinaro, E.; Giumetti, D.; Coraggio, S.; Panico, S.; Mancini, M. Serum lipid and lipoprotein profiles in childhood. Prev. Med. 1983, 12, 40–43. [Google Scholar] [CrossRef]
- Austad, S.N.; Fischer, K.E. Sex Differences in Lifespan. Cell. Metab. 2016, 23, 1022–1033. [Google Scholar] [CrossRef]
- Murphy, S.L.; Xu, J.; Kochanek, K.D.; Arias, E. Mortality in the United States, 2017. NCHS Data Brief 2018, 328, 1–8. [Google Scholar]
- Cho, K.H.; Park, H.J.; Kim, J.R. Decrease in serum HDL-C level is associated with elevation of blood pressure: Correlation analysis from the Korean National Healthh and Nutrition Examination Survey 2017. Int. J. Environ. Res. Public Health 2020, 17, 1101. [Google Scholar] [CrossRef]
- Waldron, I. Why do women live longer than men? Soc. Sci. Med. 1976, 10, 349–362. [Google Scholar] [CrossRef]
- Cho, K.H. High-Density Lipoproteins as Biomarkers and Therapeutic Tools Volume 1. Impacts of Lifestyle, Diseases, and Environmental Stressors on HDL; Springer: New York City, NY, USA, 2019. [Google Scholar]
- Cho, K.H. High-Density Lipoproteins as Biomarkers and Therapeutic Tools: Volume 2. Improvement and Enhancement of HDL and Clinical Applications; Springer: New York City, NY, USA, 2019. [Google Scholar]

| Boys (n = 1817, mean ± SD) | Girls (n = 1624, mean ± SD) | |||||
|---|---|---|---|---|---|---|
| 10–11 (n = 367) | 14–15 (n = 375) | 18–19 (n = 333) | 10–11 (n = 294) | 14–15 (n = 332) | 18–19 (n = 296) | |
| HDL-C (mg/dL) | 54.6 ± 11.1 | 48.4 ± 9.0 | 50.3 ± 9.3 | 52.8 ± 9.6 | 53.1 ± 9.7 | 55.6 ± 10.5 |
| p for trend | 10–15, decrease, <0.001 15–19, increase, 0.001 10–19, decrease, <0.001 | 10–15, no change, 0.588 15–19, increase, 0.012 10–19, increase, <0.001 | ||||
| TC (mg/dL) | 167.9 ± 24.8 | 152.6 ± 27.4 | 164.2 ± 28.8 | 167.7 ± 25.7 | 163.3 ± 26.4 | 168.9 ± 26.2 |
| p for trend | 10–15, decrease, <0.001 15–19, increase, <0.001 10–19, decrease, 0.009 | 10–15, decrease, 0.003 15–19, no change, 0.065 10–19, no change, 0.959 | ||||
| LDL-C (mg/dL) | 97.1 ± 22.5 | 86.6 ± 24.4 | 94.2 ± 25.7 | 96.3 ± 23.0 | 93.4 ± 23.1 | 96.3 ± 22.5 |
| p for trend | 10–15, decrease, <0.001 15–19, increase, 0.00008 10–19, no change, 0.062 | 10–15, decrease, 0.043 15–19, no change, 0.596 10–19, no change, 0.795 | ||||
| SBP (mmHg) | 106.5 ± 9.0 | 111.6 ± 9.9 | 114.9 ± 11.0 | 105.0 ± 9.3 | 106.2 ± 8.9 | 106.0 ± 9.0 |
| p for trend | 10–19, increase, <0.001 | 10–19, no change, 0.141 | ||||
| DBP (mmHg) | 61.7 ± 8.2 | 66.7 ± 9.0 | 71.8 ± 9.0 | 62.2 ± 8.4 | 66.8 ± 7.8 | 68.3 ± 7.4 |
| p for trend | 10–19, increase, <0.001 | 10–19, increase, <0.001 | ||||
| Age | Height | Weight | HDL-C | TC | LDL-C | TG | SBP | ||
|---|---|---|---|---|---|---|---|---|---|
| Boys (n = 1817) | Height (cm) | 0.773 *** | |||||||
| Weight (kg) | 0.596 *** | 0.718 *** | |||||||
| HDL-C (mg/dL) | −0.171 *** | −0.250 *** | −0.355 *** | ||||||
| TC (mg/dL) | −0.042 | −0.193 *** | 0.052 * | 0.269 *** | |||||
| LDL-C (mg/dL) | -0.029 | −0.150 *** | 0.097 *** | 0.053 * | 0.888 *** | ||||
| TG (mg/dL) | 0.111 *** | 0.075 ** | 0.231 *** | −0.356 *** | 0.277 *** | −0.023 | |||
| SBP (mmHg) | 0.277 *** | 0.288 *** | 0.451 *** | −0.133 *** | 0.120 *** | 0.101 *** | 0.190 *** | ||
| DBP (mmHg) | 0.406 *** | 0.339 *** | 0.329 *** | −0.122 *** | 0.061 ** | 0.054 * | 0.140 *** | 0.451 *** | |
| Girls (n = 1624) | Height (cm) | 0.575 *** | |||||||
| Weight (kg) | 0.488 *** | 0.653 *** | |||||||
| HDL-C (mg/dL) | 0.085 *** | 0.014 | −0.165 *** | ||||||
| TC (mg/dL) | 0.004 | −0.080 * | 0.025 | 0.293 *** | |||||
| LDL-C (mg/dL) | 0.003 | −0.070 ** | 0.064 ** | 0.052 * | 0.914 *** | ||||
| TG (mg/dL) | −0.086 *** | −0.067 ** | 0.086 *** | −0.354 *** | 0.253 *** | 0.043 | |||
| SBP (mmHg) | 0.041 | 0.107 *** | 0.266 *** | −0.065 ** | 0.015 | 0.005 | 0.102 *** | ||
| DBP (mmHg) | 0.254 *** | 0.249 *** | 0.290 *** | −0.016 | 0.003 | −0.001 | 0.027 | 0.482 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H.; Kim, J.-R. Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women. Med. Sci. 2021, 9, 35. https://doi.org/10.3390/medsci9020035
Cho K-H, Kim J-R. Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women. Medical Sciences. 2021; 9(2):35. https://doi.org/10.3390/medsci9020035
Chicago/Turabian StyleCho, Kyung-Hyun, and Jae-Ryong Kim. 2021. "Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women" Medical Sciences 9, no. 2: 35. https://doi.org/10.3390/medsci9020035
APA StyleCho, K.-H., & Kim, J.-R. (2021). Rapid Decrease in HDL-C in the Puberty Period of Boys Associated with an Elevation of Blood Pressure and Dyslipidemia in Korean Teenagers: An Explanation of Why and When Men Have Lower HDL-C Levels Than Women. Medical Sciences, 9(2), 35. https://doi.org/10.3390/medsci9020035







