Abstract
Background: Achalasia and other esophageal dysmotility disorders mimicking achalasia can be associated with cancer. This study aimed to review the main mechanisms for which cancer may develop in esophageal dysmotility disorder patients. Methods: A narrative review was performed. Results: The mechanism for developing squamous cell carcinoma and adenocarcinoma are discussed. Besides, achalasia-like syndromes related to familial KIT-gene mutation and pseudoachalasia are discussed. Conclusions: Knowing the main mechanism for which achalasia can be related to cancer is essential for clinicians to conduct the proper investigation, surveillance, and treatment.
1. Introduction
Achalasia is an esophageal motor disease characterized by the lack of relaxation of the lower esophageal sphincter (LES) and aperistalsis [1]. The first time this condition was described was in 1672 by Sir Thomas Willis, but the “achalasia” term was only created in 1929 by Hurst and Rake, meaning “no relaxation” [2]. The main mechanism for which achalasia appears is due to neuronal degeneration of the myenteric plexus, although the reason for it to happen is still uncertain [2]. Reduced density of Cajal cells in the gastroesophageal junction, the gastrointestinal pacemakers, is another mechanism that may be related in some patients [3].
Achalasia is more often seen in South American countries due to its association with Chagas disease [4]. Patients with achalasia can initially show dysphagia, recurrent chest pain, regurgitation, and weight loss [5]. Usually, symptoms are nonspecific, and there is a significant delay in diagnosis, so the majority of the patients begin their treatment in advanced stages of the disease, already presenting malnutrition, underweight, and recurrent pneumonia [5]. Manometry is the gold-standard diagnostic test for achalasia. The typical findings are incomplete relaxation of LES and uncoordinated esophageal body contractions. Frequently, LES is hypertensive [6]. Barium swallow tests reveal a dilated esophagus, the reason for which achalasia is often called megaesophagus. Besides, barium swallow tests may reveal uncoordinated esophageal contractions and a narrowing at the gastroesophageal junction [7]. Patients with achalasia have an increased risk for developing esophageal cancer [8,9].
Fagge was probably the first author to point out a possible association between achalasia and cancer in 1872 [10]. He described a case of a benign esophageal condition associated with a tumor. Sato et al. [11], analyzing a sample size of 2714 achalasia patients, evidenced that the risk of developing cancer was 16.7 times higher for men with achalasia and 8.8 times higher for women with achalasia.
When patients start to have symptoms of cancer, the tumor is usually already advanced. The symptoms of achalasia and esophagus cancer are similar, and the patient may undervalue the symptoms. Additionally, esophageal-related symptoms depend on the esophagus distension and longitudinal contraction of esophageal smooth muscle, and in achalasia, the esophagus is naturally enlarged [12]. In this way, achalasia patients may present only mild esophageal symptoms, even with a severe condition within it [13]. For the carcinoma to overlap the symptoms of achalasia, it must be large to obstruct a dilated esophagus [14]. Due to the delay in the differential diagnosis, many patients end up having a late cancer diagnosis, and the locally advanced disease leads to poor prognosis of these patients, often making the curative treatment unfeasible [9].
Despite many studies establishing an association between achalasia and cancer, achalasia’s actual cancer genesis mechanisms are poorly debated. This study aims to review the literature on the pathophysiology of cancer development in achalasia and other esophageal dysmotility disorders mimicking achalasia.
2. Materials and Methods
A narrative review was performed, gathering the medical evidence regarding the mechanisms for which achalasia and other esophageal dysmotility disorders that mimic achalasia may be related to cancer development. We provided an overview of the available research evidence for clinicians to know the different neoplasm types that may be associated with esophageal dysmotility disorders. The following search terms were used: “achalasia”, “megaesophagus”, “esophageal dysmotility”, “esophageal motility disorders”, “dysphagia”, “esophageal cancer”, “neoplasms”, “pseudoachalasia”. The main databases searched were PubMed, Embase, Lilacs/BVS, Cochrane Central, and Google Scholar. The study design included any observational or experimental human study and animal models.
3. Results
The present review searched for the main evidence of the mechanisms involving esophageal dysmotility and cancer. They include epithelial neoplasms, such as squamous cell carcinoma and adenocarcinoma, and also mesenchymal cancers. Both esophageal and extra-esophageal cancer were discussed.
3.1. Esophageal Squamous Cell Carcinoma
The incidence of squamous cell carcinoma in achalasia is 312/100,000 patient-years at risk [9]. Although there is no controlled study comparing different geographic regions, South American cohorts report a higher incidence of cancer per patients with achalasia than in achalasia cohorts in the rest of the world [9]. This possible geographic variability in the incidence of esophageal squamous cell carcinoma may correlate to diet aspects. In fact, in non-achalasia patients, squamous cell carcinoma is related to nutritional and dietary habits, including vitamins, minerals, meats, salted foods, mycotoxins, and even hot beverages [15].
Beyond the diet, a large number of other factors may contribute to cancer. Continuous saliva stasis and food decomposition in the esophagus may induce chronic hyperplastic esophagitis [16]. Inflammation may contribute to cancer development through several different ways, including angiogenesis, DNA damage, promotion of cellular growth and multiplication, and dysregulation of programmed cell death [17]. The esophageal stasis also predisposes to recurrent lesions in the esophagus due to the increased time of exposure of the esophageal mucosa to substances such as alcohol, tobacco, and nitrosamines, leading to chronic inflammation. Additionally, the origin of the achalasia is immune-mediated, and organ neurons and ganglions are affected by cytotoxic T-cells, mast cells, eosinophils, and antibodies, contributing to a chronic inflammatory process [18].
Overgrowth of nitrate-reducing bacteria due to esophageal food stasis leads to increased volatile N-nitrosamines concentration in the esophageal lumen [19]. N-nitrosamines originated with nitric oxide reactions [20]. Overproduction of reactive nitrogen species (RNS) leads to nitrosative stress [20]. In this circumstance, there will be damage to the lipids, proteins, and nucleic acids [20], and consequently, lead to genotoxic and carcinogenic effects, as demonstrated in animal models [21]. RNS concentration can be increased by the excess of food intake or by endogenous production in the food stasis [21]. Esophageal achalasia has specific microbiota, which may vary according to the grade of dilatation [22]. Among the microorganisms found in the achalasia esophagus, Staphylococcus sp., Corynebacterium sp., Peptostreptococcus sp., and Veillonella sp. can produce N-nitrosamines [22]. N-nitrosamines under certain pH conditions decompose into alkyldiazhydroxide species, and the alkylation of the DNA produces modified purine and pyrimidine bases [23].
Achalasia predisposes to psychiatric conditions, probably due to its influence on patient well-being [24,25]. Psychiatric syndromes can predispose these patients to alcoholism [11,26], a known risk factor for esophageal squamous cell carcinoma [11]. Alcohol in stasis may lead to much more intense esophagitis, including acute necrotizing esophagitis [27].
Some studies investigated the genetic pathways in the development of cancer in achalasia. Munari et al. [28] reported an association between Chagasic achalasia and PIK3CA mutations. Patients with these mutations presented a significantly lower survival rate. PIK3CA encodes phosphoinositide 3-kinase (PI3K), an upstream kinase that regulates cell proliferation, apoptosis, and growth [29]. However, there is no certainty if these mutations are caused by the Trypanosoma infection or by chronic esophageal dilatation.
In another study investigating genetic changes in esophageal cancer related to Chagasic achalasia, Lacerda et al. [30] showed a high rate of TP53 mutations in these cancers, but there was no difference comparing with non-achalasia squamous cell carcinoma. Safatale-Ribeiro et al. [31] showed that TP53 overexpression and mutational changes, including exons 5, 6, and 7, are more often seen in patients with high-grade inflammation achalasia of the esophagus. In addition to the TP53 gene mutation, p21, p16, and epidermal growth factor receptor may also play a role in the carcinogenesis in achalasia [16].
Patients with Chagasic achalasia have also a higher prevalence of chromosome aneusomies, including chromosomes 7, 11, and 17 [32]. These cytogenetic alterations are known to predispose patients to squamous cell carcinoma [33].
Finally, Chagasic achalasia squamous cell carcinoma patients also present a higher rate of microsatellite instability than non-achalasia squamous cell carcinoma (21% vs. 11%) [34]. This finding may point to future immunotherapy options for these patients since microsatellite instability is a surrogate prognostic biomarker for immunotherapy in other cancer types [35].
3.2. Esophageal Adenocarcinoma
While the carcinogenesis of squamous cell carcinoma in achalasia has been well investigated in previous studies, so far, there are few studies evaluating achalasia and the development of esophageal adenocarcinoma. In a national Swedish inpatient data evaluation, both esophageal squamous cell carcinoma and adenocarcinoma were increased in achalasia patients [36]. The incidence rate of esophageal adenocarcinoma is 21/100,000 achalasia patient-years at risk [9]. However, the incidence probably varies across different worldwide geographic regions [11]. Nonetheless, the mechanisms for which adenocarcinoma develop in achalasia are poorly understood.
There is a possibility that the esophageal achalasia environment acts as a cancer risk for both types of esophageal carcinoma, and that for some reason, oncogenic events may end up in squamous cell carcinoma or adenocarcinoma. Both acid reflux clearance impairment and chronic inflammation due to food stasis within the esophagus may act as carcinogenesis mechanisms for squamous cell carcinoma and adenocarcinoma [37]. As well as for squamous cell carcinoma, the bacteria metabolites, such as N-nitrosocompounds, lactic acid, and heme, have an association with Barrett’s esophagus-adenocarcinoma sequence [38,39]. In addition to the nitrosative stress, the oxidative stress in chronic esophagitis induces replacement of the stratified squamous epithelium for a metaplastic columnar epithelium, leading to Barrett’s esophagus and, eventually, adenocarcinoma [40]. In fact, achalasia associated with Barrett’s esophagus has been previously reported [41].
Besides, achalasia’s main therapeutic approaches aim to perform LES disruptures, such as myotomy or endoscopic dilation. Those procedures can contribute to gastroesophageal reflux and induce metaplastic transformation of squamous mucosa to Barrett’s esophagus [36]. In this sense, caution is needed in patients submitted to peroral endoscopic myotomy (POEM) without anti-reflux procedures. Patients with achalasia have limited esophageal sensibility to esophagitis, and often POEM patients present high-grade esophagitis with only mild or no symptoms. [13,42].
The precise mechanism for which Barrett’s esophagus appears after chronic oxidative stress is not fully understood. Chronic esophagitis promotes increased metabolism of molecular oxygen, raising the intracellular concentration of reactive oxygen species (ROS). ROS can act at several steps in multistage carcinogenesis, participating in numerous signaling cascades, inducing both initiation and progression of cancer. Most of the mutations related to ROS involves base pair substitution, and all nucleobases can be affected, producing mispaired DNA sequences [43]. The 8-OH-deoxyguanosine (8-OHdG), a biomarker of oxidative stress, is known to be elevated in both Barrett’s esophagus and esophageal adenocarcinoma [44]. In addition, the superoxide dismutase and the glutathione redox system, which acts to mitigate the oxidative stress insult, are reduced in Barrett’s esophagus tissue. [45]. Long-standing elevated ROS activate transcription factors, such as activator protein-1 (AP-1) and nuclear factor-kappaB (NF-κB). NF-κB translocates into the nucleus, where it binds to promote a target oncogene. Alternatively, upstream kinases activated by both oxidative and pro-inflammatory stimuli activate AP-1 components, promoting transcriptional activation of oncogenes, leading to cell proliferation and growth [46]. Additionally, pro-inflammatory cytokines and microRNAs have a significant role in adenocarcinoma development. Barrett’s esophagus shows mutations even before the cancer development [47]. A cumulative number of mutations is the underlining mechanism for the sequence of Barrett’s esophagus to adenocarcinoma [37]. These genetic changes include TP53 and P16 expression, copy number alterations, and altered gene transcription patterns [37]. The overexpression of TP53 and numeric abnormalities of chromosome 7 that are often seen in achalasia patients [32] are also known to be critical cytogenetic biomarkers for adenocarcinoma precursors [48].
Non-achalasia studies of adenocarcinomas without evidence of Barrett epithelium hypothesize that the neoplasm raised from heterotopic gastric mucosa or gastric mucosa extends into the esophagus or even esophageal glands. [49,50]. It is known from Helicobacter pylori and autoimmune gastritis studies that chronic inflammation increases the risk for developing gastric adenocarcinoma, and it could apply to the heterotopic gastric mucosa or esophageal glands [51]. Consequently, chronic esophagitis in achalasia is a presumable risk variable for malignization, even with no Barrett epithelium evidence. In addition, it is known that gastroesophageal reflux induces chronic inflammation and oxidative stress in the cardia and proximal gastric mucosa, and thus, procedures that disrupt the LES and favor the gastric reflux may contribute to proximal gastric cancer development [52].
3.3. Other Types of Cancer
In addition to the epithelial cancers, other achalasia-associated neoplasms have already been described, although rare.
Interstitial cells of Cajal (ICC) are present in the gastrointestinal wall and act as pacemakers, coordinating the gastrointestinal organs’ motility [53]. Malfunctioning of these cells may lead to an achalasia-like phenotype.
ICC expresses high levels of KIT (CD117) in their surface, also known as stem cell growth factor receptor [54]. KIT is a type III tyrosine kinase receptor and is expressed in several normal tissues, including melanocytes, mast cells, hematopoietic stem cells, germ cells, basal cells, and some cells in the nervous system. This receptor is encoded by the proto-oncogene c-KIT. Animal models have shown a link between c-KIT mutation and a low ICC density, leading to gastrointestinal dysmotility [55].
Besides, ICC are the progenitor cells of gastrointestinal stromal tumors (GISTs), and a gain-of-function mutation of the c-KIT may lead to these tumors [56]. The tyrosine kinase activity induces phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways [57]. There are several reports of familial achalasia-like and GISTs. Hirota et al. [58] reported familial gastrointestinal stromal tumors with achalasia-like symptoms and c-KIT mutation. These patients showed a single base pair mutation in the extracellular domain of stem cell gene mutation. Hoshino et al. [59] described two patients with familial achalasia, and one of them had gastric GIST.
Mast cells also highly express tyrosine kinase receptors on their surface, and thus, KIT-gene mutation may also induce mastocytosis [56]. Halpern et al. [60] investigated seven members of a family with achalasia-like symptoms. The patients had a unique activating mutation in exon 9 of c-KIT. All of the patients had mastocytosis, and three of them also developed GISTs. Marshall et al. [61] evaluated a family’s pedigree spanning four generations with achalasia-like symptoms and mastocytosis. Other findings in this family were diffuse esophageal leiomyomatosis, urticaria pigmentosa, neurofibroma, and mastocytosis.
The association of the KIT and the KIT ligand, also known as stem cell or mast cell growth factor, allows the signaling for the correct development, growth, migration, and differentiation of the melanocytes [62]. The association between pigment cell anomalies and c-KIT mutation is well established, including certain types of melanomas, such as acro lentiginous melanoma [63]. Neuhann et al. [64] reported a novel c-KIT mutation in family members presenting achalasia-like symptoms. The familial members had an autosomal dominant genetic disorder due to germline mutation in exon 11 of the c-KIT. All of them had multiple hyperpigmented skin macules, and some of the members also presented multiple GISTs. Ávila et al. [65] also reported a c-KIT mutation in exon 11 in an Argentinian family. Patients presented dysphagia associated with GISTs, diffuse melanosis, and lentiginosis. In a large kindred of 22 members with the c-KIT mutation, the index patient had esophageal thickening and was diagnosed with multiple smooth muscle tumors, dying within the 2 months follow-up. At the time, there was no genetic mutation suspected. The second family member called the medical staff’s attention for a possible inheritance disorder. This patient had multifocal low-grade leiomyosarcoma and hyperpigmentation of the perineum, hands, knees, and in the surrounding area of the mouth. The patient was submitted to a genomic study from leukocytes, confirming mutation in exon 11 of the KIT-gene [66] Other studies of patients with dysphagia and gastrointestinal stromal tumors or mast or melanocyte disorders linked with the c-KIT mutation have been reported [67,68,69,70]. In these cases, exons 8 [71], 9 [60], 11 [64,65,66,70], 13 [67], and 17 [58,69] mutations of the c-KIT were described.
PDGFRA is another type III tyrosine kinase receptor. The PDGFRA activates when binding to PDGFs and elicits the same pathways of the KIT signaling [72]. Consequently, mutations of PDGFRA hypothetically could lead to the same phenotype of the c-KIT mutation [72]. PDGFRA mutation syndromes account for a minority of the GISTs [72]. However, no PDGFRA mutations related to achalasia-like have been described yet.
Knowledge of the c-KIT mutation in these neoplasms opens a wide field to explore target therapy studies in the future. The use of tyrosine kinase inhibitors, such as Glivec (STI571; Imatinib. Novartis, Basel, Switzerland), was found to promote downstaging and metastasis control of GIST with c-Kit expression [73]. The low molecular weight tyrosine kinase inhibitor Imatinib blocks activation of KIT by binding the normal ATP binding site. [73]. A pooled analysis from ten European Organisation for Research and Treatment of Cancer (EORTC) databases [74] evaluated neoadjuvant Imatinib in advanced GISTs and showed excellent long-term survival outcomes and facilitation to resection. Most of the patients were preoperatively evaluated for c-KIT and PDGFRA mutations. In a recent randomized clinical trial [75], long-term use of Imatinib as adjuvant therapy for GIST improved the recurrence-free survival rates. In an open-label, non-controlled study [76], unresectable melanoma patients with a proven c-KIT mutation were evaluated for Imatinib response, demonstrating notable radiographic improvement. In a systemic mastocytosis study [77], Imatinib showed a 50% response, including 40% of sustained complete response. Ávila et al. [65] demonstrated an expressive response of the melanosis and gastrointestinal stromal tumors in the family members with achalasia-like symptoms. Moreover, tyrosine kinase inhibitors may change the Cajal cell’s pacemaker activity in the gastrointestinal system [78]. Halpern et al. [60] reported in their study that three of the patients were treated with tyrosine kinase inhibitors for GIST and mastocytosis. They noted robust response to the drug, with reduction of GIST size, reduction of bone marrow mast cells, and interestingly, patients revealed an expressive improvement in dysphagia.
Table 1 summarizes the main studies evaluating families’ pedigrees with germline c-KIT mutation, achalasia-like symptoms, and cancer.

Table 1.
Germline c-KIT mutation associated with achalasia-like symptoms and neoplasms development. Main studies.
3.4. Cancer Leading to Esophageal Dysmotility
Dysphagia mimicking achalasia may also be secondary to neoplasms instead of the other way around. Pseudoachalasia is a condition in which symptoms, manometric, endoscopic, and barium swallow test findings are pretty similar to achalasia. The first description of pseudoachalasia was in 1919 by Howarth [79]. He discussed a case report of esophageal dilatation with no anatomical stenosis as a manifestation of malignancy.
Pseudoachalasia is caused by malignancy in the majority of patients [80]. Esophageal, gastric, and esophagogastric junction neoplasms account for 46% of the causes of pseudoachalasia [80]. Following, lung (13%) and breast (6%) cancer are the most common causes of pseudoachalasia in non-gastrointestinal tumors [80]. Non-malignant causes are less frequent and include post laparoscopic gastric band, post vagotomy, descending aortic aneurysm, post sleeve gastrectomy, and stricture at the gastrojejunostomy [80].
The mechanism by which pseudoachalasia occurs can be due to direct tumor invasion and compression of the LES, or local nerves infiltration, leading to LES dysfunction. Moreover, the mass effect may block food transit and lead to progressive dilatation of the esophagus [81]. This is the main mechanism by which adenocarcinoma of the gastroesophageal junction leads to pseudoachalasia. Fabian et al. [82] reported pseudoachalasia as the first manifestation of a cardia adenocarcinoma in a patient that presented typical signs of achalasia at endoscopy, manometry, and the barium swallow test.
Besides, paraneoplastic pseudoachalasia may happen by the host’s immune response against tumor antigens, which leads to neuropathy [83]. It happens both by activation of immunocytes and by humoral response [84]. Most of the patients with neuronal paraneoplastic pseudoachalasia express serum autoantibodies, and the most common is the antineuronal nuclear antibody type-1 (ANNA-1; or Anti-Hu) [85]. Anti-acetylcholine and anti-neural calcium channel antibodies may be present in some patients as well [82,86]. Previous in vitro studies showed that these antibodies bind to neuronal antigens. The inflammatory/immune insult to the myenteric plexus could explain esophageal dysmotility [86].
A neuronal autoimmune disorder is more often associated with small-cell carcinoma, which in most cases arises in the lung [87]. Antigens from the small-cell carcinoma induce B-lymphocyte and helper T-lymphocyte responses. The first descriptions of neuronal autoimmune disorder in small-cell carcinoma were related to sensory neuronopathy and encephalomyeloradiculoneuropathy, but several other neurological conditions have already been reported, such as cerebellar ataxia, limbic encephalitis, myelopathy, Lambert–Eaton syndrome, and myopathy [87].
Immunohistochemical studies showed that ANNA-1 are RNA-binding proteins that act mainly in the nucleus of neurons in both the peripheral and central nervous systems. All myenteric ganglia may be affected, and ANNA-1 has been linked to other gastrointestinal tract dysmotility, such as gastroparesis, megacolon, and intestinal pseudo-obstruction [84]. Liu et al. [85] described a patient with severe dysphagia and a metastatic lung small cell carcinoma. Sera analysis showed circulating ANNA-1 IgG. Analysis of the specimens from myotomy showed fibrosis and perineural and intraneural lymphocytic infiltration, mainly CD8-positive T cells, and a paucity of myenteric ganglia. Interestingly, this finding resembles the degeneration of the myenteric ganglions in the primary achalasia.
For the correct differentiation of typical achalasia from pseudoachalasia, clinicians need to assess symptoms onset and manometric findings. Duration of symptoms is much shorter in pseudoachalasia than primary achalasia (median duration 13 vs. 36 months) [88]. Besides, achalasia patients are usually younger and have less severe weight loss than pseudoachalasia patients. In esophageal manometry, lack of intact peristalsis is less frequent in pseudoachalasia than in primary achalasia. Additionally, integrated relaxation pressure and esophagogastric junction contractile integral are significantly lower in pseudoachalasia than primary achalasia. [88]
The treatment of pseudoachalasia is focused on finding and treating the primary etiology. Endoscopic dilatation and botulinum toxin injection usually do not provide dysphagia relief [72]. Hirano et al. [89] reported two lung cancer patients with dysphagia. These two patients had advanced cancer and were treated with gastrostomy as palliative therapy.
Other studies also reported pseudoachalasia associated with cancer [90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122]. Table 2 summarizes the main case series and case reports of patients with pseudoachalasia caused by malignancy.

Table 2.
Pseudoachalasia secondary to malignancy. Main studies.
4. Discussion
Achalasia is a risk factor for developing cancer, including squamous cell carcinoma and adenocarcinoma. Figure 1 schemes the mechanism for DNA damage and mutations for cancer development in achalasia. Additionally, other esophageal dysmotility disorders mimicking achalasia symptoms may be associated with other malignant-related conditions, such as paraneoplastic manifestations and familial KIT-gene mutation.
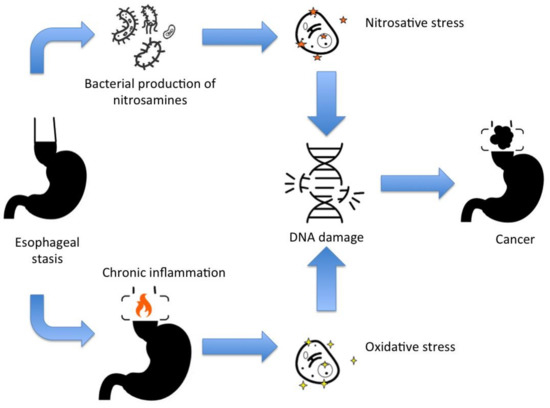
Figure 1.
The main mechanisms for DNA damage and cancer development in achalasia.
Other review studies have also issued the cancer development in achalasia. A previous meta-analysis tried to investigate the incidence of cancer in achalasia [9,123]. However, due to the relatively low incidence of achalasia, the exact incidence of cancer in achalasia is still obscure once the risk for publication and selection biases are more common for small sample-sized studies. [124]. Torres-Aguilera et al. [125] pointed the main links between achalasia and cancer. The present review attempted to provide comprehensive rationality for clinicians to understand all the mechanisms for esophageal dysmotility disorders that are cancer-related. In fact, some patients will present with only dysphagia, and clinicians should be aware of the risk of associated neoplasms and should know the principal differential diagnosis, and how to investigate, follow, surveil, or treat.
In this sense, we can highlight some take-away lessons. Firstly, patients with idiopathic or Chagasic achalasia should be under surveillance for esophageal cancer since the risk for cancer progressively increases with the time for follow-up [9]. Surveillance should be continued even if patients are appropriately treated with myotomy or esophageal endoscopic dilation since there is no current evidence that these procedures reduce the risk for carcinogenesis [9]. Under a surveillance program, patients have a higher chance of early cancer diagnosis, which is amenable for endoscopic resection [11]. In achalasia patients, chromoendoscopy may help to upgrade the accuracy of endoscopic surveillance [126]. Additionally, dysphagia worsening in achalasia should always be actively investigated for cancer.
Esophagectomy would be the unique treatment that would null the risk for malignant transformation in achalasia patients. However, esophagectomy is related to a high risk for morbidity and mortality. Esophagectomy for cancer is associated with a risk of 36% of postoperative complications, and a 30-day postoperative mortality rate of 6.7% [127].
Another take-away lesson is that clinicians need to remember that more than one member of the same family with achalasia-like symptoms, mast cell or skin pigmentation disorders, or GISTs should be investigated for c-KIT mutation. As well, patients with acute onset achalasia-like symptoms with severe weight loss or dysphagia in the elderly should call attention for pseudoachalasia. Additionally, dysphagia in non-gastroesophageal cancer should always be investigated for a paraneoplastic neuronal disorder.
However, there are still more questions than answers in the field of cancer and esophageal dysmotility. Future studies are needed to fill the medical literacy gaps. Future studies should investigate the interventions by which clinicians could reduce the risk for squamous cell carcinoma or adenocarcinoma development in achalasia. There is no evidence that myotomy or endoscopic dilatation could reduce the mechanisms for achalasia malignization [7], and controlled studies are needed to investigate it. Additionally, there is a concern regarding the long-term results of POEM. Will long-term follow-up of POEM patients show a higher risk for progressing to Barrett-adenocarcinoma sequence?
Reactions involving both ROS and N-nitrosamines are potential targets for cancer chemoprevention in achalasia. Drugs that potentially mitigate the oxidative and nitrosative stress may help not only unveil future prophylaxis for achalasia-related cancer but also Barrett’s-related cancer. An animal model study evaluated the use of NG-nitro-L-arginine methyl ester (L-NAME), a NOS inhibitor, for cancer therapy. The study demonstrated that NOS inhibitors reduced the tumor growth rate [128]. Other preclinical studies using L-NAME or a similar arginine analog have shown similar results in the tumor growth rate [129,130].
Theoretically, dietary antioxidants could act to prevent oxidative stress insults in the esophageal food stasis. Curcumin, resveratrol, epigallocatechin gallate, caffeic acid phenethyl ester, isothiocyanates are antioxidative phytochemicals that could be evaluated for cancer prevention [46].
Additionally, antimicrobial therapy for changing the esophageal stasis microbiota theoretically could reduce the colonization of bacteria that can produce N-nitrosamines.
In addition, the studies of neoplasm biomarkers, such as microsatellite instability, may point to future new therapies, including immunotherapies, for patients with achalasia-related carcinoma. Additionally, tyrosine kinase inhibitor studies may show the benefit of treating certain types of esophageal dysmotility and c-KIT mutation syndromes.
5. Conclusions
Achalasia is associated with cancer. Knowing the main mechanisms for which achalasia can be related to cancer is essential for clinicians to conduct the proper investigation, surveillance, and treatment. Besides, it may help to enkindle future studies that may provide specific interventions for cancer prophylaxis and treatment in achalasia.
Author Contributions
Conceptualization, F.T. and J.H.B.d.S.; methodology, F.T.; validation, E.T.B., N.M.D. and G.M.R.; N.M.D.—original draft preparation, G.M.R.; writing—review and editing, E.T.B.; visualization, M.S.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [F.T.], upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gyawali, C.P. Achalasia: New perspectives on an old disease. Neurogastroenterol. Motil. 2016, 28, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R. Achalasia. Gastroenterol. Hepatol. 2017, 13, 388. [Google Scholar]
- Kilic, A.; Luketich, J.D.; Landreneau, R.J.; Owens, S.R.; Krasinskas, A.M.; Schuchert, M.J. Alterations in the density of interstitial cells of Cajal in achalasia. Dig. Dis. Sci. 2008, 53, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Herbella, F.A.; Aquino, J.L.; Stefani-Nakano, S.; Artifon, E.L.; Sakai, P.; Crema, E.; Andreollo, N.A.; Lopes, L.R.; de Castro Pochini, C.; Corsi, P.R.; et al. Treatment of achalasia: Lessons learned with Chagas’ disease. Dis. Esophagus 2008, 21, 461–467. [Google Scholar] [CrossRef]
- Blam, M.E.; Delfyett, W.; Levine, M.S.; Metz, D.C.; Katzka, D.A. Achalasia: A disease of varied and subtle symptoms that do not correlate with radiographic findings. Am. J. Gastroenterol. 2002, 97, 1916–1923. [Google Scholar] [CrossRef] [PubMed]
- Müller, M. Impact of high-resolution manometry on achalasia diagnosis and treatment. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 3. [Google Scholar]
- Ott, D.J.; Richter, J.E.; Chen, Y.M.; Wu, W.C.; Gelfand, D.W.; Castell, D.O. Esophageal radiography and manometry: Correlation in 172 patients with dysphagia. Am. J. Roentgenol. 1987, 149, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Urach, D.R.; Tomlinson, G.A.; Harnish, J.L.; Martino, R.; Diamant, N.E. A measure of disease-specific health-related quality of life for achalasia. Am. J. Gastroenterol. 2005, 100, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Bernardo, W.M.; da Rocha, J.R.; Szachnowicz, S.; Seguro, F.C.; Bianchi, E.T.; Sallum, R.A.; Cecconello, I. Esophageal achalasia: A risk factor for carcinoma. A systematic review and meta-analysis. Dis. Esophagus 2017, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fagge, C.H. A case of simple stenosis of the oesophagus followed by epithelioma. Guy’s Hosp. Rep. 1872, 171, 413. [Google Scholar]
- Sato, H.; Terai, S.; Shimamura, Y.; Tanaka, S.; Shiwaku, H.; Minami, H.; Sato, C.; Ogawa, R.; Yokomichi, H.; Inoue, H. Achalasia and esophageal cancer: A large database analysis in Japan. J. Gastroenterol. 2021, 56, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Miwa, H.; Kondo, T.; Oshima, T.; Fukui, H.; Tomita, T.; Watari, J. Esophageal sensation and esophageal hypersensitivity-overview from bench to bedside. J. Neurogastroenterol. Motil. 2010, 16, 353. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Morrell, A.L.; Szor, D.J.; Dias, A.R. Achalasia: A mechanical and sensitivity disorder. United Eur. Gastroenterol. J. 2020, 8, 1126–1127. [Google Scholar] [CrossRef] [PubMed]
- Meijssen, M.A.; Tilanus, H.W.; Van Blankenstein, M.; Hop, W.C.; Ong, G.L. Achalasia complicated by oesophageal squamous cell carcinoma: A prospective study in 195 patients. Gut 1992, 33, 155–158. [Google Scholar] [CrossRef]
- Palladino-Davis, A.G.; Mendez, B.M.; Fisichella, P.M.; Davis, C.S. Dietary habits and esophageal cancer. Dis. Esophagus 2015, 28, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Chino, O.; Kijima, H.; Shimada, H.; Nishi, T.; Tanaka, H.; Oshiba, G.; Kise, Y.; Kajiwara, H.; Tsuchida, T.; Tanaka, M.; et al. Clinicopathological studies of esophageal carcinoma in achalasia: Analyses of carcinogenesis using histological and immunohistochemical procedures. Anticancer Res. 2000, 20, 3717–3722. [Google Scholar] [PubMed]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef]
- Storch, W.B.; Eckardt, V.F.; Junginger, T. Complement components and terminal complement complex in oesophageal smooth muscle of patients with achalasia. Cell. Mol. Biol. 2002, 48, 247–252. [Google Scholar]
- Pajecki, D.; Zilberstein, B.; Cecconello, I.; Dos Santos, M.A.; Yagi, O.K.; Gama-Rodrigues, J.J. Larger amounts of nitrite and nitrate-reducing bacteria in megaesophagus of Chagas’ disease than in controls. J. Gastrointest. Surg. 2007, 11, 199–203. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain, (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hoogenboom, L.; Leblanc, J.C.; Nebbia, C.S.; et al. Risk assessment of nitrate and nitrite in feed. EFSA J. 2020, 18, e06290. [Google Scholar]
- Pajecki, D.; Zilberstein, B.; Santos, M.A.; Quintanilha, A.G.; Cecconello, I.; Gama-Rodrigues, J. Megaesophagus microbiota and carcinogenesis. Arq. Gastroenterol. 2003, 40, 16–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tricker, A.R.; Preussmann, R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. Genet. Toxicol. 1991, 259, 277–289. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Massey, B.T.; McCarty, D.J.; Jacobsen, S.J. Epidemiology of hospitalization for achalasia in the United States. Dig. Dis. Sci. 1993, 38, 233–244. [Google Scholar] [CrossRef]
- Clouse, R.E. Psychiatric disorders in patients with esophageal disease. Med. Clin. N. Am. 1991, 75, 1081–1096. [Google Scholar] [CrossRef]
- Shivani, R.; Goldsmith, R.J.; Anthenelli, R.M. Alcoholism and psychiatric disorders: Diagnostic challenges. Alcohol Res. Health 2002, 26, 90. [Google Scholar]
- Lee, J.K.; Bhargava, V.; Mittal, R.K.; Ghosh, P. Achalasia, alcohol-stasis, and acute necrotizing esophagitis: Connecting the dots. Dig. Dis. Sci. 2011, 56, 612–614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munari, F.F.; Cruvinel-Carloni, A.; Lacerda, C.F.; De Oliveira, A.T.; Scapulatempo-Neto, C.; Da Silva, S.R.; Crema, E.; Adad, S.J.; Rodrigues, M.A.; Henry, M.A.; et al. PIK3CA mutations are frequent in esophageal squamous cell carcinoma associated with chagasic megaesophagus and are associated with a worse patient outcome. Infect. Agents Cancer 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K pathway in human disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef]
- Lacerda, C.F.; Cruvinel-Carloni, A.; De Oliveira, A.T.; Scapulatempo-Neto, C.; López, R.V.; Crema, E.; Adad, S.J.; Rodrigues, M.A.; Henry, M.A.; Guimaraes, D.P.; et al. Mutational profile of TP53 in esophageal squamous cell carcinoma associated with chagasic megaesophagus. Dis. Esophagus 2017, 1–9. [Google Scholar] [CrossRef]
- Safatle-Ribeiro, A.V.; Ribeiro, U., Jr.; Sakai, P.; Clarke, M.R.; Fylyk, S.N.; Ishioka, S.; Gama-Rodrigues, J.; Finkelstein, S.D.; Reynolds, J.C. Integrated p53 histopathologic/genetic analysis of premalignant lesions of the esophagus. Cancer Detect. Prev. 2000, 24, 13–23. [Google Scholar]
- da Silva Manoel-Caetano, F.; Borim, A.A.; Caetano, A.; Cury, P.M.; Silva, A.E. Cytogenetic alterations in chagasic achalasia compared to esophageal carcinoma. Cancer Genet. Cytogenet. 2004, 149, 17–22. [Google Scholar] [CrossRef]
- Bellini, M.F.; Silva, A.E.; Varella-Garcia, M. Genomic imbalances in esophageal squamous cell carcinoma identified by molecular cytogenetic techniques. Genet. Mol. Biol. 2010, 33, 205–213. [Google Scholar] [CrossRef]
- Campanella, N.C.; Lacerda, C.F.; Berardinelli, G.N.; Abrahão-Machado, L.F.; Cruvinel-Carloni, A.; De Oliveira, A.T.; Scapulatempo-Neto, C.; Crema, E.; Adad, S.J.; Rodrigues, M.A.; et al. Presence of microsatellite instability in esophageal squamous cell carcinoma associated with chagasic megaesophagus. Biomark. Med. 2018, 12, 573–582. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Le, D.T. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 373, 1979. [Google Scholar] [PubMed]
- Zendehdel, K.; Nyrén, O.; Edberg, A.; Ye, W. Risk of esophageal adenocarcinoma in achalasia patients, a retrospective cohort study in Sweden. Am. J. Gastroenterol. 2011, 106, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, K.; Spaander, M.C.; Leeuwenburgh, I.; Peppelenbosch, M.P.; Fuhler, G.M. Achalasia and associated esophageal cancer risk: What lessons can we learn from the molecular analysis of Barrett’s–associated adenocarcinoma? Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188291. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.; Furrie, E.; Macfarlane, G.T.; Dillon, J.F. Microbial colonization of the upper gastrointestinal tract in patients with Barrett’s esophagus. Clin. Infect. Dis. 2007, 45, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Li, H.; Zhang, Y.; Yang, Y.; Lu, R.; Liu, K.; Lin, S.; Lan, X.; Wang, H.; Wu, H.; et al. Transitional basal cells at the squamous–columnar junction generate Barrett’s oesophagus. Nature 2017, 550, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Han, Y.M.; Kim, W.H.; Park, J.M.; Jeong, M.; Go, E.J.; Hong, S.P.; Hahm, K.B. Oxidative stress from reflux esophagitis to esophageal cancer: The alleviation with antioxidants. Free Radic. Res. 2016, 50, 1071–1079. [Google Scholar] [CrossRef]
- Guo, J.P.; Gilman, P.B.; Thomas, R.M.; Fisher, R.S.; Parkman, H.P. Barrett’s esophagus and achalasia. J. Clin. Gastroenterol. 2002, 34, 439–443. [Google Scholar] [CrossRef]
- Karyampudi, A.; Nabi, Z.; Ramchandani, M.; Darisetty, S.; Goud, R.; Chavan, R.; Kalapala, R.; Rao, G.V.; Reddy, D.N. Gastroesophageal reflux after per-oral endoscopic myotomy is frequently asymptomatic, but leads to more severe esophagitis: A case–control study. United Eur. Gastroenterol. J. 2020, 9, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Räsänen, J.V.; Sihvo, E.I.; Ahotupa, M.O.; Färkkilä, M.A.; Salo, J.A. The expression of 8-hydroxydeoxyguanosine in oesophageal tissues and tumours. Eur. J. Surg. Oncol. EJSO 2007, 33, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Sihvo, E.I.; Salminen, J.T.; Rantanen, T.K.; Rämö, O.J.; Ahotupa, M.; Färkkilä, M.; Auvinen, M.I.; Salo, J.A. Oxidative stress has a role in malignant transformation in Barrett’s oesophagus. Int. J. Cancer 2002, 102, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K.; Lee, J.S. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J. Nutr. 2005, 135, 2993S–3001S. [Google Scholar] [CrossRef]
- Ross-Innes, C.S.; Becq, J.; Warren, A.; Cheetham, R.K.; Northen, H.; O’Donovan, M.; Malhotra, S.; Di Pietro, M.; Ivakhno, S.; He, M.; et al. Whole-genome sequencing provides new insights into the clonal architecture of Barrett’s esophagus and esophageal adenocarcinoma. Nat. Genet. 2015, 47, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Persons, D.L.; Croughan, W.S.; Borelli, K.A.; Cherian, R. Interphase cytogenetics of esophageal adenocarcinoma and precursor lesions. Cancer Genet. Cytogenet. 1998, 106, 11–17. [Google Scholar] [CrossRef]
- Sabel, M.S.; Pastore, K.; Toon, H.; Smith, J.L. Adenocarcinoma of the esophagus with and without Barrett mucosa. Arch. Surg. 2000, 135, 831–836. [Google Scholar] [CrossRef][Green Version]
- Fujishima, F.; Nakamura, Y.; Kasajima, A.; Ozawa, Y.; Ito, K.; Taniuchi, S.; Watanabe, M.; Asonuma, S.; Takubo, K.; Sasano, H. Adenocarcinoma in the squamous-lined esophagus without Barrett’s mucosa, probably arising from esophageal gland duct. Esophagus 2015, 12, 327–331. [Google Scholar] [CrossRef]
- Bockerstett, K.A.; DiPaolo, R.J. Regulation of gastric carcinogenesis by inflammatory cytokines. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kauppi, J.; Räsänen, J.; Sihvo, E.; Nieminen, U.; Arkkila, P.; Ahotupa, M.; Salo, J. Increased oxidative stress in the proximal stomach of patients with Barrett’s esophagus and adenocarcinoma of the esophagus and Esophagogastric junction. Transl. Oncol. 2016, 9, 336–339. [Google Scholar] [CrossRef]
- Thomson, L.; Robinson, T.L.; Lee, J.C.; Farraway, L.A.; Hughes, M.J.; Andrews, D.W.; Huizinga, J.D. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 1998, 4, 848–851. [Google Scholar] [CrossRef]
- Andersson, J.; Sjögren, H.; Meis-Kindblom, J.M.; Stenman, G.; Åman, P.; Kindblom, L.G. The complexity of KIT gene mutations and chromosome rearrangements and their clinical correlation in gastrointestinal stromal (pacemaker cell) tumors. Am. J. Pathol. 2002, 160, 15–22. [Google Scholar] [CrossRef]
- Hulzinga, J.D.; Thuneberg, L.; Klüppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Patruno, R.; Marech, I.; Zizzo, N.; Ammendola, M.; Nardulli, P.; Gadaleta, C.; Introna, M.; Capriuolo, G.; Rubini, R.A.; Ribatti, D.; et al. c-Kit expression, angiogenesis, and grading in canine mast cell tumour: A unique model to study c-Kit driven human malignancies. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Slipicevic, A.; Herlyn, M. KIT in melanoma: Many shades of gray. J. Investig. Dermatol. 2015, 135, 337–338. [Google Scholar] [CrossRef]
- Hirota, S.; Nishida, T.; Isozaki, K.; Taniguchi, M.; Nishikawa, K.; Ohashi, A.; Takabayashi, A.; Obayashi, T.; Okuno, T.; Kinoshita, K.; et al. Familial gastrointestinal stromal tumors associated with dysphagia and novel type germline mutation of KIT gene. Gastroenterology 2002, 122, 1493–1499. [Google Scholar] [CrossRef]
- Hoshino, M.; Omura, N.; Yano, F.; Yamamoto, S.R.; Matsuda, M.; Yanaga, K. Simultaneous diagnosis of familial achalasia: Report of two cases. Surg. Case Rep. 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.L.; Torphy, R.J.; McCarter, M.D.; Sciotto, C.G.; Glode, L.M.; Robinson, W.A. A familial germline mutation in KIT associated with achalasia, mastocytosis and gastrointestinal stromal tumors shows response to kinase inhibitors. Cancer Genet. 2019, 233, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.B.; Diaz-Arias, A.A.; Bochna, G.S.; Vogele, K.A. Achalasia due to diffuse esophageal leiomyomatosis and inherited as an autosomal dominant disorder: Report of a family study. Gastroenterology 1990, 98, 1358–1365. [Google Scholar] [CrossRef]
- Wehrle-Haller, B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Res. 2003, 16, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Doma, V.; Barbai, T.; Beleaua, M.A.; Kovalszky, I.; Rásó, E.; Tímár, J. KIT mutation incidence and pattern of melanoma in Central Europe. Pathol. Oncol. Res. 2019, 26, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Neuhann, T.M.; Mansmann, V.; Merkelbach-Bruse, S.; Klink, B.; Hellinger, A.; Höffkes, H.G.; Wardelmann, E.; Schildhaus, H.U.; Tinschert, S. A novel germline KIT mutation (p. L576P) in a family presenting with juvenile onset of multiple gastrointestinal stromal tumors, skin hyperpigmentations, and esophageal stenosis. Am. J. Surg. Pathol. 2013, 37, 898–905. [Google Scholar] [CrossRef]
- Ávila, S.A.; Peñaloza, J.; González, F.; Abdo, I.; Rainville, I.; Root, E.; Valenzuela, R.D.; Garber, J. Dysphagia, melanosis, gastrointestinal stromal tumors and a germinal mutation of the KIT gene in an Argentine family. Acta Gastroenterol. Latinoam. 2014, 44, 9–15. [Google Scholar]
- Robson, M.E.; Glogowski, E.; Sommer, G.; Antonescu, C.R.; Nafa, K.; Maki, R.G.; Ellis, N.; Besmer, P.; Brennan, M.; Offit, K. Pleomorphic characteristics of a germ-line KIT mutation in a large kindred with gastrointestinal stromal tumors, hyperpigmentation, and dysphagia. Clin. Cancer Res. 2004, 10, 1250–1254. [Google Scholar] [CrossRef]
- Yamanoi, K.; Higuchi, K.; Kishimoto, H.; Nishida, Y.; Nakamura, M.; Sudoh, M.; Hirota, S. Multiple gastrointestinal stromal tumors with novel germline c-kit gene mutation, K642T, at exon 13. Hum. Pathol. 2014, 45, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Vilain, R.E.; Dudding, T.; Braye, S.G.; Groombridge, C.; Meldrum, C.; Spigelman, A.D.; Ackland, S.; Ashman, L.; Scott, R.J. Can a familial gastrointestinal tumour syndrome be allelic with Waardenburg syndrome? Clin. Genet. 2011, 79, 554–560. [Google Scholar] [CrossRef] [PubMed]
- O’Riain, C.; Corless, C.L.; Heinrich, M.C.; Keegan, D.; Vioreanu, M.; Maguire, D.; Sheahan, K. Gastrointestinal stromal tumors: Insights from a new familial GIST kindred with unusual genetic and pathologic features. Am. J. Surg. Pathol. 2005, 29, 1680–1683. [Google Scholar] [CrossRef]
- Neves, M.C.; Stamp, G.; Mudan, S. Sporadic diffuse segmental interstitial cell of Cajal hyperplasia harbouring two gastric gastrointestinal stromal tumours (GIST) mimicking hereditary GIST syndromes. Int. J. Surg. Case Rep. 2015, 16, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Wardelmann, E.; Ma, Y.; Merkelbach–Bruse, S.; Preussner, L.M.; Woolery, C.; Baldus, S.E.; Heinicke, T.; Thiele, J.; Buettner, R.; et al. Novel germline mutation of KIT associated with familial gastrointestinal stromal tumors and mastocytosis. Gastroenterology 2005, 129, 1042–1046. [Google Scholar] [CrossRef]
- Ricci, R. Syndromic gastrointestinal stromal tumors. Hered. Cancer Clin. Pract. 2016, 14, 1–5. [Google Scholar] [CrossRef]
- Fletcher, C.D.; Berman, J.J.; Corless, C.; Gorstein, F.; Lasota, J.; Longley, B.J.; Miettinen, M.; O’Leary, T.J.; Remotti, H.; Rubin, B.P.; et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Int. J. Surg. Pathol. 2002, 10, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, P.; Gronchi, A.; Hohenberger, P.; Bonvalot, S.; Schöffski, P.; Bauer, S.; Fumagalli, E.; Nyckowski, P.; Nguyen, B.P.; Kerst, J.M.; et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): The EORTC STBSG experience. Ann. Surg. Oncol. 2013, 20, 2937–2943. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H.; Eriksson, M.; Hall, K.S.; Reichardt, A.; Hermes, B.; Schütte, J.; Cameron, S.; Hohenberger, P.; Jost, P.J.; Al-Batran, S.E.; et al. Survival Outcomes Associated With 3 Years vs 1 Year of Adjuvant Imatinib for Patients With High-Risk Gastrointestinal Stromal Tumors: An Analysis of a Randomized Clinical Trial After 10-Year Follow-up. JAMA Oncol. 2020, 6, 1241–1246. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Twose, I.; Matito, A.; Morgado, J.M.; Sánchez-Muñoz, L.; Jara-Acevedo, M.; García-Montero, A.; Mayado, A.; Caldas, C.; Teodósio, C.; Muñoz-González, J.I.; et al. Imatinib in systemic mastocytosis: A phase IV clinical trial in patients lacking exon 17 KIT mutations and review of the literature. Oncotarget 2017, 8, 68950. [Google Scholar] [CrossRef]
- Popescu, L.M.; Vidulescu, C.; Curici, A.; Caravia, L.; Simionescu, A.A.; Ciontea, S.M.; Simion, S. Imatinib inhibits spontaneous rhythmic contractions of human uterus and intestine. Eur. J. Pharmacol. 2006, 546, 177–181. [Google Scholar] [CrossRef]
- Howarth, W. Discussion on dilatation of the oesophagus without anatomical stenosis. Proc. R. Soc. Med. 1919, 12, 64. [Google Scholar] [CrossRef]
- Schizas, D.; Theochari, N.A.; Katsaros, I.; Mylonas, K.S.; Triantafyllou, T.; Michalinos, A.; Kamberoglou, D.; Tsekrekos, A.; Rouvelas, I. Pseudoachalasia: A systematic review of the literature. Esophagus 2020, 17, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ponds, F.A.; Van Raath, M.I.; Mohamed, S.M.; Smout, A.J.; Bredenoord, A.J. Diagnostic features of malignancy-associated pseudoachalasia. Aliment. Pharmacol. Ther. 2017, 45, 1449–1458. [Google Scholar] [CrossRef]
- Fabian, E.; Eherer, A.J.; Lackner, C.; Urban, C.; Smolle-Juettner, F.M.; Krejs, G.J. Pseudoachalasia as first manifestation of a malignancy. Dig. Dis. 2019, 37, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ponce, J.; Garrigues, V.; Nos, P.; García, E.; Siles, S.; del Val, A. Esophageal pseudoachalasia related to a neoplasm. Rev. Esp. Enferm. Dig. Organo Of. Soc. Esp. Patol. Dig. 1993, 83, 1–4. [Google Scholar]
- Li, Q.; Michel, K.; Annahazi, A.; Demir, I.E.; Ceyhan, G.O.; Zeller, F.; Komorowski, L.; Stöcker, W.; Beyak, M.J.; Grundy, D.; et al. Anti-Hu antibodies activate enteric and sensory neurons. Sci. Rep. 2016, 6, 1–2. [Google Scholar] [CrossRef]
- Liu, W.; Fackler, W.; Rice, T.W.; Richter, J.E.; Achkar, E.; Goldblum, J.R. The pathogenesis of pseudoachalasia: A clinicopathologic study of 13 cases of a rare entity. Am. J. Surg. Pathol. 2002, 26, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Altermatt, H.J.; Rodriguez, M.; Scheithauer, B.W.; Lennon, V.A. Paraneoplastic anti-Purkinje and type I anti-neuronal nuclear autoantibodies bind selectively to central, peripheral, and autonomic nervous system cells. Lab. Investig. J. Tech. Methods Pathol. 1991, 65, 412–420. [Google Scholar]
- Chan, K.H.; Vernino, S.; Lennon, V.A. ANNA-3 anti-neuronal nuclear antibody: Marker of lung cancer-related autoimmunity. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2001, 50, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gergely, M.; Mello, M.D.; Rengarajan, A.; Gyawali, C.P. Duration of symptoms and manometric parameters offer clues to diagnosis of pseudoachalasia. Neurogastroenterol. Motil. 2021, 33, e13965. [Google Scholar] [CrossRef]
- Hirano, T.; Miyauchi, E.; Inoue, A.; Igusa, R.; Chiba, S.; Sakamoto, K.; Sugiura, H.; Kikuchi, T.; Ichinose, M. Two cases of pseudo-achalasia with lung cancer: Case report and short literature review. Respir. Investig. 2016, 54, 494–499. [Google Scholar] [CrossRef]
- Wenzl, E.; Starlinger, M.; Feil, W.; Stacher, G.; Schiessel, R. Secondary achalasia caused by diffuse infiltrating cardial cancer. Chir. Z. Alle Geb. Oper. Medizen 1988, 59, 536–540. [Google Scholar]
- Eustace, S.; Fitzgerald, E. Primary rhabdomyosarcoma of the diaphragm: An unusual cause of adolescent pseudo-achalasia. Pediatric Radiol. 1993, 23, 622–623. [Google Scholar] [CrossRef] [PubMed]
- Nazareno, J.; Taves, D.; Preiksaitis, H.G. Metastatic breast cancer to the gastrointestinal tract: A case series and review of the literature. World J. Gastroenterol. WJG 2006, 12, 6219. [Google Scholar] [CrossRef]
- Campo, S.M.; Zullo, A.; Scandavini, C.M.; Frezza, B.; Cerro, P.; Balducci, G. Pseudoachalasia: A peculiar case report and review of the literature. World J. Gastrointest. Endosc. 2013, 5, 450. [Google Scholar] [CrossRef]
- Campo, S.M.; Lorenzetti, R.; de Matthaeis, M.; Hassan, C.; Zullo, A.; Cerro, P.; Morini, S. Palliation with oesophageal metal stent of pseudoachalasia from gastric carcinoma at the cardia: A case report. Diagn. Ther. Endosc. 2009, 2009, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Costantini, M.; Zaninotto, G.; Ruol, A.; Guirroli, E.; Rampado, S.; Ancona, E. Pseudoachalasia: Not only esophago-gastric cancer. Dis. Esophagus 2007, 20, 168–172. [Google Scholar] [CrossRef]
- Pastor, D.M.; Eggers, A.D.; Drabick, J.J.; Loughran, T.P.; Bayerl, M.G.; Shope, T.R. Retroperitoneal diffuse large B-cell lymphoma presenting as pseudoachalasia. J. Clin. Oncol. 2010, 28, e184–e187. [Google Scholar] [CrossRef]
- Moorman, A.J.; Oelschlager, B.K.; Rulyak, S.J. Pseudoachalasia caused by retroperitoneal B-cell lymphoma. Clin. Gastroenterol. Hepatol. 2008, 6, A32. [Google Scholar] [CrossRef]
- Paulsen, J.M.; Aragon, G.C.; Ali, M.A.; Brody, F.J.; Borum, M.L. Pseudoachalasia secondary to metastatic breast carcinoma. Dig. Dis. Sci. 2010, 55, 1179–1181. [Google Scholar] [CrossRef]
- Lahbabi, M.; Ihssane, M.; Adil, I.S.; Allah, B.D. Pseudoachalasia secondary to metastatic breast carcinoma mimicking radiation stenosis. Clin. Res. Hepatol. Gastroenterol. 2012, 36, e117–e121. [Google Scholar] [CrossRef] [PubMed]
- García-Alonso, F.J.; Hernández Tejero, M.; Castanon-Deprit, A. Hepatobiliary and Pancreatic: Pseudoachalasia from pancreatic cancer. J. Gastroenterol. Hepatol. 2015, 30, 1336. [Google Scholar] [CrossRef] [PubMed]
- Anaizi, A.; Rizvi-Toner, A.; Valestin, J.; Schey, R. Large cell neuroendocrine carcinoma of the lung presenting as pseudoachalasia: A case report. J. Med. Case Rep. 2015, 9, 1–3. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bholat, O.S.; Haluck, R.S. Pseudoachalasia as a result of metastatic cervical cancer. JSLS J. Soc. Laparoendosc. Surg. 2001, 5, 57. [Google Scholar]
- Haberstroh, W.; Shafa, S. Pseudoachalasia secondary to metastatic bladder cancer. BMJ Open Gastroenterol. 2019, 6, e000284. [Google Scholar] [CrossRef] [PubMed]
- Branchi, F.; Tenca, A.; Bareggi, C.; Mensi, C.; Mauro, A.; Conte, D.; Penagini, R. A case of pseudoachalasia hiding a malignant pleural mesothelioma. Tumori J. 2016, 102 (Suppl. 2), S50–S53. [Google Scholar] [CrossRef]
- Campos, C.T.; Ellis, F.H., Jr.; LoCicero, J. III. Pseudoachalasia: A report of two cases with comments on possible causes and diagnosis. Dis. Esophagus 1997, 10, 220–224. [Google Scholar] [CrossRef]
- Cotta Rebollo, J.; Toscano Castilla, E.; Lozano Lanagran, M.; Martín Ocaña, F.; Pérez Aísa, Á.C.; Fernández Cano, F.; González Artacho, C.; Rosón Rodríguez, P.; Melgarejo Corder, F. Seudoacalasia en paciente con antecedentes de linfoma no Hodgkin. Gastroenterol. Y. Hepatol. 2016, 39, 274–275. [Google Scholar] [CrossRef] [PubMed]
- Then, E.O.; Ofosu, A.; Rawla, P.; Sunkara, T.; Dadana, S.; Culliford, A.; Gaduputi, V. Burkitt’s Lymphoma of the Gastrohepatic Omentum: A Malignant Presentation of Pseudoachalasia. Case Rep. Gastrointest. Med. 2019, 2019. [Google Scholar] [CrossRef]
- Ulla, J.L.; Fernandez-Salgado, E.; Alvarez, V.; Ibañez, A.; Soto, S.; Carpio, D.; Vazquez-Sanluis, J.; Ledo, L.; Vazquez-Astray, E. Pseudoachalasia of the cardia secondary to nongastrointestinal neoplasia. Dysphagia 2008, 23, 122–126. [Google Scholar] [CrossRef]
- Ter, R.B.; Govil, Y.K.; Leite, L.; Infantolino, A.; Ghabra, M.; Galan, A.; Katz, P.O. Adenosquamous carcinoma in Barrett’s esophagus presenting as pseudoachalasia. Am. J. Gastroenterol. 1999, 94, 268–270. [Google Scholar] [CrossRef]
- Agrusa, A.; Romano, G.; Frazzetta, G.; De Vita, G.; Chianetta, D.; Di Buono, G.; Di Giovanni, S.; Sorce, V.; Gulotta, G. Achalasia secondary to submucosal invasion by poorly differentiated adenocarcinoma of the cardia, Siewert II: Consideration on preoperative workup. Case Rep. Surg. 2014, 2014. [Google Scholar] [CrossRef]
- Song, C.W.; Chun, H.J.; Kim, C.D.; Ryu, H.S.; Hyun, J.H.; Kahrilas, P.J. Association of pseudoachalasia with advancing cancer of the gastric cardia. Gastrointest. Endosc. 1999, 50, 486–491. [Google Scholar] [CrossRef]
- de Borst, J.M.; Wagtmans, M.J.; Fockens, P.; van Lanschot, J.J.; West, R.; Boeckxstaens, G.E. Pseudoachalasia caused by pancreatic carcinoma. Eur. J. Gastroenterol. Hepatol. 2003, 15, 825–828. [Google Scholar] [CrossRef]
- El-Newihi, H.M.; Dellinger, G.W.; Mihas, A.A.; Achord, J.L. Gastric cancer and pernicious anemia appearing as pseudoachalasia. South. Med. J. 1996, 89, 906–910. [Google Scholar] [CrossRef]
- Stone, M.L.; Kilic, A.; Jones, D.R.; Lau, C.L.; Kozower, B.D. A diagnostic consideration for all ages: Pseudoachalasia in a 22-year-old male. Ann. Thorac. Surg. 2012, 93, e11–e12. [Google Scholar] [CrossRef][Green Version]
- Moonka, R.; Pellegrini, C.A. Malignant pseudoachalasia. Surg. Endosc. 1999, 13, 273–275. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, G.H.; Am Song, G.; Nam, H.S.; Yi, Y.S.; Ahn, K.H.; Kim, S.; Kim, J.Y.; Park, D.Y. Primary squamous cell carcinoma of the liver initially presenting with pseudoachalasia. Gut Liver 2012, 6, 275. [Google Scholar] [CrossRef]
- Hejazi, R.A.; Zhang, D.; McCallum, R.W. Gastroparesis, pseudoachalasia and impaired intestinal motility as paraneoplastic manifestations of small cell lung cancer. Am. J. Med. Sci. 2009, 338, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Kotoulas, C.; Galanis, G.; Yannopoulos, P. Secondary achalasia due to a mesenchymal tumour of the oesophagus. Eur. J. Surg. Oncol. 2000, 26, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Iascone, C.; Maffi, C.; Pascazio, C.; Sciacca, V. Recurrent gastric carcinoma causing pseudoachalasia: Case report. Dis. Esophagus 2000, 13, 87–90. [Google Scholar] [CrossRef]
- Leung, V.K.; Kan, P.S.; Lai, M.S. Cholangiocarcinoma presenting as pseudoachalasia and gastroparesis. Hong Kong Med. J. 2003, 9, 296–298. [Google Scholar] [PubMed]
- Hsu, Y.C.; Li, A.F.; Lin, H.J. Pseudoachalasia from gastric cancer. Clin. Gastroenterol. Hepatol. 2009, 7, A24. [Google Scholar] [CrossRef]
- Lawal, A.; Antonik, S.; Dua, K.; Massey, B.T. Esophageal adenocarcinoma: Pseudo-nutcracker esophagus. Dysphagia 2009, 24, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Gillies, C.L.; Farrukh, A.; Abrams, K.R.; Mayberry, J.F. Risk of esophageal cancer in achalasia cardia: A meta-analysis. JGH Open 2019, 3, 196–200. [Google Scholar] [CrossRef]
- Sterne, J.A.; Gavaghan, D.; Egger, M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000, 53, 1119–1129. [Google Scholar] [CrossRef]
- Torres-Aguilera, M.; Troche, J.M. Achalasia and esophageal cancer: Risks and links. Clin. Exp. Gastroenterol. 2018, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Boller, D.A.; Borovicka, J.; Studer, R.; Zuercher, U.; Kradolfer, D.; Neuweiler, J.; Spieler, P.; Schoenegg, R.; Grossenbacher, R.; Meyenberger, C. Lugol chromoendoscopy combined with image cytometry and p53 LOH in patients at risk for esophageal squamous cell carcinoma. Gastrointest. Endosc. 2006, 63, AB130. [Google Scholar] [CrossRef]
- Gockel, I.; Exner, C.; Junginger, T. Morbidity and mortality after esophagectomy for esophageal carcinoma: A risk analysis. World J. Surg. Oncol. 2005, 3, 1–8. [Google Scholar]
- Kennovin, G.D.; Hirst, D.G.; Stratford, M.R.; Flitney, F.W. Inducible nitric oxide synthase is expressed in tumor-associated vasculature: Inhibition retards tumor growth in vivo. Biol. Nitric Oxide Part 1994, 4, 473–479. [Google Scholar]
- De Wilt, J.H.; Manusama, E.R.; Van Etten, B.; Van Tiel, S.T.; Jorna, A.S.; Seynhaeve, A.L.; ten Hagen, T.L. Nitric oxide synthase inhibition results in synergistic anti-tumour activity with melphalan and tumour necrosis factor alpha-based isolated limb perfusions. Br. J. Cancer 2000, 83, 1176–1182. [Google Scholar] [CrossRef][Green Version]
- Orucevic, A.; Lala, P.K. Effects ofNG-Nitro-L-arginine Methyl Ester, an Inhibitor of Nitric Oxide Synthesis, on IL-2-Induced LAK Cell Generationin Vivoandin Vitroin Healthy and Tumor-Bearing Mice. Cell. Immunol. 1996, 169, 125–132. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).