Abstract
A patent foramen ovale (PFO) is a common, incidental echocardiographic finding in otherwise healthy and asymptomatic infants and children. However, a variety of clinical conditions have been ascribed to the presence of a PFO in childhood, such as cryptogenic stroke, platypnea-orthodeoxia syndrome, decompression sickness and migraine, although the data on these are controversial and sometimes contradictory. This review discusses embryology and correlation with post-natal anatomy, anatomical variations of the atrial septum, diagnostic modalities in special circumstances of PFO associated clinical syndromes, and the role of PFO in congenital heart disease, pulmonary hypertension, dilated cardiomyopathy and heart failure in children who require an extracorporeal membrane oxygenator or ventricular assist device as life support.
1. Introduction
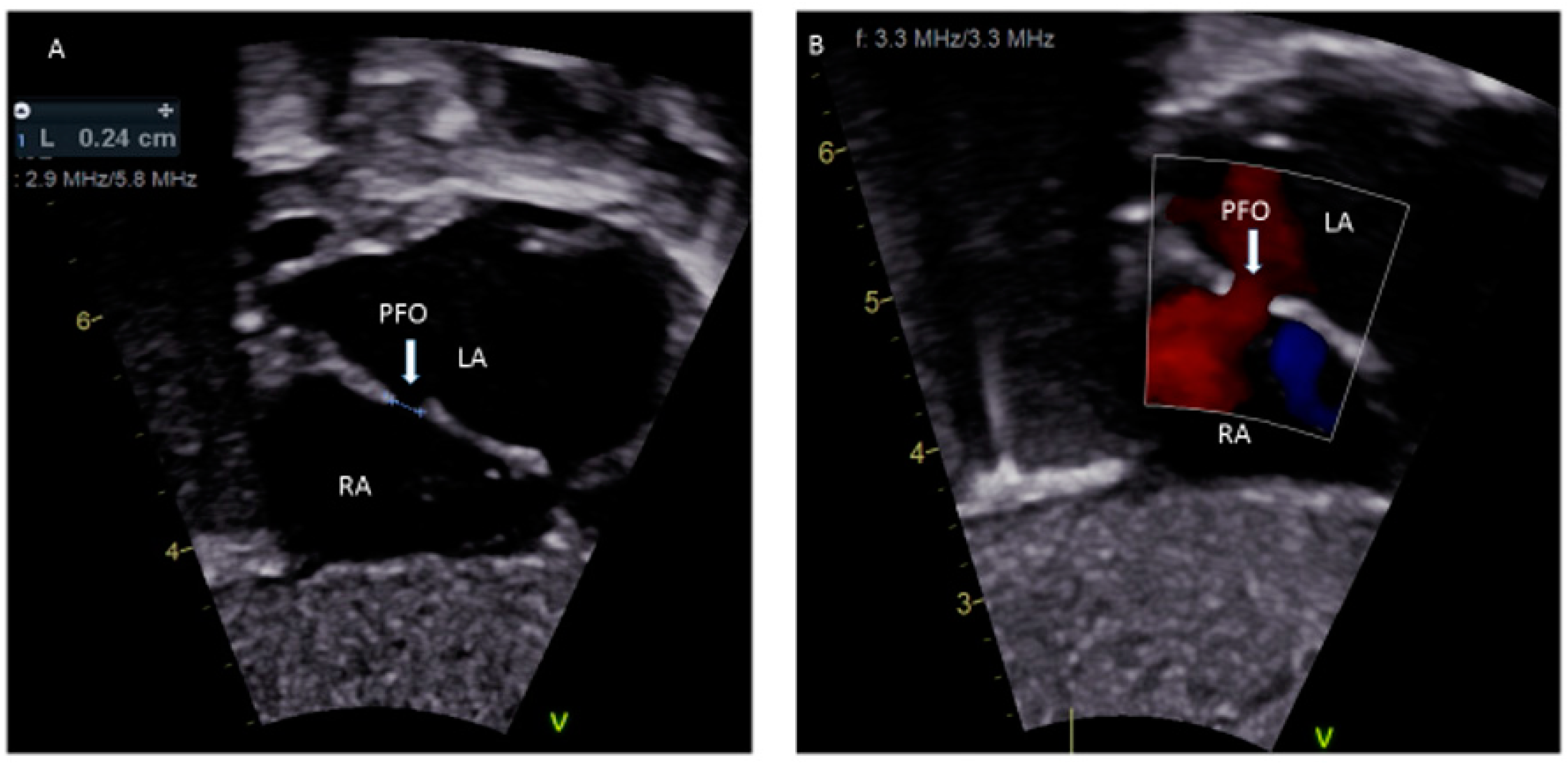
A patent foramen ovale (PFO) is a commonly discovered potential opening between the right atrium (RA) and left atrium (LA) on routine echocardiographic surveillance in an otherwise asymptomatic infant or child (Figure 1A,B). Screening for a PFO in an asymptomatic patient or follow-up for an isolated PFO is not justified [1]. There are limited data on the underlying causes of a PFO, although there is some good evidence that genetic factors play a role [2]. The prevalence of PFO in the general population is 25% and the median diameter of a PFO is 5 mm (mean ± standard deviation, 4.9 ± 2.6 mm); very rarely, a PFO can enlarge on follow-up [3,4]. There has been much discourse about the differences between a benign PFO and a pathological secundum atrial septal defect (ASD). The criteria to differentiate these two are the size of the hole, the presence or absence of a flap of septal tissue and the size of the interatrial shunt [5]. A systematic search of “foramen ovale (FO)”, “FO in fetal life”, “PFO”, “high risk-PFO in children”, “diagnosis of PFO”, “PFO and congenital heart defect (CHD)”, “PFO and migraine”, “stroke/transient ischemic attack (TIA) in children”, “platypnea-orthodeoxia”, “residual PFO after CHD repair”, “PFO and decompression illness”, “PFO and hypoxemia”, “PFO and thromboembolism” and “treatment of PFO in children” was performed through the PubMed and Cochrane databases to obtain information relevant to children, which is included in this review. Randomized control trials (RCT) comparing the treatment of PFO using either devices or medical therapy in children with many PFO-associated syndromes are not available. PFO-associated clinical syndromes are rare, most studies are observational, and it is difficult to inform a relative risk, absolute risk, odds ratio or a p-value of the disease associations with PFO based on which a definite clinical recommendation can be made. Existing guidelines are mainly for adults with cryptogenic strokes in the setting of PFO, and many trials are still on-going in adults. It is not ideal to extrapolate adult studies to children as there is a significant difference in the physiology contributing to thrombus formation and paradoxical embolism in adults compared to children. There is a need to conduct RCT of the role of PFO in clinical syndromes in children.
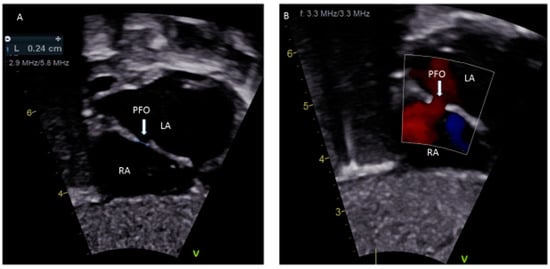
Figure 1.
(A) Sub-costal view showing a 2.4 mm PFO; (B) Color Doppler demonstrating left to right shunting.
The importance of a PFO in some critical congenital heart defects (CHD), especially in neonates, is well recognized. However, there is controversy regarding the clinical significance of a PFO with vascular events [6,7,8], specifically when accompanied by anatomic anomalies [9] such as atrial septal aneurysms, Chiari networks and prominent Eustachian valves. A PFO associated with CHD is common and the physiology is very different from an isolated PFO [10]. The presence of a PFO has also been implicated in the pathogenesis of medical conditions such as platypnea-orthodeoxia syndrome [11], decompression sickness [3] or migraine [12] in children. Currently, there are no accepted guidelines for the management of PFO in the pediatric age group. This review describes the embryology, anatomy, variations of atrial septum associated with PFO, the relevance of PFO in fetal life, diagnostic modalities in special circumstances when a PFO is associated with CHD or other clinical scenarios during infancy and childhood and outlines an overall approach to the management of PFO-associated syndromes.
2. PFO in Fetal Life
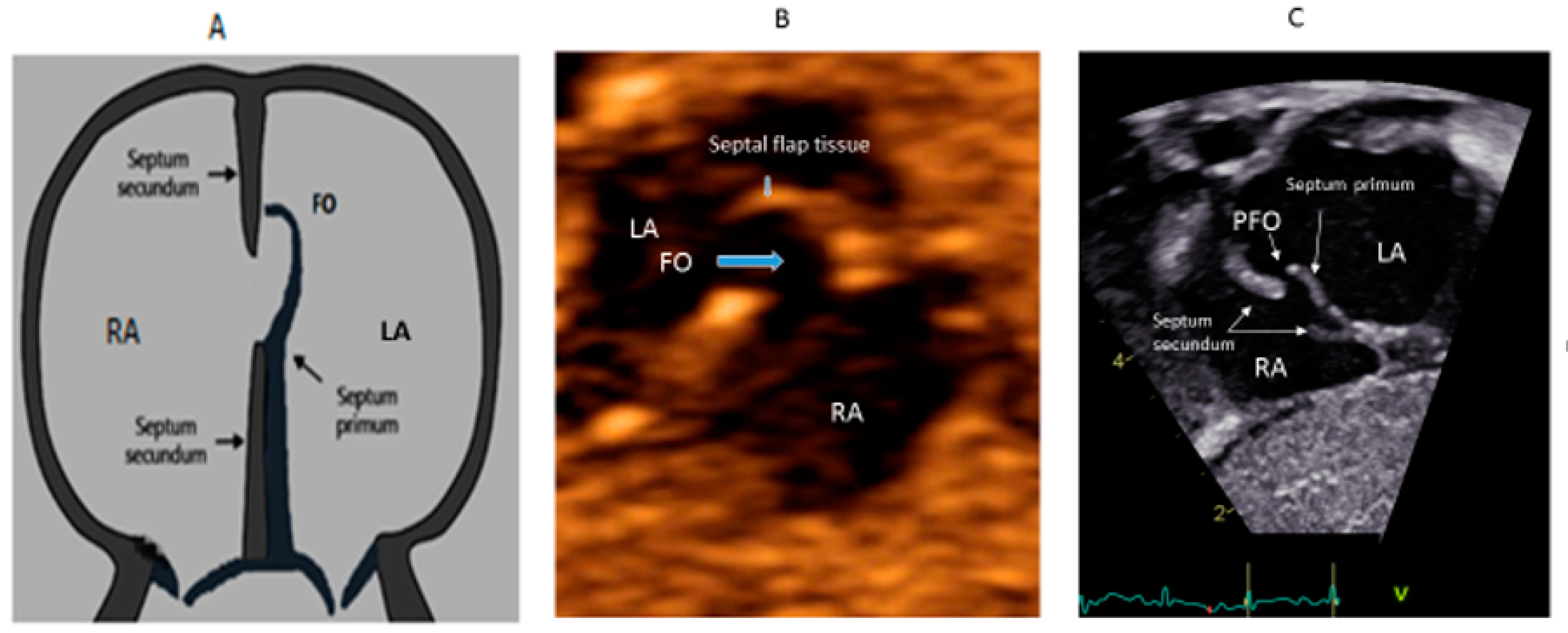
In the embryonic stage, between 4 and 5 weeks of gestation, the septum primum begins to grow from the posterosuperior region of the primitive common atrium and grows in the midline towards the endocardial cushions at the crux of the heart. Smaller fenestrations develop in the mid-portion of the septum primum, even as the most inferior part merges with the developing endocardial cushions. The septum secundum also arises from the posterosuperior region of the common atrial wall, just to the right of the septum primum, and grows towards the endocardial cushions. Its inferior edge is concave and forms the crista dividens (limbus of the fossa ovalis). A second orifice forms as a flap valve mechanism in the upper portion of the septum primum (Figure 2A), which remains patent during fetal life as an FO (Figure 2B).
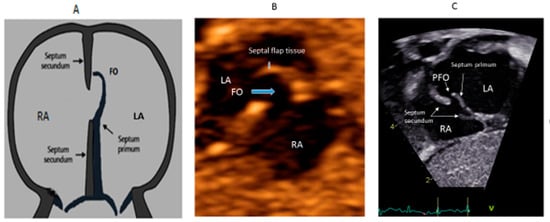
Figure 2.
Correlation between development of atrial septum (A), Fetal (B) and post-natal anatomy (C).
During fetal life, the Eustachian valve on the leftward ridge of the inferior vena cava facilitates the shunting of oxygenated blood from the umbilical vein to the left atrium (LA) via FO. This allows more oxygenated blood to return to the left ventricle (LV). This physiological right to left shunt in fetal life is large, unobstructed and essential [13]. At birth, the instant increase in pulmonary blood flow due to the onset of spontaneous ventilation is associated with increased venous return to LA. The distension of the LA with higher volume, along with the decreased vena caval flow into RA after cord clamping, results in the movement of the primum septum rightwards and allows the apposition and subsequent fusion of the primum with the secundum membranes. A deficiency in the membranous portion of the primum and the limbus results in a PFO (Figure 2C).
The FO in fetal life can be reliably demonstrated by echocardiography using ultrasound modalities such as 2D, color and spectral Doppler during the second half of pregnancy [14]. The fetal FO is however difficult to demonstrate circumferentially despite advances in ultrasound imaging; hence, the transverse diameter is commonly used [14]. Fetal premature closure or restriction of the FO has been reported at any gestational age with variable effects on fetal hemodynamics and adverse fetal and postnatal outcomes. The spectrum of clinical presentation is related to the gestational age when the restriction first occurs and the severity of restriction. Significant flow restriction results in an additional amount of blood being re-directed to the right ventricle (RV) as it bypasses the LA, causing right heart failure manifesting as pleural/pericardial effusions, ascites, necrotizing enterocolitis and non-immune hydrops fetalis. There is also an association between the premature closure of the FO with neonatal mortality and complications such as prematurity, maternal preeclampsia and placental abruption [15].
Premature FO closure has been postulated as a cause of hypoplastic left heart syndrome (HLHS) [16] and neonatal pulmonary hypertension [17]. The diagnostic criteria for restrictive fetal FO include an FO diameter of <2 mm, a Doppler velocity >1.2 m/s, RA and RV dilatation, tricuspid valve regurgitation, pericardial effusion and/or hydrops fetalis [14]. More advanced quantification using color and spectral Doppler in the pulmonary artery and pulmonary veins, using the pulsatility index, is useful to monitor FOs [18]. Restriction of a fetal FO can however be difficult to ascertain in the presence of an atrial septal aneurysm because the fossa ovalis may appear widely patent. Close surveillance using fetal echocardiography could help determine progression, prognosticate outcomes and hence guide the management of the fetus and infant. Fetal cardiac intervention is an evolving management tool that has been used in HLHS with an intact or restrictive FO to alleviate or reduce the hemodynamic consequences [19]. The risk assessment of the fetus and the mother influences the optimal timing and method of delivery of the infant [20].
3. PFO in Neonates and Infants
The hemodynamic changes after birth lead to a smooth transition of fetal circulation and primarily depend upon the drop in pulmonary vascular resistance (PVR) and an increase in systemic vascular resistance (SVR). The presence of PFO plays an important role in understanding the normal cardiovascular transition physiology and its adverse adaptation. The shunt across the PFO is either left-to-right or bidirectional but never purely right-to-left. A pure right-to-left shunt across the PFO should raise suspicion of underlying CHDs such as total anomalous pulmonary venous drainage or tricuspid atresia. Assessment of the flow across the PFO, especially when it is bidirectional, may indicate persistent pulmonary hypertension of the newborn, and it contributes to monitoring in premature infants and infants with perinatal asphyxia.
In neonates with right heart obstructive lesions, such as tricuspid or pulmonary atresia, and left heart obstructive lesions, such as HLHS, the continued patency of the FO is critical so that an obligatory right-to-left or left-to-right shunt, respectively, across the atrial septum, can take place. In neonates with transposition of the great arteries (TGA), the circulation is parallel (instead of normal in-series circulation) and mixing across the circulations is essential for survival; this is usually provided by the ASD/PFO. With TGA, it is important to assess the atrial level shunt and a PFO ≤2mm warrants an urgent balloon atrial septostomy to improve survival [21]. The atrial septum in infants with HLHS can be very thick and, if PFO is restrictive, these newborns become critically ill [22]. In this case, instead of a balloon atrial septostomy, a blade septostomy or the creation of a separate defect in the atrial septum by Brockenbrough (needle atrial transeptal) puncture or radiofrequency wire perforation is needed.
4. PFO in Childhood
A PFO is frequently detected during childhood by transthoracic echocardiography (TTE) for the evaluation of a murmur or other medical condition. In the majority of children, TTE with or without contrast saline is sufficient to diagnose a PFO because of the excellent acoustic windows [23]. The most ideal window to visualize the PFO is the subcostal acoustic window. This is because the septum is relatively perpendicular to the transducer and adequately echo-reflective from that position. This reduces the likelihood of a false dropout and a misdiagnosis of a PFO. The confirmation of the PFO is required by documenting evidence of the transseptal flow by color Doppler. Both the subcostal long-axis and short-axis views can be utilized for a confirmatory diagnosis. The apical views often have false dropouts of the acoustic signals due to the parallel orientation of the transducer beam and the atrial septum position. This is not the most ideal location for the evaluation of a PFO. However, the apical-4 chamber view is helpful during the saline contrast “bubble” study in looking for the appearance of acoustic reflective signals (“bubbles”) in the LA and LV (representing right-to-left flow). There is also a need for the standardization of PFO identification and quantification for a saline contrast bubble study, which at present does not exist. In the French PFO- Atrial Septal Aneurysm (ASA) study, a PFO is identified if at least three bubbles appear in the LA. The degree of shunting is defined to be small if three to nine bubbles appear, it is moderate if 10–30 bubbles appear and large if >30 bubbles appear in the LA [24]. According to PFO in cryptogenic stroke study (PICSS), a PFO is considered to be present if more than one bubble appears in the LA and if >10 appear in the LA, it is considered as a large PFO [25]. Recently, it was shown that for a given PFO, the amount of right-to-left contrast shunting is a matter of expiratory pressure during the Valsalva maneuver [26].
Trans-esophageal echocardiogram (TEE) is traditionally considered the gold standard for detection of a PFO in the setting of stroke in the adult patients [27,28]. The TEE is necessary for children in order to delineate the anatomy of the interatrial septum during a surgical or interventional cardiac catheterization procedure. In some surgeries, such as tetralogy of Fallot (TOF) repair, a small PFO may be left behind or a punch hole made in the interatrial septum to allow right-to-left shunting and maintain cardiac output in the context of a hypertrophied RV with poor relaxation or potential increased afterload from smallish pulmonary arteries. In these situations, a right-to-left shunt or a bidirectional atrial shunt can be seen on the color Doppler. In other situations, such as a Shone complex with small left-sided structures, there is left-to-right atrial shunting at higher velocities and this evaluation needs to be included in the assessment of transmitral gradients. One major drawback of routine TEE in children is the need for deep sedation or general anesthesia. TEE is a reliable imaging modality to assist with the device closure of a PFO in the cardiac catheterization lab, particularly if a young patient is being anesthetized. In these cases, TEE is useful for determining the precise location (usually the cranial edge of the fossa ovalis), anatomy (slit-like, tunnel-like, aneurysmal or fenestrated) and the direction of shunting, and it can also assist in wire, sheath and device manipulations [29,30,31]. TEE is helpful to evaluate rarely for extension of vegetation in endocarditis, thrombus or left atrial myxoma. TEE is also a useful adjunct to procedures in which the PFO is traversed or dilated or an atrial shunt is created to allow access to the LA to obtain left-sided hemodynamic data and to perform left-sided interventions to the mitral valve, pulmonary vein stents, etc.
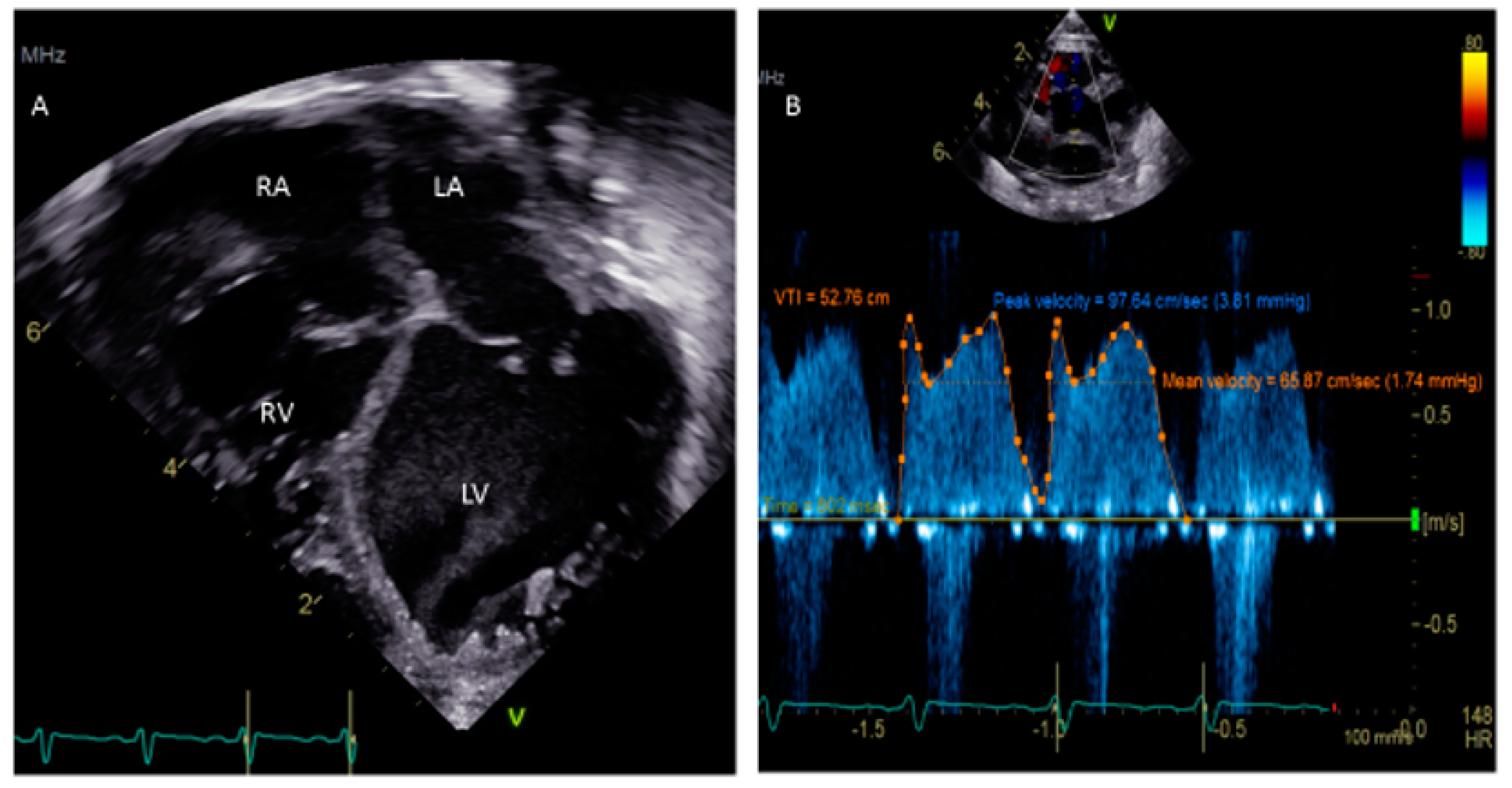
One critical scenario in which a PFO needs to be looked for is in any kind of dilated cardiomyopathy (Figure 3), in which atrial distension is associated with decreased myocardial perfusion. The presence of a PFO or the creation of an atrial level shunt can decrease atrial distension and encourage myocardial recovery in some cases, especially after the placement of an extracorporeal membrane oxygenator (ECMO) [32]. Left ventricular assist device (VAD) implantation is another option for some pediatric patients with refractory heart failure. In these patients, the implantation of a VAD abruptly decreases diastolic filling pressures and can cause right-to-left shunting with profound systemic hypoxemia. The pre-VAD TEE diagnosis of a PFO may be challenged by left atrial distension compressing the defect, which may become more apparent when the LA filling pressures decrease after the VAD placement. A PFO is closed at the time of VAD placement if its presence is detected in time; otherwise, device closure may be subsequently needed [33].
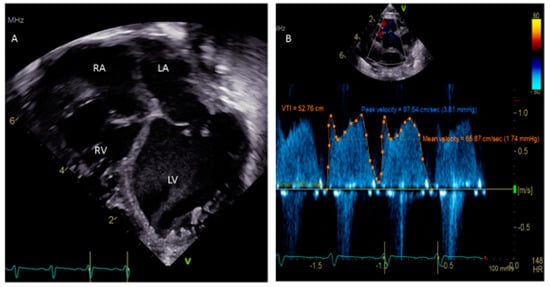
Figure 3.
(A) Apical 4-chamber view showing dilated cardiomyopathy in a 5 week old child. (B) Doppler of PFO showing peak velocity 3.81 mm Hg and mean 1.74 mm Hg.
Advanced imaging techniques for the diagnosis of PFO: Other alternative modalities are rarely employed in the evaluation of PFOs in certain settings, particularly in pediatric patients with cryptogenic strokes. Transcranial Doppler (TCD) echocardiography allows the noninvasive diagnosis of a right-to-left shunt caused by a PFO by detecting bubble signals in the middle cerebral artery after the injection of agitated saline into the antecubital vein [34]. The authors of this study have reported excellent sensitivity and specificity of 97% and 93%, respectively, in detecting PFO in stroke patients. It is also recommended that that the injection be given at rest and accompanied by the Valsalva maneuver if necessary [35]. The principal limitations of TCD are that it only indicates the existence of right-to-left shunting, it does not distinguish between intracardiac and other extracardiac shunting and it provides no anatomical information about the PFO at all. Cardiac magnetic resonance (CMR) is used for the evaluation of complex CHD anatomies; many times, the PFO is visualized. In adults, CMR was found to be inferior to TEE in the detection of right-to-left shunting and in identifying atrial septal aneurysm, but recent CMR studies with ultrafast software technology have shown improved sensitivity compared to TEE for the diagnosis of PFO in adults [36,37]. Three-dimensional echocardiography (3D Echo) has been reported to accurately depict the anatomy of the PFO and atrial septum, but experience in children is limited [38]. Intracardiac echography (ICE) is rarely used during interventional cardiac catheterization in children and adolescents [39]. The advantage of ICE is that it may avoid the need for general anesthesia and is reported to be a reasonable alternative to TEE for the catheter-based procedure in children. This technique enables the adequate characterization of the shunting across the PFO and the better visualization of septal rims, especially in the case of the tunnel type PFO during percutaneous closure device deployment [40]. Limitations include the cost of the probe, the need for second venous access to use a large-sized catheter (at least 8 Fr) and the need for an experienced operator. This procedure can also be associated with rare complications, which include vascular injury, cardiac perforation and atrial arrhythmias.
5. PFO-Associated Medical Diseases
The left-to-right shunt across the PFO is trivial and not hemodynamically significant, and no treatment is necessary for otherwise asymptomatic children. However, PFOs in the presence of CHDs can be a source of the right-to-left shunt producing paradoxical embolism with resultant cerebrovascular accidents (CVAs)/transient ischemic attacks (TIAs), brain abscess or other problems such as migraine, Caisson’s disease or platypnea-orthodeoxia syndrome.
5.1. Residual PFOs in Previously Treated Complex CHD
Systemic desaturation and paradoxical embolism can occur because of the right-to-left shunt via PFO in patients who have been previously treated for complex CHD. It has been reported that the PFO should be closed when there are low oxygen saturations, preferably with a trans-catheter device [41]. As an example, in some TOF patients, when the PFO is left for the benefit of immediate post-operative management, this can sometimes result in paradoxical embolism due to elevated right side filling pressure because of the diastolic dysfunction of the RV, and the PFO will need to be closed [42].
5.2. Cerebrovascular Accidents (CVAs)/Transient Ischemic Attacks (TIAs)
The PFO ‘‘triad’’ has been reported, which combines raised RA pressure, a venous source of thrombosis and the presence of a PFO as the requirements to determine if paradoxical embolism is a potential cause of CVA or TIA [43]. The meta-analysis of pediatric studies revealed that there was no defining role of a PFO in pediatric stroke [44,45]. There is an interaction of risk factors for hypercoagulation, arteriopathy and genetic factors that contribute to most strokes in children with PFOs [46,47,48,49,50]. An atrial septal aneurysm in the adult patient population is defined as a risk factor for stroke, but not during childhood [51]. A PFO could be a risk factor for stroke in children with sickle cell anemia because they are predisposed to thrombosis and elevations of right heart pressure, which could promote paradoxical embolization [52]. Based on current guidelines from the European position paper, primary prevention measures are not recommended for cryptogenic stroke in patients with PFO [43]. Secondary prevention measures include medical treatment (antiplatelet drugs and anticoagulants) and percutaneous or surgical closure of the PFO after an event occurs in order to prevent the recurrence of TIAs or CVAs. To date, no randomized study has compared medical treatment with percutaneous device closure. There is a lack of data comparing antiplatelet drugs and anticoagulant treatments to make evidenced-based recommendations in children, unlike adults. Therefore, the choice of treatment for PFO should be made on an individual basis, assessing the risks and benefits for each patient.
5.3. Migraine
The mechanism by which PFO may promote migraine remains speculative. One postulated mechanism is transient hypoxemia, due to the right-to-left intracardiac shunting of blood through the PFO causing microinfarcts in the brain, leading to an increased tendency towards migraine. Another theory is that blood components which otherwise would be screened out within the lungs are shunted from the RA to the LA and thus bypass the pulmonary vascular bed and are directed towards the eyes and brain; they may serve as a vaso-constrictor, which generates migraines. These speculations and other clinical observations have sparked interest in the potential usefulness of PFO closure for migraine prevention. Several non-randomized studies have described the association of migraine with right-to-left shunting across the PFO and improved migraine symptoms after PFO closure in children [12,53,54,55,56]. The only prospective, randomized, double-blinded study, the Migraine Intervention with STARFlex Technology (MIST) trial in 2008, failed to reach the primary endpoint of the complete cessation of migraine symptoms for which the study was underpowered. However, the study found a much higher incidence of PFO with a right-to-left shunt in migraine with aura in patients than reported in the general population. The study did not support the efficacy seen in previous observational reports [53,54,55,56]. Recently, the Percutaneous Closure of PFO in Patients with Migraine (PREMIUM) trial did not meet the primary endpoint of reduction in the responder rate in patients with a frequent migraine after the closure of PFO [57]. Both the MIST and PREMIUM trials showed no relationship between migraine and shunting across the PFO.
5.4. Platypnea-Orthodeoxia Syndrome
This is a relatively uncommon but serious syndrome of systemic hypoxia and breathlessness in the upright position caused by the interatrial or intrapulmonary shunting of either a PFO or an ASD [58,59]. Symptoms of platypnea-orthodeoxia are thought to be due to positional right-to-left shunting. Standing upright can stretch the PFO, thus allowing more streaming of venous blood from the inferior venacava through the defect, whether or not a persistent Eustachian valve coexists. The diagnosis of a PFO with platypnea-orthodeoxia syndrome can be confirmed during a tilt-test, measuring arterial saturation, and a contrast echocardiography showing right-to-left shunting [59]. Platypnea-orhtodeoxia has also been reported as a complication in Fontan patients with pulmonary arteriovenous fistulas and/or the presence of a Fontan fenestration communicating with the RA [60]. The treatment for this syndrome is closure of the PFO or an intra-atrial shunt using a percutaneous device [11,61].
5.5. Role of a PFO in Exacerbating Hypoxemia
PFO-mediated hypoxemia can occur when there is RA to LA shunting especially during exercise. Patients with a right-to-left shunt across the PFO may have profound hypoxemia out of proportion to underlying primary lung disease. In a subset of these patients who have normal right-sided pressures, percutaneous PFO closure may result in the marked improvement of their symptoms [62]. The presence of a PFO is about four times more frequent and hypoxemia is more severe in patients who are susceptible to high-altitude pulmonary edema than those living at high altitudes, who are resistant to this condition [63]. Interestingly, the presence of a PFO did not impact survival in primary pulmonary hypertension patients in one study [64]. The study’s authors speculated that the incidence of PFO increases in pulmonary hypertension patients with a more dilated and dysfunctional RV rather than it is congenital.
5.6. Decompression (Caisson’s) Syndrome
The association between PFO and decompression illness suffered by divers and high-altitude pilots who rapidly transition from high- to low-pressure environments has been documented [65]. The sudden decrease in pressure if the ascent from depth in the case of divers is too rapid results in the formation of nitrogen bubbles within tissues, and gas bubbles enter the arterial circulation, which can result in vessel occlusion. This can produce a range of symptoms, including muscle and joint pain, headache, dizziness, fatigue, rash, paresthesia, breathing difficulties, confusion, motor incoordination and paralysis [66,67]. A longitudinal, non-randomized follow-up study showed a reduction in both symptomatic neurological events and total brain lesions among recreational divers with PFO and decompression illness who had PFO closure, compared with those continuing to dive without closure [68]. According to this study, a PFO closure could be recommended for professional divers. However, there is controversy about whether young adults who are aviators, astronauts and scuba divers should undergo routine screening for PFO.
5.7. Systemic Embolism
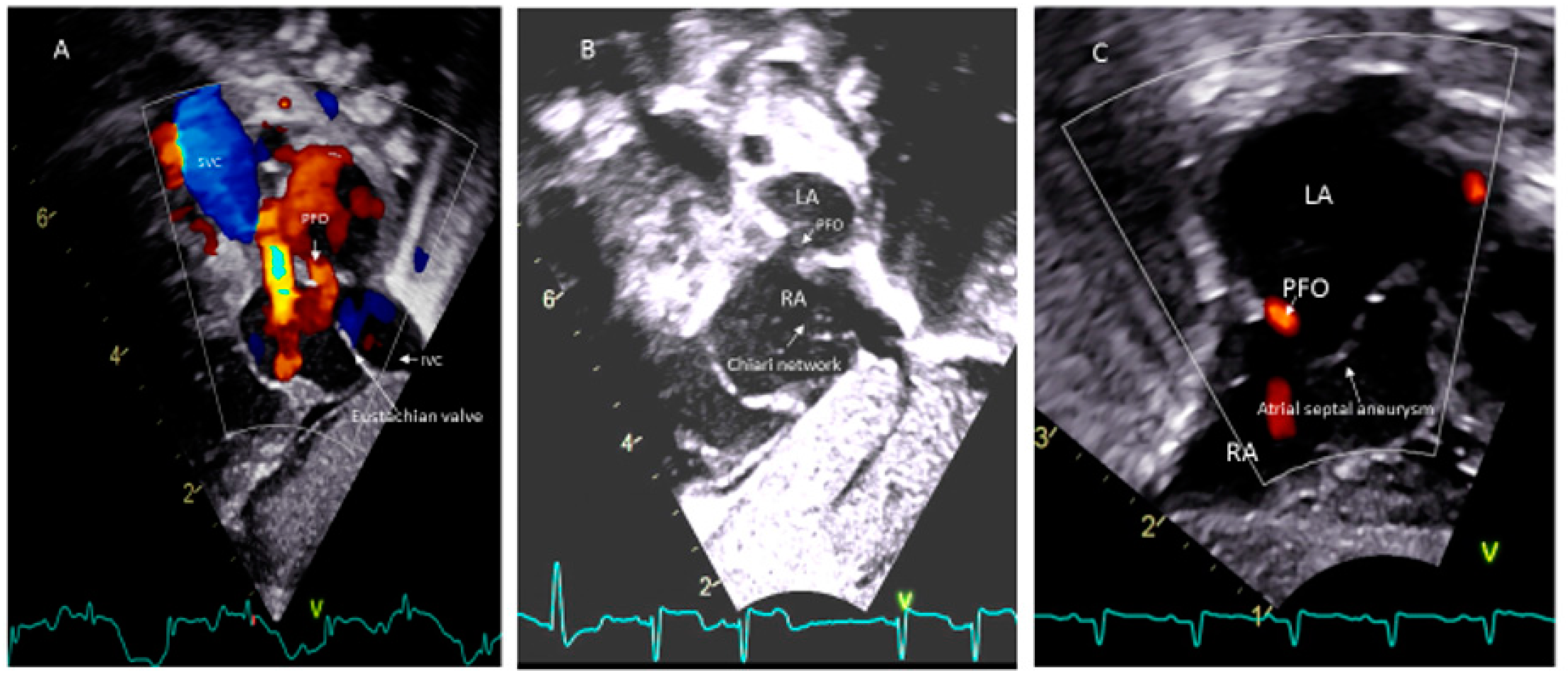
Paradoxical systemic embolism through PFOs resulting in renal and myocardial infarctions have been reported [43]. However, there is no evidence from randomized controlled trials that closure of the PFO is protective against systemic embolization in any patients. The presence of a Eustachian valve (Figure 4A) and Chiari’s network (Figure 4B) may favor and facilitate paradoxical embolism [69,70]. However, unlike in adults, atrial septal aneurysm (Figure 4C) is not considered as a risk for thromboembolism in children [51].
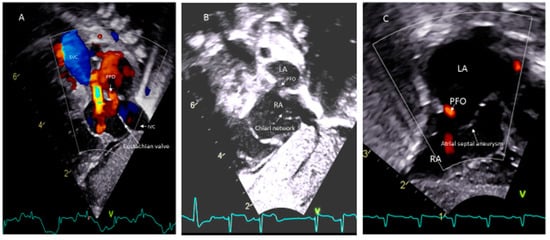
Figure 4.
PFO associated with (A) Eustachian vale; (B) Chiari network; (C) Atrial septal aneurysm.
6. Management of PFO
Currently, there are two approaches to closing a PFO: surgical and transcatheter closures. Surgical closure can be done simultaneously if other surgical interventions are necessary; otherwise, most cardiologists prefer the transcatheter closure of an isolated PFO in special circumstances such as platypnea-orhtodeoxia or when a PFO is causing the exacerbation of systemic hypoxia. Currently available percutaneous devices are the Amplatzer PFO occluder (St. Jude Medical, Inc., Golden Valley, MN, USA) and the Gore Cardioform Septal Occluder and Gore HELEX® devices (W.L. Gore and Associates, Flagstaff, AZ, USA). Other therapeutic options are under development and include HeartStitch PFO (Sutura Inc, Fountain Valley, CA, USA), or BioTREK (NMT Medical, Boston, MA, USA) [71]. There is a lack of scientific evidence for the need to close PFOs or the use of antiplatelets or anticoagulants in asymptomatic children. The treatment of PFOs in patients with associated clinical syndromes is discussed under each specific condition. In children, there is no RCT to compare antiplatelets/anticoagulants with device therapy for the treatment of PFOs associated with CVA or TIA. Future trials should include children with migraine, decompression syndrome and other medical conditions associated with PFO in order to explore the benefits of PFO closure using percutaneous devices versus using medical therapy.
7. Conclusions
Patent foramen ovale in infants and children is a frequent finding but, in most patients, it presents no clinical implications. Due to the controversy regarding the clinical significance of PFOs in many diseases described in this review and the role of PFOs in the settings of dilated cardiomyopathy and pulmonary hypertension, and with the wide use of ECMO and VAD for pediatric decompensated heart failure, increased attention has been directed towards the presence of PFO in last few years. Generally speaking, an interdisciplinary and personalized approach is required for the management of PFO in certain special circumstances during fetal life and in infants and children, as outlined in this review.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
| LA | left atrium |
| RA | right atrium |
| LV | left ventricle |
| RV | right ventricle |
| CHD | congenital heart disease |
| FO | foramen ovale |
| PFO | patent foramen ovale |
| ASD | atrial septal defect |
| ECMO | extracorporeal membrane oxygenation |
| VAD | ventricular assist device |
| HLHS | hypoplastic left heart syndrome |
| TGA | transposition of great arteries |
| PVR | pulmonary vascular resistance |
| SVR | systemic vascular resistance |
| CVA | cerebrovascular accident |
| TIA | transient ischemic attack |
| TTE | transthoracic echocardiography |
| TEE | transesophageal echocardiography |
| TCD | transcranial Doppler |
| ICE | intracardiac echocardiography |
| CMR | cardiac magnetic resonance |
References
- Sachdeva, R.; Valente, A.M.; Armstrong, A.K.; Cook, S.C.; Han, B.K.; Lopez, L.; Lui, G.K.; Pickard, S.S.; Powell, A.J.; Bhave, N.M.; et al. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT /SCMR/AHA/SOPE 2020 appropriate use of criteria for multimodality imaging during the follow-up care of patients with congenital heart disease. J. Am. Coll. Cardiol. 2020, 75, 657–703. [Google Scholar] [CrossRef] [PubMed]
- Kirk, E.P.; Sunde, M.; Costa, M.W.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; et al. Mutations in cardiac T-box factor gene TBX20 are associated with diverse cardiac pathologies, including defects of septation and vasculogenesis and cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291. [Google Scholar] [CrossRef]
- Wilmshurst, P.T.; Morrison, W.L.; Walsh, K.P. Comparison of the size of persistent foramen ovale and atrial septal defect in divers with shunt-related decompression illness and the general population. Diving Hyperb. Med. 2015, 45, 89–93. [Google Scholar]
- Hagen, P.T.; Scholz, D.G.; Edwards, W.D. Incidence and size of patent foramen ovale during the first 10 decades of life: An autopsy study of 965 normal hearts. Mayo Clin. Proc. 1984, 59, 17–20. [Google Scholar] [CrossRef]
- Allen, H.D.; Shaddy, R.E.; Penny, D.J.; Feltes, T.F.; Cetta, F. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult, 9th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2016. [Google Scholar]
- Khan, R.; Chan, A.K.; Mondal, T.K.; Paes, B.A. Thrombosis and hemostasis in newborns (THIN) group. Patent foramen ovale and stroke in childhood: A systemic review of the literature. Eur. J. Pediatric Neurol. 2016, 20, 500–511. [Google Scholar] [CrossRef]
- Shih, E.K.; Natrajan, S.; Falkensammer, C.; Beslow, L.A.; Messe, S.R.; Ichord, R.N. Role of patent foramen ovale in first and recurrent childhood cryptogenic arterial ischemic stroke. Stroke 2017, 48, A170. [Google Scholar]
- Mojadidi, M.K.; Zaman, M.O.; Elgendy, I.Y.; Mahmoud, A.N.; Patel, N.K.; Agarwal, N.; Tobis, J.M.; Meier, B. Cryptogenic stroke and PFO. J. Am. Coll. Cardiol. 2018, 71, 1035–1043. [Google Scholar] [CrossRef]
- Holda, M.K.; Koziej, M. Morphometric features of patent foramen ovale as a risk factor of cerebrovascular accidents: A systemic review and meta-analysis. Cereb. Dis. 2020, 49, 1–9. [Google Scholar] [CrossRef]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Coman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC guidelines for the management of adults with congenital heart disease. Circulation 2019, 139, e698–e800. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Osten, M.; Leventhal, A.; Bach, Y.; Yoo, D.; Mansour, D.; Benson, L.; Wilson, W.M.; Holick, E. Percutaneous intervention to treat platypnea-orthodeoxia syndrome: The Toronto experience. JACC Cardiovasc. Interv. 2016, 9, 1928–1938. [Google Scholar] [CrossRef]
- Mc Candless, R.T.; Arrington, C.B.; Nielson, D.C.; Bale, J.F.; Minich, L.L. Patent foramen ovale in children with migraine headaches. J. Pediatr. 2011, 159, 243–247. [Google Scholar] [CrossRef]
- Moore, K.L.; Persaud, V. The Developing Human: Clinically Oriented Embryology, 2nd ed.; W.B. Saunders: Philadelphia, PA, USA, 2003. [Google Scholar]
- Kiserud, T.; Rasmussen, S. Ultrasound assessment of the fetal foramen ovale. Ultrasound Obstet. Gynecol. 2001, 17, 119–124. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, Y.; Han, J.; Liu, X.; He, Y. Isolated premature restriction or closure of foramen ovale in fetuses: Echocardiographic characteristics and outcome. Echocardiography 2018, 35, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Nowlen, T.T.; Ayres, N.A.; Kearney, D.L.; Nihill, M.R.; Grifka, R.G. Premature closure of foramen ovale associated with aortic stenosis, left ventricular dilation with thrombus, and early mortality. Am. J. Cardiol. 2000, 85, 1159–1161. [Google Scholar] [CrossRef]
- Teroba, S.S.; Oulego, E.; Lobete, P.C.; Alonso, O.P. Intrauterine restriction of foramen ovale: Can cause neonatal pulmonary hypertension. Arch. Argent Pediatr. 2019, 117, e626–e630. [Google Scholar]
- Campbell, S.; Vyas, S.; Nicolaides, K.H. Doppler investigation of the fetal circulation. J. Perinat. Med. 1991, 19, 21–26. [Google Scholar] [CrossRef]
- Friedman, K.G.; Towretzky, W. Fetal cardiac interventions: Where do we stand? Arch. Cardiovasc. Dis. 2020, 113, 121–128. [Google Scholar] [CrossRef]
- Respondek-Liberska, M.; Płużańska, J.; Słodki, M.; Czichos, E.; Moll, J.; Zych-Krekora, K. Early neonatal surgery for congenital heart defects after prenatal diagnosis of restricted foramen ovale as the priority procedure? Prenat. Cardio 2015, 5, 24–29. [Google Scholar]
- Songswang, J.; Adatia, I.; Newman, C.; Smallhorn, J.F.; Willaims, W.G.; Freeem, R.M. Mortality in potential arterial switch candidates with transposition of great arteries. J. Am. College Cardiol. 1998, 32, 753–757. [Google Scholar] [CrossRef]
- Jantzen, D.W.; Moon-Grady, A.J.; Morris, S.A.; Armstrong, A.K.; Berg, C.; Gangel, J.; Fifer, C.G.; Frommelt, M.; Gembruch, U.; Herberg, U.; et al. Hypoplastic left heart syndrome with intact or restrictive atrial septum. A report from the International Fetal Cardiac Intervention Registry. Circulation 2017, 136, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Hubail, Z.; Lemler, M.; Ramciotti, C.; Moore, J.; Ikemba, C. Diagnosing a patent foramen ovale in children. Is transesophageal echocardiography necessary? Stroke 2011, 42, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Mas, J.L.; Arquizan, C.; Lamy, C.; Zuber, M.; Cabanes, L.; Derumeaux, G.; Coste, J. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N. Engl. J. Med. 2001, 345, 1740–1746. [Google Scholar] [CrossRef]
- Homma, S.; Sacco, R.L.; Di Tullio, M.R.; Sciacca, R.R.; Mohr, J.P. Effect of medical treatment in stroke patients with patent foramen ovale. Circulation 2002, 105, 2625–2631. [Google Scholar] [CrossRef]
- Moses, K.L.; Seymour, M.; Beshish, A.; Baker, K.R.; Pegelow, D.F.; Lamers, L.J.; Eldridge, M.W.; Bates, M.L. Inspiratory and expiratory resistance cause right-to-left bubble passage through the foramen ovale. Physiol. Rep. 2018, 6, e13719. [Google Scholar] [CrossRef]
- Lee, R.J.; Bartzokis, T.; Yeoh, T.K.; Frogin, H.R.; Choi, D.; Schnitter, J. Enhanced detection of intracardiac sources of cerebral emboli by transesophageal echocardiography. Stroke 1991, 22, 734–739. [Google Scholar] [CrossRef]
- Chenzbraun, A.; Pinto, F.J.; Schnittger, I. Biplane transesophageal echocardiography in the diagnosis of patent foramen ovale. J. Am. Soc. Echocardiogr. 1993, 6, 417–421. [Google Scholar] [CrossRef]
- Silvestry, F.E. Guidelines for the echocardiographic assessment of atrial septal defect and patent foramen ovale from the American Society of Echocardiography for cardiac angiography and interventions. J. Am. Soc. Echocardiogr. 2015, 28, 910–985. [Google Scholar] [CrossRef]
- Hara, H.; Virmani, R.; Ladich, E.; Mackey-Bojack, S.; Titus, J.; Reisman, M.; Gray, W.; Nakamura, M.; Mooney, M.; Poulose, A.; et al. Patent foramen ovale: Current pathology, pathophysiology, and clinical status. J. Am. Coll. Cardiol. 2005, 46, 1768–1776. [Google Scholar] [CrossRef]
- Hanley, P.C.; Tajik, A.J.; Hynes, J.K.; Edwards, W.D.; Reeder, G.S.; Hagler, D.J.; Seward, J.B. Diagnosis and classification of atrial septal aneurysm by two-dimensional echocardiography: Report of 80 consecutive cases. J. Am. Coll. Cardiol. 1985, 6, 1370–1382. [Google Scholar] [CrossRef]
- Kotani, Y.; Chetan, D.; Rodrigues, W.; Sivarajan, V.B.; Gruenwald, C.; Guerguerian, A.M.; Arsdell, G.S.V.; Honjo, O. Left atrial decompression during venoarterial extracorporeal membrane oxygenation for left ventricular failure in children: Current strategy and clinical outcomes. Artif. Organs 2013, 37, 29–36. [Google Scholar] [CrossRef]
- Bacich, D.; Fiorencis, A.; Braggion, G.; Zuin, M.; Rigatelli, G. Patent foramen ovale-related complications in left ventricular assist device patients: A reappraisal for cardiovascular professionals. J. Artif. Organs 2019, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Komar, M.; Olszowska, M.; Przewlocki, T.; Podolec, J.; Stepniewski, J.; Sobien, B.; Badacz, R.; Kablak-Ziembicka, A.; Tomkiewicz-Pajak, L.; Podolec, P. Transcranial Doppler ultrasonography should it be the first choice for persistent foramen ovale screening. Cardiovasc. Ultrasound 2014, 12, 16. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Robets, S.C.; Winoker, J.S.; Romero, J.; Goodman-Meza, D.; Gevorgyaan, R.; Tobis, J.M. Accuracy of transcranial Doppler for the diagnosis of intracardiac right to left shunt: A bivariate meta-analysis of prospective studies. JACC Cardiovasc. Imaging 2014, 7, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Nusser, T.; Hoher, M.; Merkle, N.; Gerbe, O.C.; Spiess, J.; Kestler, H.A.; Rasche, V.; Koch, M.; Hombach, V.; Wohrle, J. Cardiac magnetic resonance imaging and transesophageal echocardiography in patients with transcatheter closure of patent foramen ovale. J. Am. Coll. Cardiol. 2006, 48, 322–329. [Google Scholar] [CrossRef]
- Mohrs, O.K.; Petersen, S.E.; Erkapic, D.; Rabel, C.; Schrader, R.; Nowak, B.; Fach, N.A.; Kauczor, H.U.; Voigtlaender, T. Diagnosis of patent foramen ovale using contrast-enhanced dynamic MRI: A pilot study. AJR Am. J. Roentgenol. 2005, 184, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Rana, B.S.; Shapiro, L.M.; McCarthy, K.P.; Ho, S.Y. Three-dimensional imaging of the atrial septum and patent foramen ovale anatomy: Defining the morphological phenotypes of patent foramen ovale. Eur. J. Echocardiogr. 2010, 11, 119–125. [Google Scholar] [CrossRef]
- Medford, B.A.; Taggart, N.W.; Cabalka, A.K.; Cetta, F.; Reeder, G.S.; Hagler, D.J.; Johnson, J.N. Intracardiac echocardiography during atrial septal defect and patent foramen ovale device closure in pediatric and adolescent patients. J. Am. Soc. Echocardgr. 2014, 27, 984–990. [Google Scholar] [CrossRef]
- Ponnuthurai, F.A.; van Gaal, W.J.; Burchell, A.; Mitchell, A.R.; Wilson, N.; Ormerod, O.J. Safety and feasibility of day case patent foramen ovale (PFO) closure facilitated by intracardiac echocardiography. Int. J. Cardiol. 2007. [Google Scholar] [CrossRef]
- Rao, P.S.; Chandar, J.S.; Sideris, E.B. Role of an inverted buttoned device in trans-catheter occlusion of atrial septal defect or patent foramen ovale with right-to-left shunting associated with previously operated complex congenital cardiac anomalies. Am. J. Cardiol. 1997, 80, 914–921. [Google Scholar] [CrossRef]
- Levine, C.F.H.; Reis, R.L.; Morrow, A.G. Incidence and significance of PFO after the correction of TOF. Ann. Thorac Surg. 1972, 13, 464–467. [Google Scholar] [CrossRef]
- Pristipino, C.; Sievert, H.; D’Ascenzo, F.; Louis, M.J.; Meier, B.; Scacciatella, P.; Hildick-Smith, D.; Gaita, F.; Toni, D.; Kyrle, P.; et al. Evidence Synthesis Team. Eapci Scientific Documents and Initiatives Committee. International Experts. European position paper on the management of patients with patent foramen ovale. General approach and left circulation thromboembolism. Eur. Heart J. 2019, 40, 3182–3195. [Google Scholar] [CrossRef] [PubMed]
- Dowling, M.M.; Ikemba, C.M. Intracardiac shunting and stroke in children: A systemic review. J. Child Neurol. 2011, 26, 72–82. [Google Scholar] [CrossRef]
- Torbey, E.; Thompson, P.D. Patent foramen ovale: Thromboembolic structure or incidental finding? Conn. Med. 2011, 75, 97–105. [Google Scholar] [PubMed]
- Lantz, M.; Sjostrand, C.; Kostlas, K. Ischemic stroke and patent foramen ovale: Risk factors and genetic profile. J. Stroke Cerebrovasc. Dis. 2013, 22, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Elliott, G.C.; Sargurupremraj, M.; Velupandian, U.; Gurtu, R.; Trump, D.; Newman, W.; Wang, T.; McCollum, C. Genetics of patent foramen ovale-NKX2-5 and beyond. Clin. Neurol. Neurosurg. 2010, 11, 457–458. [Google Scholar] [CrossRef]
- Gerstl, L.; Weinberger, R.; von Kries, R.; Heiner, F.; Schroeder, A.S.; Bonfert, M.V.; Broggraefe, F.; Tracke, M.; Vill, K.; Landgraf, M.N.; et al. Risk factors in childhood arterial ischemic stroke: Findings from a population-based study in Germany. Eur. J. Paediatr. Neurol. 2018, 22, 380–386. [Google Scholar] [CrossRef]
- Cerrato, P.; Imperiale, D.; Bazzan, M.; Baima, C.; Grasso, M.; Morello, M.; Bergamasco, B. Inherited thrombophilic conditions, patent foramen ovale and juvenile ischemic stroke. Cerbrovasc. Dis. 2001, 11, 140–141. [Google Scholar] [CrossRef]
- Botto, N.; Ait-Ali, L.; Sicari, R.; Spadoni, I.; Andreassi, M.G. Patent foramen ovale: Lies liasons dangereuses between anatomy and genetics. Recenti Prog. Med. 2009, 199, 356–360. [Google Scholar]
- Giannopoulos, A.; Gavras, C.; Sarioglu, S.; Agathagelou, F.; Kassapogelou, I.; Athanassiadou, F. Atrial septal aneurysms in childhood: Prevalence, classification, and current abnormalities. Cardiol. Young 2014, 24, 453–458. [Google Scholar] [CrossRef]
- Dowling, M.M.; Quinn, C.T.; Ramaciotti, C.; Kanter, J.; Osunkwo, I.; Inusa, B.; Iyer, R.; Kwiatkowski, J.L.; Johnson, C.; Rhodes, M.; et al. Increased prevalence of potential right to left shunting in children with sickle cell anemia and stroke. Br. J. Hematol. 2017, 176, 300–308. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, R.; Han, X.; Su, M.; Cao, X.; Wang, G.; Cao, F.; Yu, S. A new perspective of migraine symptoms in patients with congenital heart defect. Headache 2018, 58, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Spalice, A.; Del Balzo, F.; Papetti, L.; Zicari, A.M.; Properzi, E.; Occasi, F.; Nicita, F.; Duse, M. Stroke and migraine is there a possible comorbidity? Ital. J. Pediatr. 2016, 42, 41. [Google Scholar] [CrossRef] [PubMed]
- Bellini, B.; Arruda, M.; Cescut, A.; Saulle, C.; Persico, A.; Carotenuto, M.; Gatta, M.; Nacinovich, R.; Piazza, F.P.; Termine, C.; et al. Headache and comorbidity in children and adolescents. J. Headache Pain 2013, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.Y.; Shin, D.H.; Cho, K.H.; Lee, S.P.; Park, S. Migraine with aura: A predictor of patent foramen ovale in children and adolescents. Cephalalgia 2013, 33, 63–68. [Google Scholar] [CrossRef]
- Tobis, J.M.; Charles, A.; Siberstein, S.D.; Sorensen, S.; Maini, B.; Horwitz, P.A.; Gurley, J.C. Percutaneous closure of patent foramen ovale in patients with migraine: The PREMIUM trial. J. Am. Coll. Cardiol. 2017, 70, 2766–2774. [Google Scholar] [CrossRef]
- Bitar, S.; Rao, P.S. Platypnea-orthodeoxia syndrome: Transcatheter management. In Catheter Based Devices for Treatment of Noncoronary Cardiovascular Disease in Adults and Children; Rao, P.S., Kern, M.J., Eds.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2003; Chapter 15; pp. 2766–2774. [Google Scholar]
- Cheng, T.O. Platypnea-orthodeoxia syndrome: Etiology, differential diagnosis, and management. Catheter. Cardiovasc. Interv. 1999, 47, 64–66. [Google Scholar] [CrossRef]
- Suzuki, H.; Ohuchi, H.; Hiraumi, Y.; Yasuda, K.; Echigo, S. Effects of postural change on oxygen saturation and respiration in patients after Fontan operation: Platypnea and orthodeoxia. Int. J. Cardiol. 2006, 106, 211–217. [Google Scholar] [CrossRef]
- Cheng, T.O. Transcatheter closure of patent foramen ovale: A definitive treatment for platypnea-orthodeoxia. Catheter. Cardiovasc, Interv. 2000, 100, 215–220. [Google Scholar] [CrossRef]
- Mojadidi, M.K.; Ruiz, J.C.; Chertoff, J.; Zaman, M.O.; Elgendy, I.Y.; Mohmoud, A.N.; Mohamad, A.A.; Elgendy, A.Y.; Patel, N.K.; Shantha, G.; et al. Patent foramen ovale and hypoxemia. Cardiol. Rev. 2019, 1, 34–40. [Google Scholar] [CrossRef]
- Allemann, Y.; Hutter, D.; Lipp, E.; Sartori, C.; Duplian, H.; Egli, M.; Cook, S.; Scherrer, U.; Seiler, C. Patent foramen ovale and high-altitude pulmonary edema. JAMA 2006, 296, 2954–2958. [Google Scholar] [CrossRef]
- Sharan, L.; Stackhouse, K.; Awerbach, J.D.; Bashore, T.M.; Krasuski, R.A. Effect of patent foramen ovale with pulmonary hypertension. Am. J. Cardio.l 2018, 122, 505–551. [Google Scholar] [CrossRef] [PubMed]
- Butler, B.D.; Hills, B.A. The lung as a filter for micro bubbles. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 537–543. [Google Scholar] [PubMed]
- Wilmshurt, P.T.; Byrne, J.C.; Webb-Pepole, M.M. Relation between interatriual shuns and decompression sickness in divers. Lancet 1989, 2, 1302–1306. [Google Scholar] [CrossRef]
- Ali, A.; Rothner, A.D. Migraine with neurological features in a scuba driver with a patent foramen ovale. Headache 2017, 57, 658–662. [Google Scholar] [CrossRef]
- Billinger, M.; Zbinden, R.; Mordasini, R.; Windecker, S.; Schmezmann, M.; Meier, B.; Seiler, C. Patent foramen ovale closure in recreational divers: Effect on decompression illness and ischemic brain lesions during long-term follow-up. Heart 2011, 97, 1932–1937. [Google Scholar] [CrossRef] [PubMed]
- Schneider, B.; Hofmann, T.; Justen MHMeinertz, T. Chiari’s network: Normal anatomic variant or risk factor for arterial embolic events. J. Am. Coll. Cardiol. 1995, 26, 203. [Google Scholar] [CrossRef]
- Rigtelli, G.; Dell’avvocata, F.; Braggion, G.; Giordan, M.; Chinaglia, M.; Cardaioli, P. Persistent venous valves correlate with increased shunt and multiple preceding cryptogenic embolic events in patients with patent foramen ovale: An intracardiac echocardiographic study. Catheter. Cardiovasc. Interv. 2008, 72, 97. [Google Scholar] [CrossRef]
- Majunke, N.; Sievert, H. ASD/PFO devices: What is in the pipeline? J. Interv. Cardiol. 2007, 20, 517–523. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).