Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators
Abstract
:1. Introduction
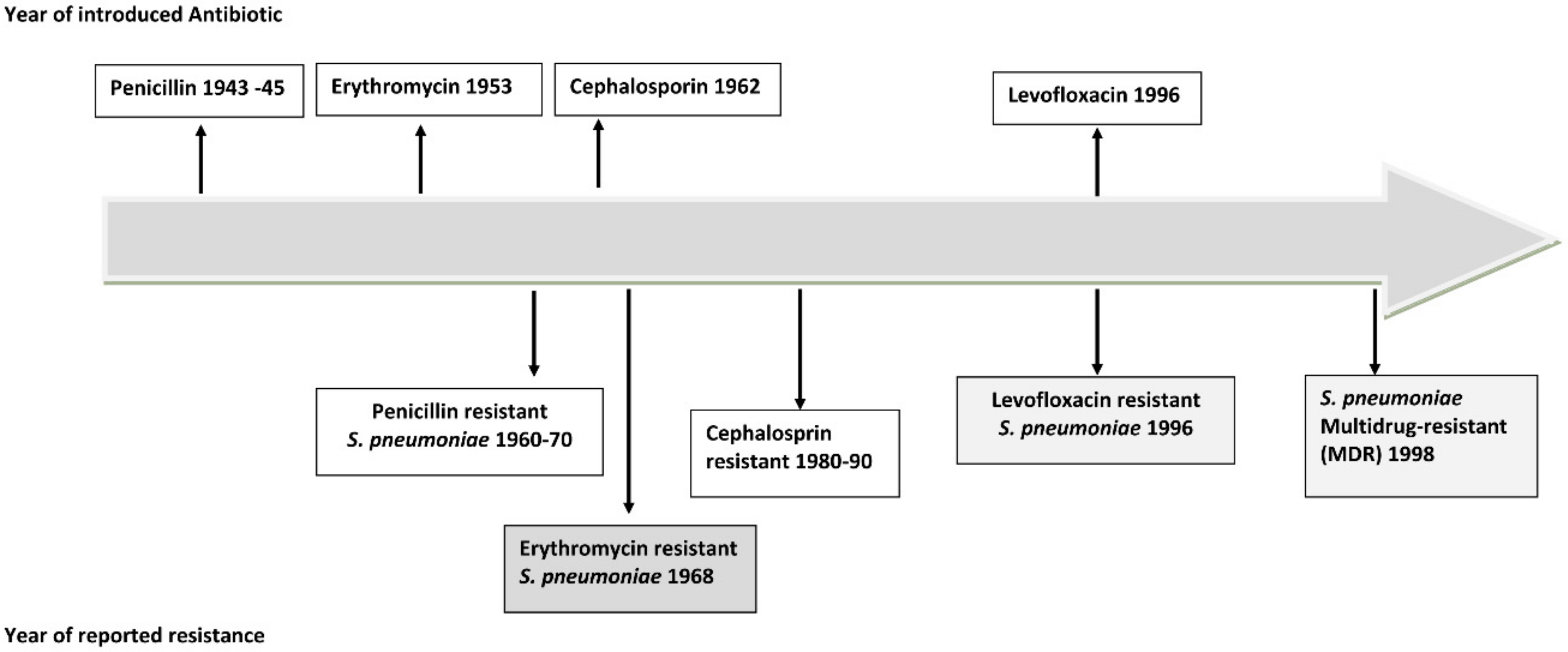
2. CAP Affects Everyone: Why Do We Not Recognize the Threat?
3. The Role of Pneumonia and Secondary Bacterial Infection in the H1N1 Influenza A Pandemic
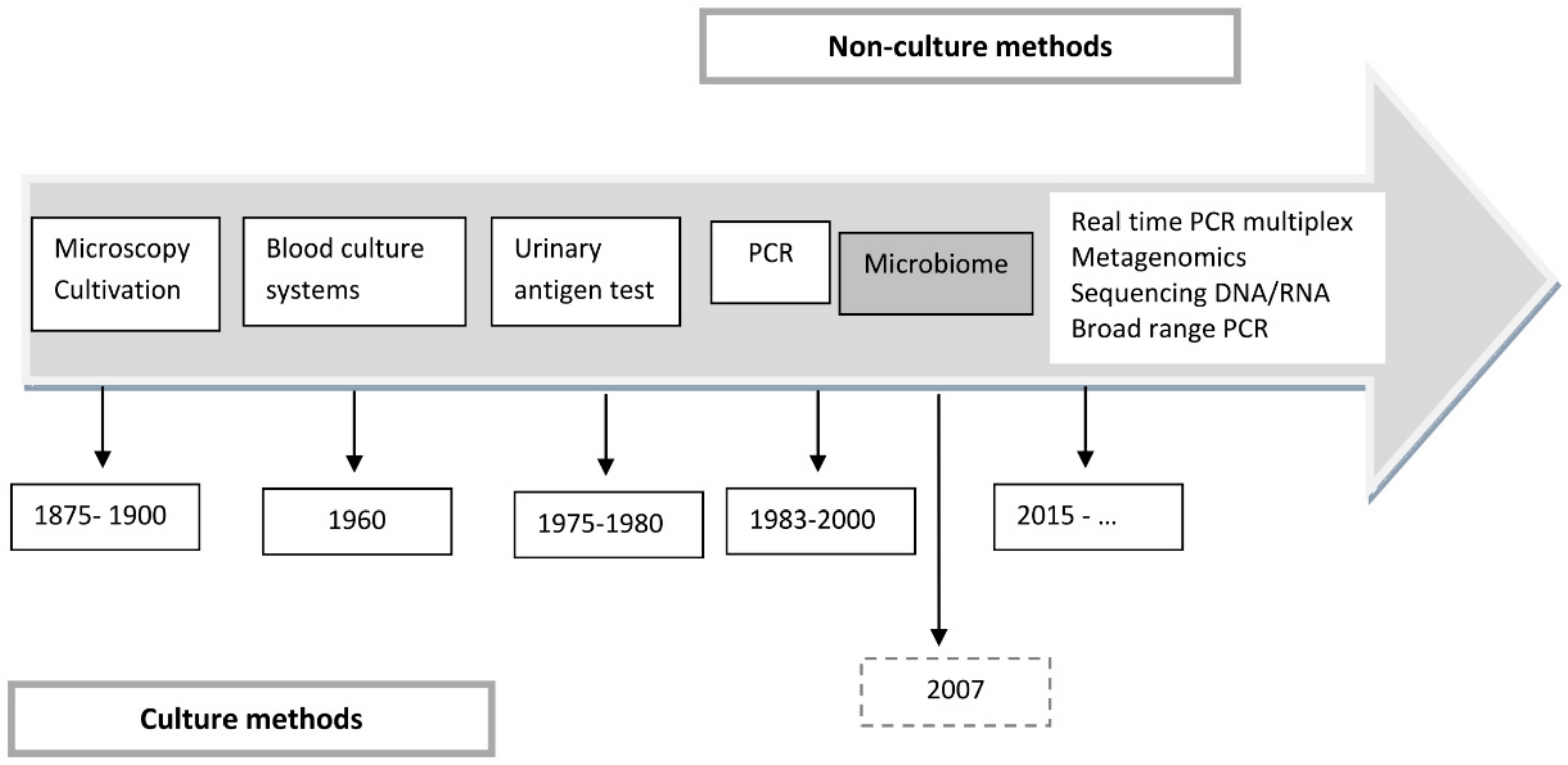
4. The Development of New Technologies
5. New Concepts, When Times Change
6. Raising Awareness about Pneumonia
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [Green Version]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soriano, J.B.; Rojas-Rueda, D.; Alonso, J.; Antó, J.M.; Cardona, P.-J.; Fernández, E.; Garcia-Basteiro, A.L.; Benavides, F.G.; Glenn, S.D.; Krish, V.; et al. The burden of disease in Spain: Results from the Global Burden of Disease 2016. Med. Clin. 2018, 151, 171–190. [Google Scholar] [CrossRef]
- Rivero-Calle, I.; Pardo-Seco, J.; Aldaz, P.; Vargas, D.A.; Mascarós, E.; Redondo, E.; Díaz-Maroto, J.L.; Linares-Rufo, M.; Fierro-Alacio, M.J.; Gil, A.; et al. Incidence and risk factor prevalence of community-acquired pneumonia in adults in primary care in Spain (NEUMO-ES-RISK project). BMC Infect. Dis. 2016, 16, 645. [Google Scholar] [CrossRef] [Green Version]
- Rivero-Calle, I.; Cebey-López, M.; Pardo-Seco, J.; Yuste, J.; Redondo, E.; Vargas, D.A.; Mascarós, E.; Díaz-Maroto, J.L.; Linares-Rufo, M.; Jimeno, I.; et al. Lifestyle and comorbid conditions as risk factors for community-acquired pneumonia in outpatient adults (NEUMO-ES-RISK project). BMJ Open Respir. Res. 2019, 6, e000359. [Google Scholar] [CrossRef] [Green Version]
- De Miguel-Díez, J.; Jiménez-García, R.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; de Miguel-Yanes, J.M.; Méndez-Bailón, M.; López-de-Andrés, A. Trends in hospitalizations for community-acquired pneumonia in Spain: 2004 to 2013. Eur. J. Intern. Med. 2017, 40, 64–71. [Google Scholar] [CrossRef]
- De Miguel-Díez, J.; López-de-Andrés, A.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; Méndez-Bailón, M.; de Miguel-Yanes, J.M.; Jiménez-García, R. Impact of COPD on outcomes in hospitalized patients with community-acquired pneumonia: Analysis of the Spanish national hospital discharge database (2004–2013). Eur. J. Intern. Med. 2017, 43, 69–76. [Google Scholar] [CrossRef]
- López-de-Andrés, A.; de Miguel-Díez, J.; Jiménez-Trujillo, I.; Hernández-Barrera, V.; de Miguel-Yanes, J.M.; Méndez-Bailón, M.; Pérez-Farinós, N.; Salinero-Fort, M.Á.N.; Jiménez-García, R. Hospitalisation with community-acquired pneumonia among patients with type 2 diabetes: An observational population-based study in Spain from 2004 to 2013. BMJ Open 2017, 7, e013097. [Google Scholar] [CrossRef]
- Turktan, M.; Ak, O.; Erdem, H.; Ozcengiz, D.; Hargreaves, S.; Kaya, S.; Karakoc, E.; Ozkan-Kuscu, O.; Tuncer-Ertem, G.; Tekin, R.; et al. Community acquired infections among refugees leading to Intensive Care Unit admissions in Turkey. Int. J. Infect. Dis. 2017, 58, 111–114. [Google Scholar] [CrossRef] [Green Version]
- Jones, B.; Gundlapalli, A.V.; Jones, J.P.; Brown, S.M.; Dean, N.C. Admission decisions and outcomes of community-acquired pneumonia in the homeless population: A review of 172 patients in an urban setting. Am. J. Public Health 2013, 103 (Suppl 2), S289–S293. [Google Scholar] [CrossRef] [PubMed]
- Bigé, N.; Hejblum, G.; Baudel, J.-L.; Carron, A.; Chevalier, S.; Pichereau, C.; Maury, E.; Guidet, B. Homeless Patients in the ICU: An Observational Propensity-Matched Cohort Study. Crit. Care Med. 2015, 43, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Smith, O.M.; Chant, C.; Burns, K.E.A.; Kaur, M.; Ashraf, S.; DosSantos, C.C.; Hwang, S.W.; Friedrich, J.O. Characteristics, clinical course, and outcomes of homeless and non-homeless patients admitted to ICU: A retrospective cohort study. PLoS ONE 2017, 12, e0179207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemay, J.-A.; Ricketson, L.J.; Zwicker, L.; Kellner, J.D. Homelessness in Adults with Invasive Pneumococcal Disease (IPD) in Calgary, Canada. Open Forum. Infect. Dis. 2019, 6, ofz362. [Google Scholar] [CrossRef]
- Olubamwo, O.O.; Onyeka, I.N.; Aregbesola, A.; Ronkainen, K.; Tiihonen, J.; Föhr, J.; Kauhanen, J. Determinants of hospitalizations for pneumonia among Finnish drug users. SAGE Open Med. 2018, 6, 2050312118784311. [Google Scholar] [CrossRef]
- Boschini, A.; Smacchia, C.; Di Fine, M.; Schiesari, A.; Ballarini, P.; Arlotti, M.; Gabrielli, C.; Castellani, G.; Genova, M.; Pantani, P.; et al. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: Incidence, etiologies, and clinical aspects. Clin. Infect. Dis. 1996, 23, 107–113. [Google Scholar] [CrossRef]
- Jahanihashemi, H.; Babaie, M.; Bijani, S.; Bazzazan, M.; Bijani, B. Poverty as an independent risk factor for in-hospital mortality in community-acquired pneumonia: A study in a developing country population. Int. J. Clin. Pract. 2018, 72, e13085. [Google Scholar] [CrossRef]
- Chalmers, J.; Campling, J.; Ellsbury, G.; Hawkey, P.M.; Madhava, H.; Slack, M. Community-acquired pneumonia in the United Kingdom: A call to action. Pneumonia 2017, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Prina, E.; Ranzani, O.T.; Polverino, E.; Cillóniz, C.; Ferrer, M.; Fernandez, L.; Puig de la Bellacasa, J.; Menéndez, R.; Mensa, J.; Torres, A. Risk factors associated with potentially antibiotic-resistant pathogens in community-acquired pneumonia. Ann. Am. Thorac. Soc. 2015, 12, 153–160. [Google Scholar] [CrossRef]
- Cillóniz, C.; Gabarrús, A.; Ferrer, M.; Puig de la Bellacasa, J.; Rinaudo, M.; Mensa, J.; Niederman, M.S.; Torres, A. Community-Acquired Pneumonia Due to Multidrug- and Non-Multidrug-Resistant Pseudomonas aeruginosa. Chest 2016, 150, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Cillóniz, C.; Dominedò, C.; Torres, A. Multidrug Resistant Gram-Negative Bacteria in Community-Acquired Pneumonia. Crit. Care 2019, 23, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliberti, S.; Cook, G.S.; Babu, B.L.; Reyes, L.F.; Rodriguez, A.; Sanz, F.; Soni, N.J.; Anzueto, A.; Faverio, P.; Sadud, R.F.; et al. International prevalence and risk factors evaluation for Drug-Resistant Streptococcus pneumoniae pneumonia. J. Infect. 2019, 79, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, D.; Aliberti, S.; Soni, N.J.; Faverio, P.; Marcos, P.J.; Wunderink, R.G.; Rodriguez, A.; Sibila, O.; Sanz, F.; Martin-Loeches, I.; et al. Prevalence and risk factors for Enterobacteriaceae in patients hospitalized with community-acquired pneumonia. Respirology 2019. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Lee, N.; Cilloniz, C.; Vila, J.; Van der Eerden, M. Laboratory diagnosis of pneumonia in the molecular age. Eur. Respir. J. 2016, 48, 1764–1778. [Google Scholar] [CrossRef] [Green Version]
- Gadsby, N.J.; Russell, C.D.; McHugh, M.P.; Mark, H.; Conway Morris, A.; Laurenson, I.F.; Hill, A.T.; Templeton, K.E. Comprehensive Molecular Testing for Respiratory Pathogens in Community-Acquired Pneumonia. Clin. Infect. Dis. 2016, 62, 817–823. [Google Scholar] [CrossRef] [Green Version]
- Feldman, C.; Anderson, R. Pneumonia as a systemic illness. Curr. Opin. Pulm. Med. 2018, 24, 237–243. [Google Scholar] [CrossRef]
- Menéndez, R.; Montull, B.; Reyes, S.; Amara-Elori, I.; Zalacain, R.; Capelastegui, A.; Aspa, J.; Borderías, L.; Martín-Villasclaras, J.J.; Bello, S.; et al. Pneumonia presenting with organ dysfunctions: Causative microorganisms, host factors and outcome. J. Infect. 2016, 73, 419–426. [Google Scholar] [CrossRef]
- Eitze, S.; Fleischmann-Struzek, C.; Betsch, C.; Reinhart, K. Vaccination60+ study group Determinants of sepsis knowledge: A representative survey of the elderly population in Germany. Crit. Care 2018, 22, 273. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, A.; Chanyasanha, C.; Sujirarat, D.; Matsumoto, N.; Nakazato, M. Factors associated with pneumococcal vaccination in elderly people: A cross-sectional study among elderly club members in Miyakonojo City, Japan. BMC Public Health 2018, 18, 1172. [Google Scholar] [CrossRef]
- Ridda, I.; Motbey, C.; Lam, L.; Lindley, I.R.; McIntyre, P.B.; Macintyre, C.R. Factors associated with pneumococcal immunisation among hospitalised elderly persons: A survey of patient’s perception, attitude, and knowledge. Vaccine 2008, 26, 234–240. [Google Scholar] [CrossRef]
- Klett-Tammen, C.J.; Krause, G.; Seefeld, L.; Ott, J.J. Determinants of tetanus, pneumococcal and influenza vaccination in the elderly: A representative cross-sectional study on knowledge, attitude and practice (KAP). BMC Public Health 2016, 16, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montull, B.; Menéndez, R.; Torres, A.; Reyes, S.; Méndez, R.; Zalacaín, R.; Capelastegui, A.; Rajas, O.; Borderías, L.; Martin-Villasclaras, J.; et al. Predictors of Severe Sepsis among Patients Hospitalized for Community-Acquired Pneumonia. PLoS ONE 2016, 11, e0145929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cilloniz, C.; Dominedò, C.; Ielpo, A.; Ferrer, M.; Gabarrus, A.; Battaglini, D.; Bermejo-Martin, J.; Meli, A.; Garcia-Vidal, C.; Liapikou, A.; et al. Risk and Prognostic Factors in Very Old Patients with Sepsis Secondary to Community-Acquired pneumonia. J Clin Med. 2019, 8, E961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliberti, S.; Dela Cruz, C.S.; Sotgiu, G.; Restrepo, M.I. Pneumonia is a neglected problem: It is now time to act. Lancet Respir. Med. 2019, 7, 10–11. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Falcone, M.; Taliani, G.; Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Venditti, M.; Chirinos, J.A.; Corrales-Medina, V.F.; et al. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 64, 1486–1493. [Google Scholar] [CrossRef]
- Musher, D.M.; Rueda, A.M.; Kaka, A.S.; Mapara, S.M. The association between pneumococcal pneumonia and acute cardiac events. Clin. Infect. Dis. 2007, 45, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 27–33. [Google Scholar] [CrossRef]
- Menéndez, R.; Méndez, R.; Aldás, I.; Reyes, S.; Gonzalez-Jimenez, P.; España, P.P.; Almirall, J.; Alonso, R.; Suescun, M.; Martinez-Dolz, L.; et al. Community-Acquired Pneumonia Patients at Risk for Early and Long-term Cardiovascular Events Are Identified by Cardiac Biomarkers. Chest 2019, 6, 1080–1091. [Google Scholar] [CrossRef]
- Denning, N.-L.; Aziz, M.; Gurien, S.D.; Wang, P. DAMPs and NETs in Sepsis. Front Immunol 2019, 10, 2536. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, J.; Eurich, D.T.; Majumdar, S.R.; Jin, Y.; Marrie, T.J. Long-term morbidity and mortality after hospitalization with community-acquired pneumonia: A population-based cohort study. Medicine 2008, 87, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Bordon, J.; Wiemken, T.; Peyrani, P.; Paz, M.L.; Gnoni, M.; Cabral, P.; Venero, M.d.C.; Ramirez, J. CAPO Study Group Decrease in long-term survival for hospitalized patients with community-acquired pneumonia. Chest 2010, 138, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Waterer, G.W.; Kessler, L.A.; Wunderink, R.G. Medium-term survival after hospitalization with community-acquired pneumonia. Am. J. Respir. Crit. Care Med. 2004, 169, 910–914. [Google Scholar] [CrossRef]
- Ciruela, P.; Izquierdo, C.; Broner, S.; Muñoz-Almagro, C.; Hernández, S.; Ardanuy, C.; Pallarés, R.; Domínguez, A.; Jané, M.; Catalan Working Group on Invasive Pneumococcal Disease. The changing epidemiology of invasive pneumococcal disease after PCV13 vaccination in a country with intermediate vaccination coverage. Vaccine 2018, 36, 7744–7752. [Google Scholar] [CrossRef]
- Martinez-Huedo, M.A.; Lopez-de-Andrés, A.; Mora-Zamorano, E.; Hernández-Barrera, V.; Jiménez-Trujillo, I.; Zamorano-Leon, J.J.; Jiménez-García, R. Decreasing influenza vaccine coverage among adults with high-risk chronic diseases in Spain from 2014 to 2017. Hum. Vaccin. Immunother. 2019. [Google Scholar] [CrossRef]
- Vacunación en población adulta. Recomendaciones. September 2018. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/prevPromocion/vacunaciones/docs/Vacunacion_poblacion_adulta.pdf (accessed on 4 December 2019).
- González-Romo, F.; Picazo, J.J.; García Rojas, A.; Labrador Horrillo, M.; Barrios, V.; Magro, M.C.; Gil Gregorio, P.; de la Cámara, R.; Rodríguez, A.; Barberán, J.; et al. Consensus document on pneumococcal vaccination in adults at risk by age and underlying clinical conditions. 2017 Update. Rev. Esp. Quimioter. 2017, 30, 142–168. [Google Scholar]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.-Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Cherit, G.; De la Torre, A.; Rishu, A.; Pinto, R.; Ñamendys-Silva, S.A.; Camacho-Ortiz, A.; Silva-Medina, M.A.; Hernández-Cárdenas, C.; Martínez-Franco, M.; Quesada-Sánchez, A.; et al. Influenza A (H1N1pdm09)-Related Critical Illness and Mortality in Mexico and Canada, 2014. Crit. Care Med. 2016, 44, 1861–1870. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018, 18, 637. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.W.; Rubinson, L.; Uyeki, T.M.; Vaughn, F.L.; John, B.B.; Miller, R.R.; Higgs, E.; Randolph, A.G.; Smoot, B.E.; Thompson, B.T. Critical Illness from 2009 Pandemic Influenza A (H1N1) Virus and Bacterial Co-Infection in the United States. Crit. Care Med. 2012, 40, 1487–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cillóniz, C.; Ewig, S.; Menéndez, R.; Ferrer, M.; Polverino, E.; Reyes, S.; Gabarrús, A.; Marcos, M.A.; Cordoba, J.; Mensa, J.; et al. Bacterial co-infection with H1N1 infection in patients admitted with community acquired pneumonia. J. Infect. 2012, 65, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC) Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1)—United States, May-August 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 1071–1074.
- Jeyaseelan, S. Bacteria: The silent killer during flu pandemics? Am. J. Respir. Crit. Care Med. 2010, 181, 874. [Google Scholar] [CrossRef]
- Zangrillo, A.; Biondi-Zoccai, G.; Landoni, G.; Frati, G.; Patroniti, N.; Pesenti, A.; Pappalardo, F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: A systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Crit. Care 2013, 17, R30. [Google Scholar] [CrossRef] [Green Version]
- Davies, A.; Jones, D.; Bailey, M.; Beca, J.; Bellomo, R.; Blackwell, N.; Forrest, P.; Gattas, D.; Granger, E.; Herkes, R.; et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009, 302, 1888–1895. [Google Scholar]
- Van de Veerdonk, F.L.; Kolwijck, E.; Lestrade, P.P.A.; Hodiamont, C.J.; Rijnders, B.J.A.; van Paassen, J.; Haas, P.-J.; Oliveira dos Santos, C.; Kampinga, G.A.; Bergmans, D.C.J.J.; et al. Influenza-associated Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2017, 196, 524–527. [Google Scholar] [CrossRef]
- Shah, M.M.; Hsiao, E.I.; Kirsch, C.M.; Gohil, A.; Narasimhan, S.; Stevens, D.A. Invasive pulmonary aspergillosis and Influenza co-infection in immunocompetent hosts: Case reports and review of the literature. Diagn. Microbiol. Infect. Dis. 2018, 91, 147–152. [Google Scholar] [CrossRef]
- Jefferson, T.; Jones, M.; Doshi, P.; Spencer, E.A.; Onakpoya, I.; Heneghan, C.J. Oseltamivir for influenza in adults and children: Systematic review of clinical study reports and summary of regulatory comments. BMJ 2014, 348, g2545. [Google Scholar] [CrossRef] [Green Version]
- Cillóniz, C.; Ewig, S.; Ferrer, M.; Polverino, E.; Gabarrús, A.; Puig de la Bellacasa, J.; Mensa, J.; Torres, A. Community-acquired polymicrobial pneumonia in the intensive care unit: Aetiology and prognosis. Crit. Care 2011, 15, R209. [Google Scholar] [CrossRef] [Green Version]
- Siegel, S.J.; Roche, A.M.; Weiser, J.N. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe 2014, 16, 55–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Toit, A. Bacterial pathogenesis: Pneumococci find a sugar daddy in influenza. Nat. Rev. Microbiol. 2014, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Civljak, R.; Nicolini, A.; Torres, A. Polymicrobial community-acquired pneumonia: An emerging entity. Respirology 2016, 21, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Ader, F.; Faure, K.; Guery, B.; Nseir, S. Pseudomonas aeruginosa and Candida albicans interaction in the respiratory tract: From pathophysiology to a therapeutic perspective. Pathol. Biol. 2008, 56, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Méar, J.-B.; Kipnis, E.; Faure, E.; Dessein, R.; Schurtz, G.; Faure, K.; Guery, B. Candida albicans and Pseudomonas aeruginosa interactions: More than an opportunistic criminal association? Med. Mal. Infect. 2013, 43, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Almirall, J.; Blanquer, J.; Bello, S. Community-acquired pneumonia among smokers. Arch. Bronconeumol. 2014, 50, 250–254. [Google Scholar] [CrossRef]
- Shen, P.; Morissette, M.C.; Vanderstocken, G.; Gao, Y.; Hassan, M.; Roos, A.; Thayaparan, D.; Merlano, M.; Dorrington, M.G.; Nikota, J.K.; et al. Cigarette Smoke Attenuates the Nasal Host Response to Streptococcus pneumoniae and Predisposes to Invasive Pneumococcal Disease in Mice. Infect. Immun. 2016, 84, 1536–1547. [Google Scholar] [CrossRef] [Green Version]
- Almirall, J.; Serra-Prat, M.; Bolíbar, I.; Balasso, V. Risk Factors for Community-Acquired Pneumonia in Adults: A Systematic Review of Observational Studies. Respiration 2017, 94, 299–311. [Google Scholar] [CrossRef]
- Bello, S.; Menéndez, R.; Antoni, T.; Reyes, S.; Zalacain, R.; Capelastegui, A.; Aspa, J.; Borderías, L.; Martin-Villasclaras, J.J.; Alfageme, I.; et al. Tobacco smoking increases the risk for death from pneumococcal pneumonia. Chest 2014, 146, 1029–1037. [Google Scholar] [CrossRef]
- Baskaran, V.; Murray, R.L.; Hunter, A.; Lim, W.S.; McKeever, T.M. Effect of tobacco smoking on the risk of developing community acquired pneumonia: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0220204. [Google Scholar] [CrossRef] [Green Version]
- Pirozzi, C.S.; Jones, B.E.; VanDerslice, J.A.; Zhang, Y.; Paine, R.; Dean, N.C. Short-Term Air Pollution and Incident Pneumonia. A Case-Crossover Study. Ann. Am. Thorac. Soc. 2018, 15, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Chanderraj, R.; Dickson, R.P. Rethinking pneumonia: A paradigm shift with practical utility. Proc. Natl. Acad. Sci. USA 2018, 115, 13148–13150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44 (Suppl 2), S27–S72. [Google Scholar] [CrossRef]
- Lim, W.S.; Baudouin, S.V.; George, R.C.; Hill, A.T.; Jamieson, C.; Le Jeune, I.; Macfarlane, J.T.; Read, R.C.; Roberts, H.J.; Levy, M.L.; et al. BTS guidelines for the management of community acquired pneumonia in adults: Update 2009. Thorax 2009, 64 (Suppl 3), iii1–iii55. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, R.; Torres, A.; Aspa, J.; Capelastegui, A.; Prat, C.; Rodríguez de Castro, F.; Sociedad Española de Neumología y Cirugía Torácica. Community acquired pneumonia. New guidelines of the Spanish Society of Chest Diseases and Thoracic Surgery (SEPAR). Arch. Bronconeumol. 2010, 46, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Priest, P.C.; Slow, S.; Chambers, S.T.; Cameron, C.M.; Balm, M.N.; Beale, M.W.; Blackmore, T.K.; Burns, A.D.; Drinković, D.; Elvy, J.A.; et al. The burden of Legionnaires’ disease in New Zealand (LegiNZ): A national surveillance study. Lancet Infect. Dis. 2019, 19, 770–777. [Google Scholar] [CrossRef]
- Bermejo-Martin, J.F.; Cilloniz, C.; Mendez, R.; Almansa, R.; Gabarrus, A.; Ceccato, A.; Torres, A.; Menendez, R.; NEUMONAC group. Lymphopenic Community Acquired Pneumonia (L-CAP), an Immunological Phenotype Associated with Higher Risk of Mortality. EBioMedicine 2017, 24, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez, R.; Menéndez, R.; Amara-Elori, I.; Feced, L.; Piró, A.; Ramírez, P.; Sempere, A.; Ortega, A.; Bermejo-Martín, J.F.; Torres, A. Lymphopenic community-acquired pneumonia is associated with a dysregulated immune response and increased severity and mortality. J. Infect. 2019, 78, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Güell, E.; Martín-Fernandez, M.; De la Torre, M.C.; Palomera, E.; Serra, M.; Martinez, R.; Solsona, M.; Miró, G.; Vallès, J.; Fernández, S.; et al. Impact of Lymphocyte and Neutrophil Counts on Mortality Risk in Severe Community-Acquired Pneumonia with or without Septic Shock. J. Clin. Med. 2019, 8, E754. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-Y. Pneumonia, Acute Respiratory Distress Syndrome, and Early Immune-Modulator Therapy. Int. J. Mol. Sci. 2017, 18, E388. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-A.; Kang, H.-M.; Rhim, J.-W.; Kang, J.-H.; Lee, K.-Y. Early Corticosteroid Therapy for Mycoplasma pneumoniae Pneumonia Irrespective of Used Antibiotics in Children. J. Clin. Med. 2019, 8, E726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, W.H.; de Steenhuijsen Piters, W.A.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.G.; Segal, L.N. The Lung Microbiome and Its Role in Pneumonia. Clin. Chest Med. 2018, 39, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Woo, T.E.; Lim, R.; Heirali, A.A.; Acosta, N.; Rabin, H.R.; Mody, C.H.; Somayaji, R.; Surette, M.G.; Sibley, C.D.; Storey, D.G.; et al. A longitudinal characterization of the Non-Cystic Fibrosis Bronchiectasis airway microbiome. Sci. Rep. 2019, 9, 6871. [Google Scholar] [CrossRef]
- Wang, Z.; Maschera, B.; Lea, S.; Kolsum, U.; Michalovich, D.; Van Horn, S.; Traini, C.; Brown, J.R.; Hessel, E.M.; Singh, D. Airway host-microbiome interactions in chronic obstructive pulmonary disease. Respir. Res. 2019, 20, 113. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Laxman, B.; Naureckas, E.T.; Hogarth, D.K.; Sperling, A.I.; Solway, J.; Ober, C.; Gilbert, J.A.; White, S.R. Associations between fungal and bacterial microbiota of airways and asthma endotypes. J. Allergy Clin. Immunol. 2019, 144, 1214–1227.e7. [Google Scholar] [CrossRef] [Green Version]
- Pallecchi, L.; Bartoloni, A.; Paradisi, F.; Rossolini, G.M. Antibiotic resistance in the absence of antimicrobial use: Mechanisms and implications. Expert Rev. Anti-Infect. Ther. 2008, 6, 725–732. [Google Scholar] [CrossRef]
- Abdissa, A.; Asrat, D.; Kronvall, G.; Shitu, B.; Achiko, D.; Zeidan, M.; Yamuah, L.K.; Aseffa, A. Throat carriage rate and antimicrobial susceptibility pattern of group A Streptococci (GAS) in healthy Ethiopian school children. Ethiop. Med J. 2011, 49, 125–130. [Google Scholar] [PubMed]
- Adler, H.; Nikolaou, E.; Gould, K.; Hinds, J.; Collins, A.M.; Connor, V.; Hales, C.; Hill, H.; Hyder-Wright, A.D.; Zaidi, S.R.; et al. Pneumococcal Colonization in Healthy Adult Research Participants in the Conjugate Vaccine Era, United Kingdom, 2010-2017. J. Infect. Dis. 2019, 219, 1989–1993. [Google Scholar] [CrossRef]
- Otani, S.; Chihade, D.B.; Coopersmith, C.M. Critical illness and the role of the microbiome. Acute Med. Surg. 2018, 6, 91–94. [Google Scholar] [CrossRef] [Green Version]
- Just Actions. The Missing Piece. Why Continued Neglect of Pneumonia Threatens the Achievement of Health Goals. 2018. Available online: http://justactions.org/wp-content/uploads/2018/11/The-Missing-PieceReport.pdf (accessed on 4 December 2019).
| Study | Immunological Profile | Clinical Correlate |
|---|---|---|
| Bermejo-Martin et al. [77] (n = 4396; CAP immunocompetent) | Lymphocytes (<724 cells/mm3) L-CAP phenotype | Increased ICU admission |
| More complications | ||
| Increased 30-day mortality | ||
| Mendez et al. [78] (n = 217, CAP immunocompetent) | L-CAP phenotype present: CD4+ depletion, higher inflammatory response, and low IgG2 levels | Increased severity of pneumonia at presentation |
| Increased treatment failure | ||
| Increased 30-day mortality | ||
| Güell et al. [79] (n = 710; CAP admitted to ICU) | Lymphopenia <675 cells/mm3 or <501 cells/mm3 | 2.32- and 3.76-fold risk of mortality in patients with or without septic shock |
| Neutrophils <8850 cells/mm3 | 3.6-fold risk of mortality |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cillóniz, C.; Menéndez, R.; García-Vidal, C.; Péricas, J.M.; Torres, A. Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators. Med. Sci. 2020, 8, 6. https://doi.org/10.3390/medsci8010006
Cillóniz C, Menéndez R, García-Vidal C, Péricas JM, Torres A. Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators. Medical Sciences. 2020; 8(1):6. https://doi.org/10.3390/medsci8010006
Chicago/Turabian StyleCillóniz, Catia, Rosario Menéndez, Carolina García-Vidal, Juan Manuel Péricas, and Antoni Torres. 2020. "Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators" Medical Sciences 8, no. 1: 6. https://doi.org/10.3390/medsci8010006
APA StyleCillóniz, C., Menéndez, R., García-Vidal, C., Péricas, J. M., & Torres, A. (2020). Defining Community-Acquired Pneumonia as a Public Health Threat: Arguments in Favor from Spanish Investigators. Medical Sciences, 8(1), 6. https://doi.org/10.3390/medsci8010006







