The Presence of Immature GV− Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ovarian Stimulation Protocol
2.3. Human Oocytes
2.4. Clinical Outcomes
2.5. Immunostaining of GV− Oocytes and Quantification of DNA Damage
2.6. Statistical Analyses
3. Results
3.1. Overall Characteristics of Study Population and of Treatment Cycles
3.2. Clinical Outcomes for GV+ and GV− Patients
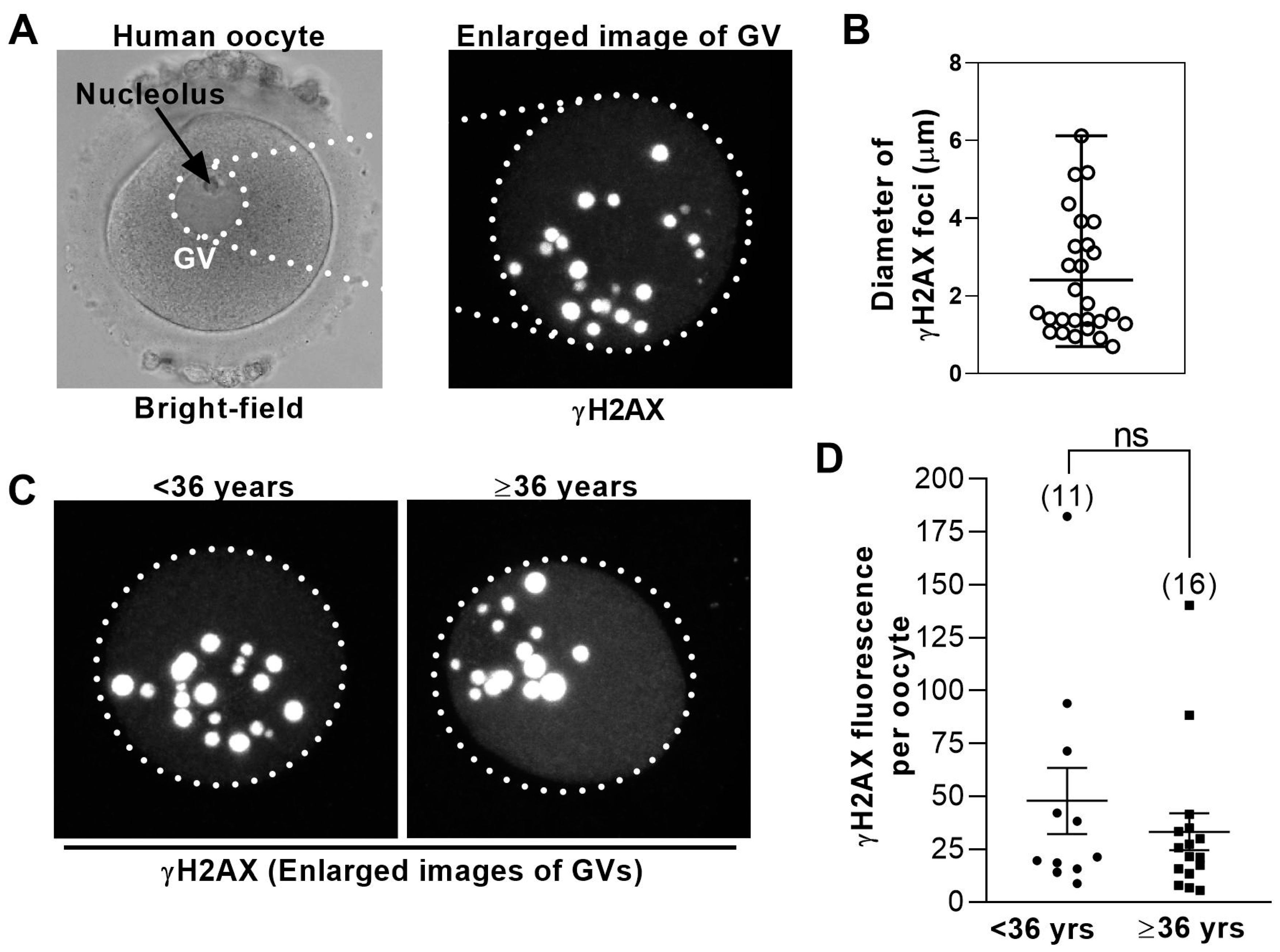
3.3. DNA Damage Levels in Human GV− Oocytes Are Uniformly High
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S.; et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci. Transl. Med. 2013, 5, 172ra121. [Google Scholar] [CrossRef] [PubMed]
- Mihalas, B.P.; Redgrove, K.A.; McLaughlin, E.A.; Nixon, B. Molecular Mechanisms Responsible for Increased Vulnerability of the Ageing Oocyte to Oxidative Damage. Oxid. Med. Cell. Longev. 2017, 2017, 4015874. [Google Scholar] [CrossRef] [PubMed]
- Winship, A.L.; Stringer, J.M.; Liew, S.H.; Hutt, K.J. The importance of DNA repair for maintaining oocyte quality in response to anti-cancer treatments, environmental toxins and maternal ageing. Hum. Reprod. Update 2018, 24, 119–134. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; Chao de la Barca, J.M.; Desquiret-Dumas, V.; Ferre-L’Hotellier, V.; Moriniere, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; McKenzie, L.J.; Matzuk, M.M. Revisiting oocyte-somatic cell interactions: In search of novel intrafollicular predictors and regulators of oocyte developmental competence. Mol. Hum. Reprod. 2008, 14, 673–678. [Google Scholar] [CrossRef]
- Alcoba, D.D.; Pimentel, A.M.; Brum, I.S.; Corleta, H.E. Developmental potential of in vitro or in vivo matured oocytes. Zygote 2015, 23, 93–98. [Google Scholar] [CrossRef]
- Halvaei, I.; Ali Khalili, M.; Razi, M.H.; Nottola, S.A. The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J. Assist. Reprod. Genet. 2012, 29, 803–810. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.C.; Kim, K.J.; Han, C.H.; Kim, J.H. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil. Steril. 2000, 74, 1153–1158. [Google Scholar] [CrossRef]
- De Vos, A.; Van de Velde, H.; Joris, H.; Van Steirteghem, A. In-vitro matured metaphase-I oocytes have a lower fertilization rate but similar embryo quality as mature metaphase-II oocytes after intracytoplasmic sperm injection. Hum. Reprod. 1999, 14, 1859–1863. [Google Scholar] [CrossRef]
- Teramoto, S.; Osada, H.; Sato, Y.; Shozu, M. Nondominant small follicles are a promising source of mature oocytes in modified natural cycle in vitro fertilization and embryo transfer. Fertil. Steril. 2016, 106, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.D.; Looman, C.W.; Weima, S.M.; te Velde, E.R. A high number of oocytes obtained after ovarian hyperstimulation for in vitro fertilization or intracytoplasmic sperm injection is not associated with decreased pregnancy outcome. Fertil. Steril. 2006, 85, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.H.; Lau, E.Y.; Yeung, W.S.; Ho, P.C. Oocyte and embryo quality in patients with excessive ovarian response during in vitro fertilization treatment. J. Assist. Reprod. Genet. 2003, 20, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Coticchio, G.; Dal Canto, M.; Guglielmo, M.C.; Albertini, D.F.; Mignini Renzini, M.; Merola, M.; Lain, M.; Sottocornola, M.; De Ponti, E.; Fadini, R. Double-strand DNA breaks and repair response in human immature oocytes and their relevance to meiotic resumption. J. Assist. Reprod. Genet. 2015, 32, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Patrizio, P.; Sakkas, D. From oocyte to baby: A clinical evaluation of the biological efficiency of in vitro fertilization. Fertil. Steril. 2009, 91, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Bromer, J.G.; Sakkas, D.; Patrizio, P. Live babies born per oocyte retrieved in a subpopulation of oocyte donors with repetitive reproductive success. Fertil. Steril. 2010, 94, 2064–2068. [Google Scholar] [CrossRef]
- Greaney, J.; Wei, Z.; Homer, H. Immunofluorescence Staining of K-Fibers in Mouse Oocytes Using Cold Fixation. Methods Mol. Biol. 2018, 1818, 77–87. [Google Scholar] [CrossRef]
- Gui, L.; Homer, H. Spindle assembly checkpoint signalling is uncoupled from chromosomal position in mouse oocytes. Development 2012, 139, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Homer, H. Hec1-dependent cyclin B2 stabilization regulates the G2-M transition and early prometaphase in mouse oocytes. Dev. Cell 2013, 25, 43–54. [Google Scholar] [CrossRef]
- Homer, H.; Gui, L.; Carroll, J. A spindle assembly checkpoint protein functions in prophase I arrest and prometaphase progression. Science 2009, 326, 991–994. [Google Scholar] [CrossRef]
- Riris, S.; Cawood, S.; Gui, L.; Serhal, P.; Homer, H.A. Immunofluorescence staining of spindles, chromosomes, and kinetochores in human oocytes. Methods Mol. Biol. 2013, 957, 179–187. [Google Scholar] [PubMed]
- Wei, Z.; Greaney, J.; Zhou, C.; Homer, H.A. Cdk1 inactivation induces post-anaphase-onset spindle migration and membrane protrusion required for extreme asymmetry in mouse oocytes. Nat. Commun. 2018, 9, 4029. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J. Cell. Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Fertility Problems: Assessment and Treatment (NICE Guideline 156) 2013. Available online: https://www.nice.org.uk/guidance/conditions-and-diseases/fertility-pregnancy-and-childbirth/fertility (accessed on 2 January 2020).
- Greaney, J.; Wei, Z.; Homer, H. Regulation of chromosome segregation in oocytes and the cellular basis for female meiotic errors. Hum. Reprod. Update 2018, 24, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Franasiak, J.M.; Forman, E.J.; Hong, K.H.; Werner, M.D.; Upham, K.M.; Treff, N.R.; Scott, R.T., Jr. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil. Steril. 2014, 101, 656–663.e1. [Google Scholar] [CrossRef] [PubMed]
| GV+ | GV− | p Value | |
|---|---|---|---|
| Number of patients | 29 | 31 | |
| Age (mean ± SD) | 36.4 ± 4.8 | 35.4 ± 4.4 | 0.45 |
| ICSI (%) | 24 (82.8) | 22 (71) | 0.22 |
| Total embryo transfer events | 46 | 40 | |
| Total embryos transferred (%) | 51 (68.9) | 43 (57.3) | 0.1 |
| Total embryos used (%) | 55 (74.3) | 52 (69.3) | 0.25 |
| Number of embryos per transfer (mean ± SD) | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.6 |
| Single embryo transfers (%) | 41 (89.1) | 37 (92.5) | 0.43 |
| Blastocyst-stage transfers (%) | 40 (78.4) | 36 (83.7) | 0.44 |
| Complete embryo utilisation (%) | 23 (79.3) | 23 (74.2) | 0.44 |
| GV+ | GV− | |
|---|---|---|
| Polycystic ovary syndrome (PCOS) (%) | 3 (10) | 4 (13) |
| Tubal factor (%) | 6 (21) | 5 (16) |
| Severe male factor (%) | 6 (21) | 5 (16) |
| Unexplained (%) | 9 (31) | 10 (32) |
| Endometriosis (%) | 5 (17) | 7 (23) |
| GV+ | GV− | p Value | |
|---|---|---|---|
| Total oocytes (GV + MI + MII) | 405 (14 ± 7.4) | 270 (8.7 ± 5) | 0.002 |
| Total GV− oocytes | 70 (2.4 ± 2.0) | ||
| Total MI-oocytes | 16 (0.5 ± 0.7) | 14 (0.4 ± 0.9) | 0.65 |
| Total MII-oocytes | 319 (11.0 ± 6.9) | 256 (8.3 ± 4.8) | 0.08 |
| Corrected MII-oocytes | 259 (8.9 ± 6.1) | 192 (6.18 ± 3.7) | 0.04 |
| GV+ | GV− | p Value | |
|---|---|---|---|
| Fertilisation rates (%) | 65.2 | 65.6 | 0.49 |
| Number of usable embryos | 74 | 75 | |
| Oocyte utilisation rate–usable embryos (%) | 23.2 (18.7–28.2) | 29.3 (23.8–35.3) | 0.049 |
| Oocyte utilisation rate–clinical pregnancy (%) | 2.3 (0.85–5) | 6.8 (3.6–11.3) | 0.02 |
| Oocyte utilisation rate–live-birth (%) | 1.9 (0.6–4.4) | 5.7 (2.9–10.0) | 0.03 |
| Implantation rate (%) | 11.8 (4.4–23.9) | 30.2 (17.2–50.8) | 0.02 |
| Live-birth rate per embryo transferred (%) | 9.8 (3.3–21.4) | 25.6 (13.5–41.2) | 0.04 |
| Miscarriage rates (%) | 16.7 (4.2–64.1) | 15.4 (1.9–45.4) | 0.47 |
| GV+ | GV− | p Value | |
|---|---|---|---|
| Oocyte utilisation rate—clinical pregnancy (%) | 1.4 (0.3–3.9) | 7.9 (3.8–14) | 0.003 |
| Oocyte utilisation rate—live-birth (%) | 0.9 (0.1–3.2) | 6.3 (2.8–12.03) | 0.005 |
| Implantation rate (%) | 7 (1.5–19.1) | 33.3 (17.3–52.8) | 0.005 |
| Live-birth rate per embryo transferred (%) | 4.9 (0.6–16.5) | 26.7 (12.3–45.9) | 0.009 |
| Miscarriage rates (%) | 33.3 (0.8–90.6) | 20 (2.5–55.6) | 0.32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astbury, P.; Subramanian, G.N.; Greaney, J.; Roling, C.; Irving, J.; Homer, H.A. The Presence of Immature GV− Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study. Med. Sci. 2020, 8, 4. https://doi.org/10.3390/medsci8010004
Astbury P, Subramanian GN, Greaney J, Roling C, Irving J, Homer HA. The Presence of Immature GV− Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study. Medical Sciences. 2020; 8(1):4. https://doi.org/10.3390/medsci8010004
Chicago/Turabian StyleAstbury, Pia, Goutham N. Subramanian, Jessica Greaney, Chris Roling, Jacqui Irving, and Hayden A. Homer. 2020. "The Presence of Immature GV− Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study" Medical Sciences 8, no. 1: 4. https://doi.org/10.3390/medsci8010004
APA StyleAstbury, P., Subramanian, G. N., Greaney, J., Roling, C., Irving, J., & Homer, H. A. (2020). The Presence of Immature GV− Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study. Medical Sciences, 8(1), 4. https://doi.org/10.3390/medsci8010004





