Persistent Urogenital Schistosomiasis and Its Associated Morbidity in Endemic Communities within Southern Ghana: Suspected Praziquantel Resistance or Reinfection?
Abstract
1. Introduction
2. Materials and Methods
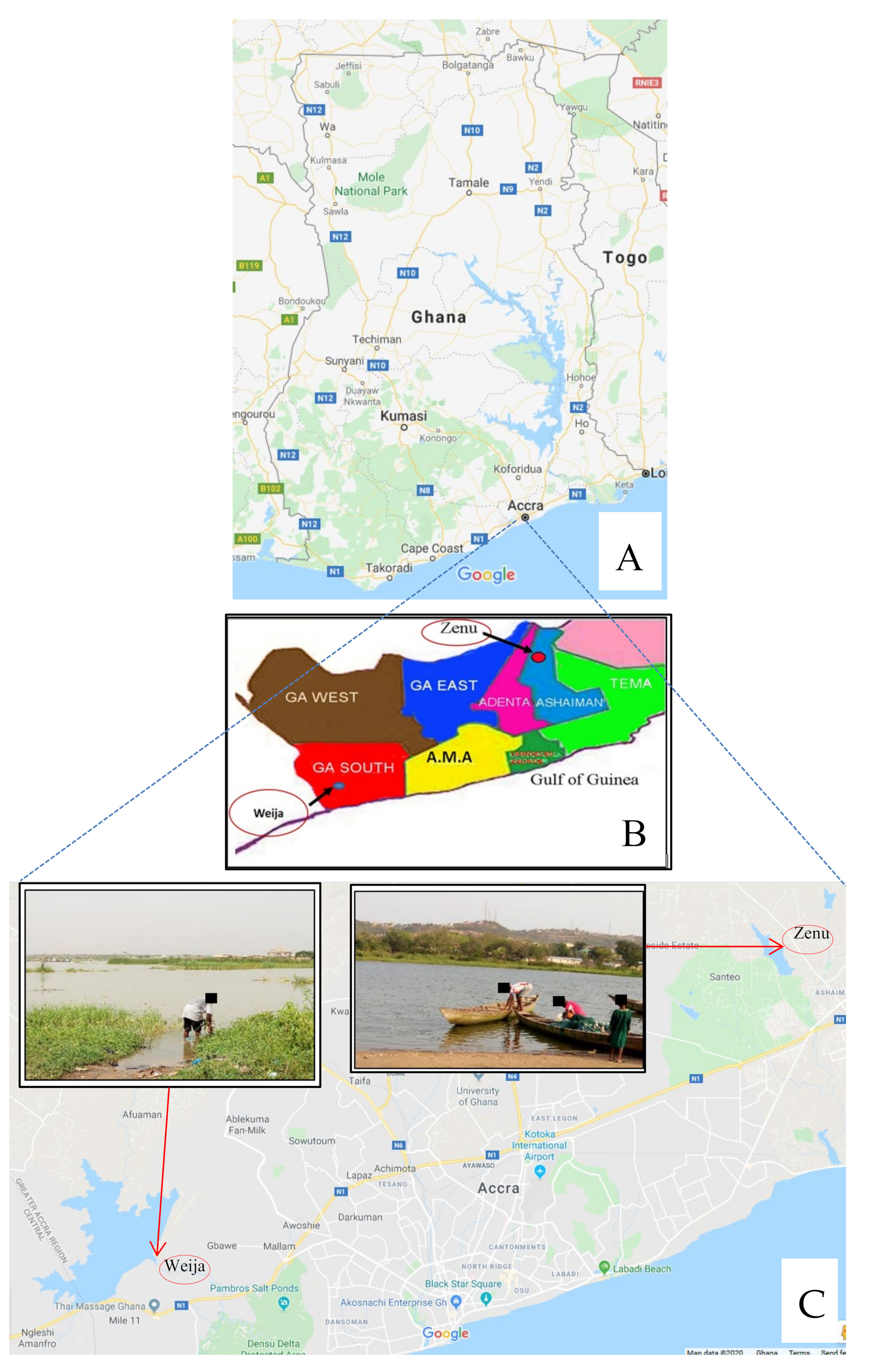
2.1. Design and Study Site
2.2. Sample Collection, Determination of Morbidity Parameters and Treatment of Infected Participants
2.3. Assessment of Reinfection
2.4. Assessment of Suspected Resistance
2.5. Egg Viability Tests
2.6. Analyses of Data
3. Results
3.1. Reinfection or Suspected Resistance
3.2. Mean Egg Count and Egg Reduction Rates
3.3. Assessment of Morbidity Parameters
3.4. Viability at Baseline and Post-Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gryseels, B.; Polman, K.; Clerinx, J.; Kestens, L. Human Schistosomiasis. Lancet 2006, 368, 1106–1118. [Google Scholar] [CrossRef]
- Hotez, P.J.; Brindley, P.J.; Bethony, J.M.; King, C.H.; Pearce, E.J. Helminth infections: The great neglected tropical diseases. J. Clin. Invest. 2008, 118, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Stothard, J.R. Improving control of African schistosomiasis: Towards effective use of rapid diagnostic tests within an appropriate disease surveillance model. Trans. R Soc. Trop. Med. Hyg. 2009, 103, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Janovy, J. Foundations of Parasitology, 6th ed.; McGraw Hill Companies Incorporated: Boston, MA, USA, 2000; Available online: https://azdoc.tips/documents/foundations-of-parasitology-2-5c15f885b25cf (accessed on 13 September 2019).
- Jordan, P.; Webbe, G.; Sturrock, R. Human schistosomiasis. Wallingford, England. CAB. 1993. Available online: https://academic.oup.com/trstmh/article-abstract/88/6/716/1917879?redirectedFrom=fulltext (accessed on 24 September 2019).
- Mostafa, M.H.; Sheweita, S.A.; O’Connor, P.J. Relationship between schistosomiasis and bladder cancer. Clin. Microbiol. Rev. 1999, 12, 97–111. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Schistosomiasis: Population requiring preventive chemotherapy and number of people treated in 2010. Wkly. Epidemiol. Rec 2012, 87, 37–44. [Google Scholar]
- Amuta, E.U.; Houmsou, R.S. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac. J. Trop. Med. 2014, 7, 34–39. [Google Scholar] [CrossRef]
- World Health Organization. The Control of Schistosomiasis. Second Report of the WHO Expert Committee, Technical Report Series no. 830. Geneva. 1993. Available online: https://apps.who.int/iris/handle/10665/37115 (accessed on 14 September 2019).
- World Health Organization. Preventive chemotherapy in human helminthiasis. 2006. Available online: https://www.paho.org/hq/index.php?option=com_docman&view=list&slug=guidelines-3954&Itemid=270&lang=en (accessed on 18 October 2019).
- Jeziorski, M.C.; Greenberg, R.M. Voltage-gated calcium channel subunits from Platyhelminthes: Potential role in Praziquantel action. Int. J. Parasitol. 2006, 36, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; Conceição, M.J. Specific Schistosomiasis treatment as a strategy for disease control. Mem. Inst. Oswaldo Cruz Rio de Janeiro 2010, 105, 598–603. [Google Scholar] [CrossRef]
- Alonso, M.; Muñoz, J.; Gascon, J.; Valls, E.M.; Corachan, M. Failure of Standard Treatment with Praziquantel in Two Returned Travelers with Schistosoma haematobium Infection. Am. J. Trop. Med. Hyg. 2006, 74, 342–344. [Google Scholar] [CrossRef]
- da Silva, I.; Thiengo, R.; Conceição, M.J.; Rey, L.; Lenzi, H.L.; Filho, P.E.; Ribeiro, P.C. Therapeutic failure of Praziquantel in the treatment of Schistosoma haematobium infection in Brazilians returning from Africa. Mem. do Inst. Oswaldo Cruz. 2005, 100, 445–449. [Google Scholar] [CrossRef]
- Oniya, M.O.; Odaibo, A.B. Reinfection Pattern and Predictors of Urinary Schistosomiasis among School Pupils from a Southwestern Village in Nigeria. Int. J. Trop. Dis. Health 2006, 1, 173–177. [Google Scholar]
- Ezeh, O.K.; Agho, K.E.; Dibley, M.J.; Hall, J.; Page, A.N. Determinants of neonatal mortality in Nigeria: Evidence from the 2008 demographic and health survey. BMC Public Health 2015, 14, 521. [Google Scholar] [CrossRef] [PubMed]
- Webster, B.L.; Diaw, O.T.; Seye, M.M.; Faye, D.S.; Stothard, J.R.; Sousa-Figueiredo, J.C.; Rollinson, D. Praziquantel treatment of School children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: Monitoring treatment success and re-infection patterns. Acta Tropica 2013, 128, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Richards, F.J.; Sullivan, J.; Ruiz-Tiben, E.; Eberhard, M.; Bishop, H. Effect of Praziquantel on the eggs of Schistosoma mansoni, with a note on the implications for managing central nervous system Schistosomiasis. Ann. Trop. Med. Ph. 1989, 83, 465–472. [Google Scholar] [CrossRef]
- Matsuda, H.; Tanaka, H.; Nogami, S.; Muto, M. Mechanism of action of Praziquantel on the eggs of Schistosoma japonicum. J. Exp. Med. 1983, 53, 271–274. [Google Scholar]
- Elfaki, T.E.M.; Osman, A.; EL Sayid, M. The Effect of Treatment on Viability of Eggs among Urinary Schistosomiasis Patients in Al-Shajara Area, Khartoum State-Sudan. Eur. Acad. Res. 2015. Vol III. [Google Scholar]
- Tetteh-Quarcoo, B.P.; Simon, K.A.; Eric, S.D.; Marian, N.; Andrew, A.M.; Afutu, E.; Edward, T.H.; Patrick, F.A.K. Urinary Schistosomiasis in Children—Still a Concern in Part of the Ghanaian Capital City. Open J. Med. Microbiol 2013, 3, 151–158. [Google Scholar] [CrossRef]
- Ampofo, J.A.; Zuta, P.C. Schistosomiasis in the Weija Lake: A case study of the public health importance of man-made lakes. Lake Reserv. Res. Management 1995, 1, 191–195. [Google Scholar] [CrossRef]
- Aboagye, I.F.; Edoh, D. Investigation of the Risk of Infection of Urinary Schistosomiasis at Mahem and Galilea Communities in the Greater Accra Region of Ghana. WAJAE 2009, 15. [Google Scholar] [CrossRef]
- Anim-Baidoo, I.; Gadri, L.; Asmah, R.H.; Owusu, E.; Markakpo, U.S.; Donkor, E.S.; Ayeh-Kumi, P. Urinary Schistosomiasis and Its Related Anaemia among Children in a High Risk Community in Ghana. Int. J. Trop. Dis. Health 2017, 22, 1–9. [Google Scholar] [CrossRef]
- Weber, M.D.; Blair, D.M.; Clarke, V. The pattern of schistosome egg distribution in a micturition flow. Cent. Afr. J. Med. 1969, 13, 75–88. [Google Scholar]
- Samie, A.; Nchachi, D.J.; Obi, C.L.; Igumbor, E.O. Prevalence and temporal distribution of Schistosoma haematobium infections in the Vhembe district, Limpopo Province, South Africa. Afr. J. Biotechnol. 2010, 42, 7157–7164. [Google Scholar]
- Zhu, H.; Xu, J.; Zhu, R.; Cao, C.; Bao, Z.; Yu, Q. Comparison of the miracidium hatching test and modified Kato-Katz method for detecting Schistosoma japonicum in low prevalence areas of China. Southeast Asian J. Trop. Med. Public Health 2014, 45, 20–25. [Google Scholar] [PubMed]
- Forson, P.O.; Tetteh-Quarcoo, P.B.; Ahenkorah, J.; Aryee, R.; Okine, E.N.; Afutu, E.; Djameh, G.I.; Agyapong, J.; Anang, A.K.; Ayeh-Kumi, P.F. Ability of Vital and Fluorescent Staining in the Differentiation of Schistosoma haematobium Live and Dead Eggs. Med. Sci. (Basel) 2019, 7, 64. [Google Scholar] [CrossRef]
- Senghor, B.; Diaw, O.T.; Doucoure, S.; Sylla, S.N.; Seye, M.; Talla, I.; Sokhna, C. Efficacy of Praziquantel against urinary schistosomiasis and reinfection in Senegalese school children where there is a single well-defined transmission period. Parasit Vectors 2015, 8, 32. [Google Scholar] [CrossRef]
- Kabuyaya, M.; Chimbari, M.J.; Manyangadze, T.; Mukaratirwa, S. Efficacy of Praziquantel on Schistosoma haematobium and reinfection rates among school-going children in the Ndumo area of uMkhanyakude district, KwaZulu-Natal, South Africa. Infect. Dis. Poverty 2017, 6, 83. [Google Scholar] [CrossRef]
- Woldegerima, E.; Bayih, A.G.; Tegegne, Y.; Aemero, M.; Zeleke, A.J. Prevalence and Reinfection Rates of Schistosoma mansoni and Praziquantel Efficacy against the Parasite among Primary School Children in Sanja Town, Northwest Ethiopia. J. Parasitol. Res. 2019, 8. [Google Scholar] [CrossRef]
- King, C.H.; Muchiri, E.M.; Ouma, J.H. Evidence against rapid emergence of Praziquantel resistance in Schistosoma haematobium, Kenya. Emerg. Infect. Dis. 2000, 6, 585–594. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Abbas, H.; Mansour, F.A.; Gasim, G.I.; Adam, I. Schistosoma haematobium infections among school children in Central Sudan one year after treatment with praziquantel. Parasit Vectors. 2012, 5, 108. [Google Scholar] [CrossRef]
- Midzi, N.; Sangweme, D.; Zinyowera, S.; Mapingure, M.P.; Brouwere, K.C.; Kumarb, N.; Mutapi, F.; Woelk, G.; Mduluzad, T. Efficacy and side effects of Praziquantel treatment against Schistosoma haematobium infection among primary school children in Zimbabwe. Trans. R Soc. Trop. Med. Hyg. 2008, 102, 759–766. [Google Scholar] [CrossRef]
- N’Goran, E.N.; Utzinger, J.; N’Guessan, A.N.; MuÈller, I.; Zamble, I.; Lohourignon, K.L.; Traore, M.; Sostheane, A.N.; Lengeler, C.; Tanner, M. Reinfection with Schistosoma haematobium following school-based chemotherapy with Praziquantel in four highly endemic villages in Coãte d’Ivoire. Trop. Med. Int. Health 2001, 6, 817–825. [Google Scholar]
- Saathoff, E.; Olsen, A.; Magnussen, P.; Kvalsvig, J.D.; Becker, W.; Appleton, C.C. Patterns of Schistosoma haematobium infection, impact of Praziquantel treatment and re-infection after treatment in a cohort of schoolchildren from rural KwaZulu-Natal/South Africa. BMC Infect. Dis. 2004, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Ojurongbe, O.; Sina-agbaje, O.R.; Busari, A.; Okorie, P.N.; Ojurongbe, T.A.; Akindele, A.A. Efficacy of Praziquantel in the treatment of Schistosoma haematobium infection among school-age children in rural communities of Abeokuta, Nigeria. Infect. Dis. Poverty 2014, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A.; Ross, G.C. Hybrids between Schistosoma haematobium and Schistosoma mattheei and their identification by isoelectric focusing of enzymes. Trans. R Soc. Trop. Med. Hyg. 1980, 14, 326–332. [Google Scholar] [CrossRef]
- Stete, K.; Krauth, S.J.; Coulibaly, J.T.; Knopp, S.; Hattendorf, J.; Müller, I.; Utzinger, J. Dynamics of Schistosoma haematobium egg output and associated infection parameters following treatment with Praziquantel in school-aged children. Parasit Vectors 2012, 5, 298. [Google Scholar] [CrossRef]
- Mekonnen, A.; Legesse, M.; Belay, M.; Tadesse, K.; Torben, W.; Teklemariam, Z.; Erko, B. Efficacy of Praziquantel against Schistosoma haematobium in Dulshatalo village, western Ethiopia. BMC Res. Notes 2013, 6, 392. [Google Scholar] [CrossRef][Green Version]
- Wami, W.M.; Nausch, N.; Midzi, N.; Gwisai, R.; Mduluza, T.; Woolhouse, M.E.J.; Mutapi, F. Comparative Assessment of Health Benefits of Praziquantel Treatment of Urogenital Schistosomiasis in Preschool and Primary School-Aged Children. Biomed. Res. Int. 2016, 2016. Article ID 9162631. [Google Scholar] [CrossRef]
- Kahama, A.I.; Vennervald, B.J.; Kombe, Y.; Kihara, R.W.; Ndzoru, M.; Mungai, P.; Ouma, J.H. Parameters associated with Schistosoma haematobium infection before and after chemotherapy in school children from two villages in the Coast province of Kenya. Trop. Med. Int. Health. 1999, 4, 335–340. [Google Scholar] [CrossRef]




| Method Used # | Baseline (%) (Pre-Treatment) | Post-Treatment (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Average * | |||||||
| Live | Dead | Live | Dead | Live | Dead | Live | Dead | Live | Dead | |
| Trypan blue | 60.25 | 39.75 | 19.16 | 80.84 | 27.27 | 72.27 | 0.00 | 100.00 | 15.47 | 84.53 |
| Neutral red | 70.81 | 29.19 | 12.05 | 87.95 | 16.70 | 83.33 | 11.11 | 88.89 | 13.28 | 86.72 |
| Fluorescent microscopy | 80.12 | 19.88 | 13.86 | 86.14 | 13.33 | 86.67 | 11.11 | 88.89 | 12.76 | 87.24 |
| Modified hatchability | 70.19 | 29.81 | 13.86 | 86.14 | 13.33 | 86.67 | 11.11 | 88.89 | 12.77 | 87.23 |
| Mean | 70.34 | 29.66 | 14.73 | 85.27 | 17.78 | 82.09 | 8.33 | 91.67 | 13.57 | 86.43 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tetteh-Quarcoo, P.B.; Forson, P.O.; Amponsah, S.K.; Ahenkorah, J.; Opintan, J.A.; Ocloo, J.E.Y.; Okine, E.N.; Aryee, R.; Afutu, E.; Anang, A.K.; et al. Persistent Urogenital Schistosomiasis and Its Associated Morbidity in Endemic Communities within Southern Ghana: Suspected Praziquantel Resistance or Reinfection? Med. Sci. 2020, 8, 10. https://doi.org/10.3390/medsci8010010
Tetteh-Quarcoo PB, Forson PO, Amponsah SK, Ahenkorah J, Opintan JA, Ocloo JEY, Okine EN, Aryee R, Afutu E, Anang AK, et al. Persistent Urogenital Schistosomiasis and Its Associated Morbidity in Endemic Communities within Southern Ghana: Suspected Praziquantel Resistance or Reinfection? Medical Sciences. 2020; 8(1):10. https://doi.org/10.3390/medsci8010010
Chicago/Turabian StyleTetteh-Quarcoo, Patience B., Peter O. Forson, Seth K. Amponsah, John Ahenkorah, Japheth A. Opintan, Janet E. Y. Ocloo, Esther N. Okine, Robert Aryee, Emmanuel Afutu, Abraham K. Anang, and et al. 2020. "Persistent Urogenital Schistosomiasis and Its Associated Morbidity in Endemic Communities within Southern Ghana: Suspected Praziquantel Resistance or Reinfection?" Medical Sciences 8, no. 1: 10. https://doi.org/10.3390/medsci8010010
APA StyleTetteh-Quarcoo, P. B., Forson, P. O., Amponsah, S. K., Ahenkorah, J., Opintan, J. A., Ocloo, J. E. Y., Okine, E. N., Aryee, R., Afutu, E., Anang, A. K., & Ayeh-Kumi, P. F. (2020). Persistent Urogenital Schistosomiasis and Its Associated Morbidity in Endemic Communities within Southern Ghana: Suspected Praziquantel Resistance or Reinfection? Medical Sciences, 8(1), 10. https://doi.org/10.3390/medsci8010010





