In Silico Insights into HIV-1 Vpu-Tetherin Interactions and Its Mutational Counterparts
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence and Structure Retrieval
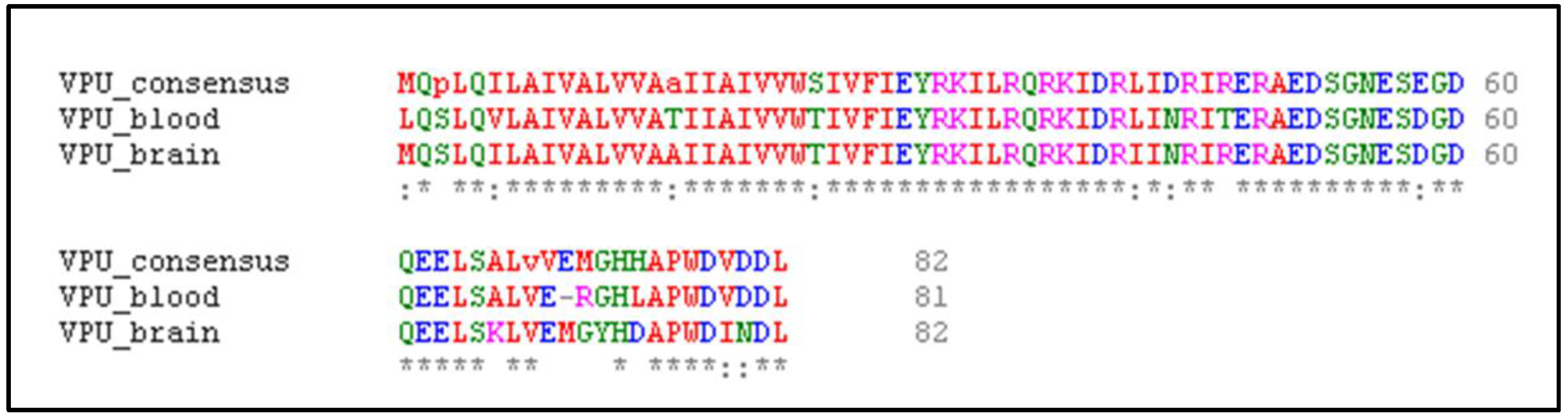
2.2. Multiple Sequence Alignment and Generation of Consensus Vpu Sequence
2.3. Protein Structure Modeling and Validation
2.4. Protein–Protein Interaction
2.5. Mutational Study
2.6. Aggregation Potential Prediction of Tetherin
3. Results
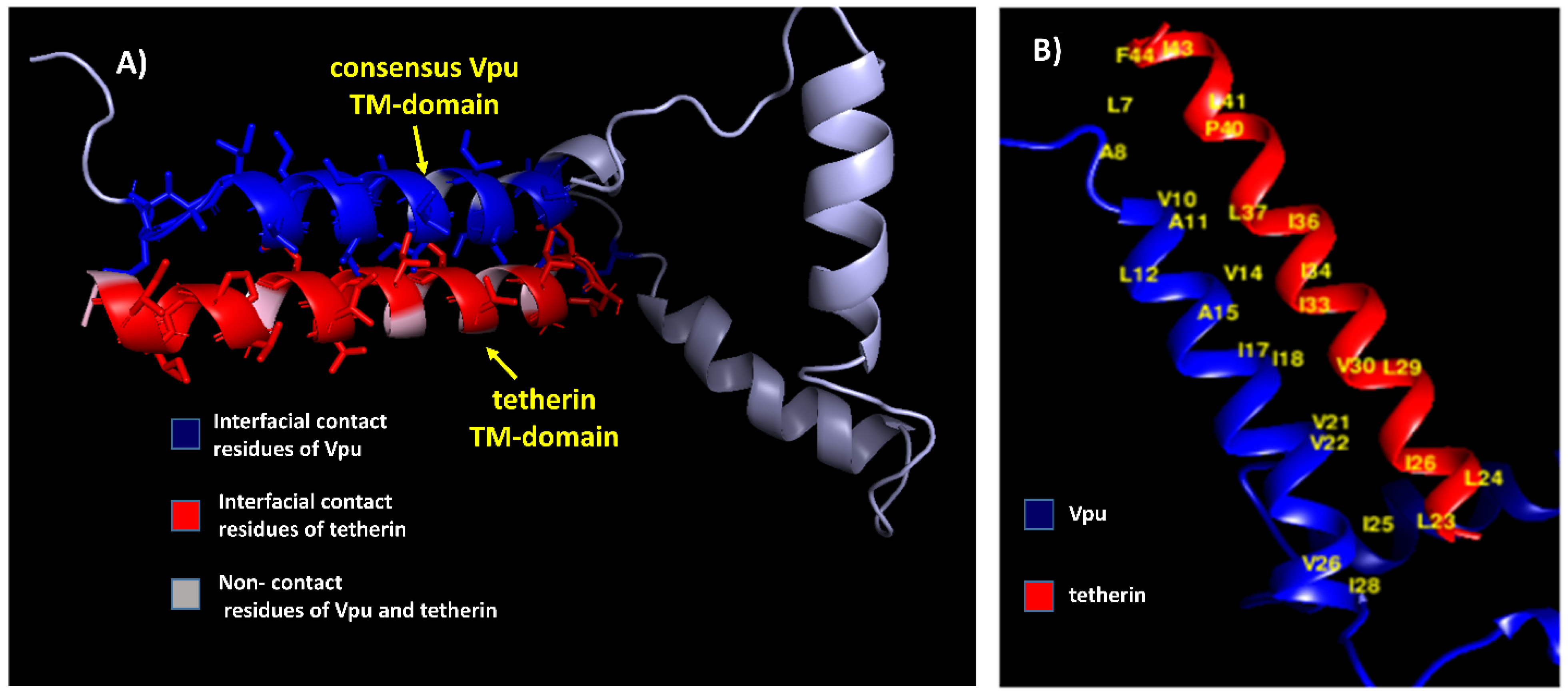
3.1. Modeled Protein Structures and Evaluation
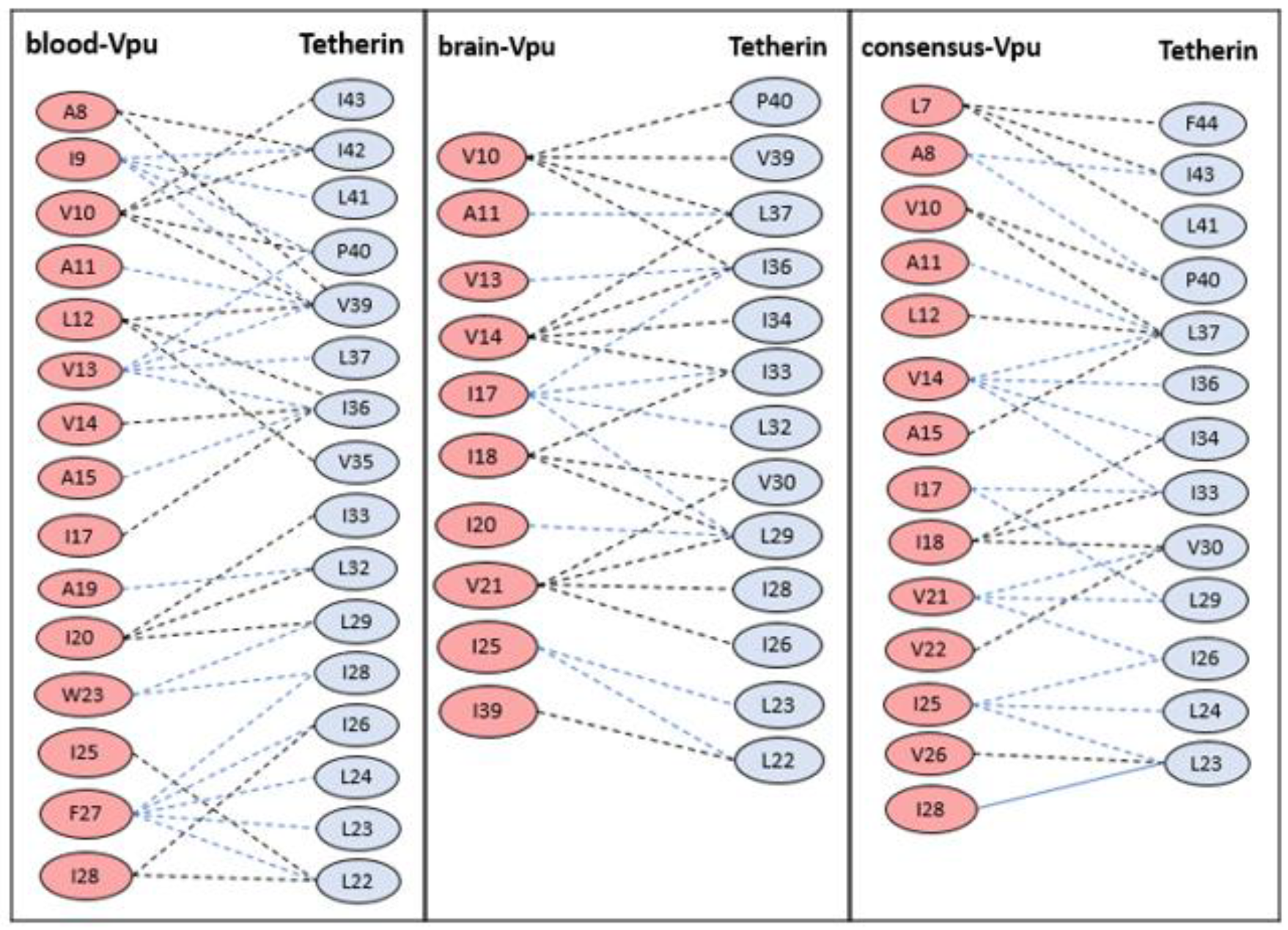
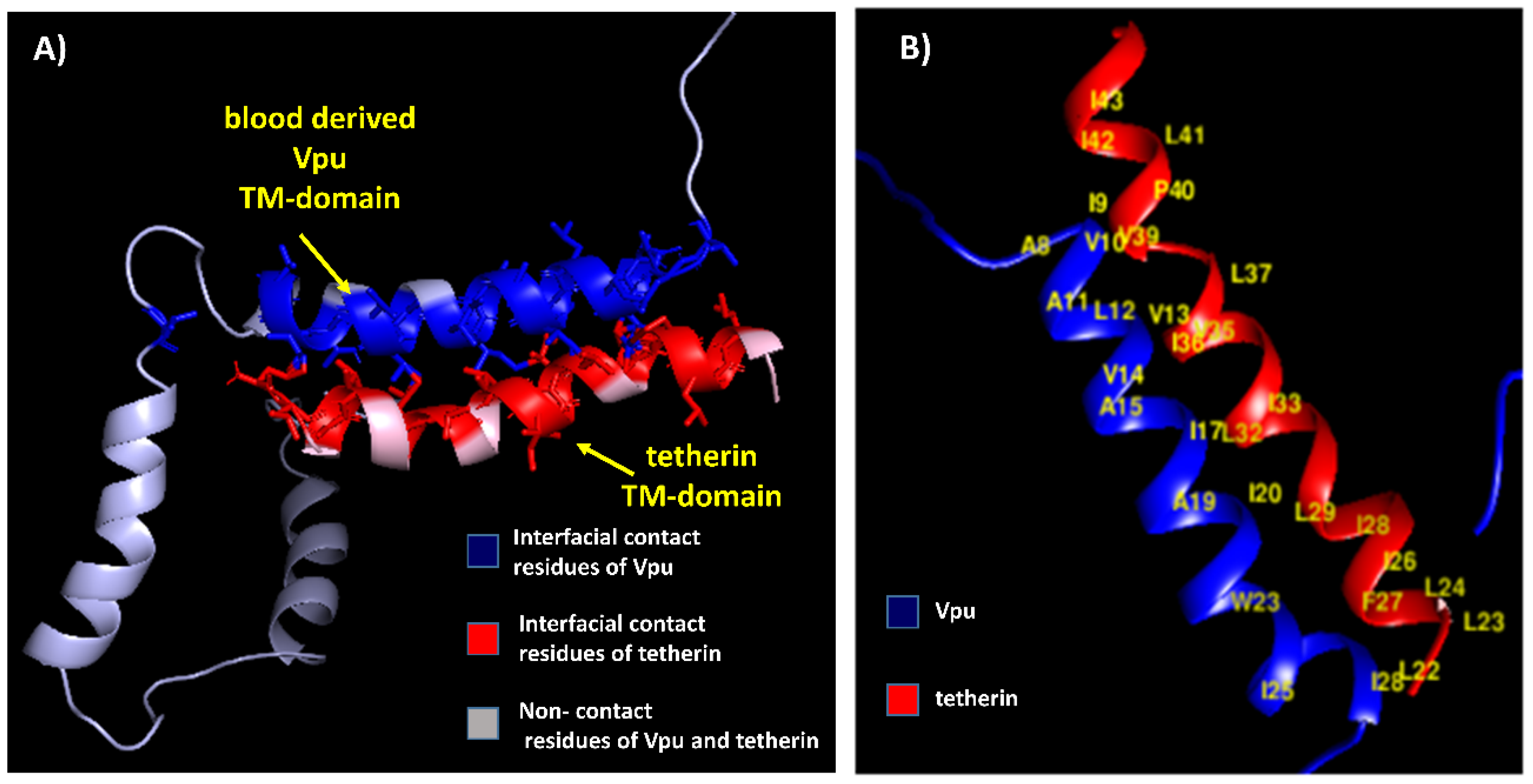
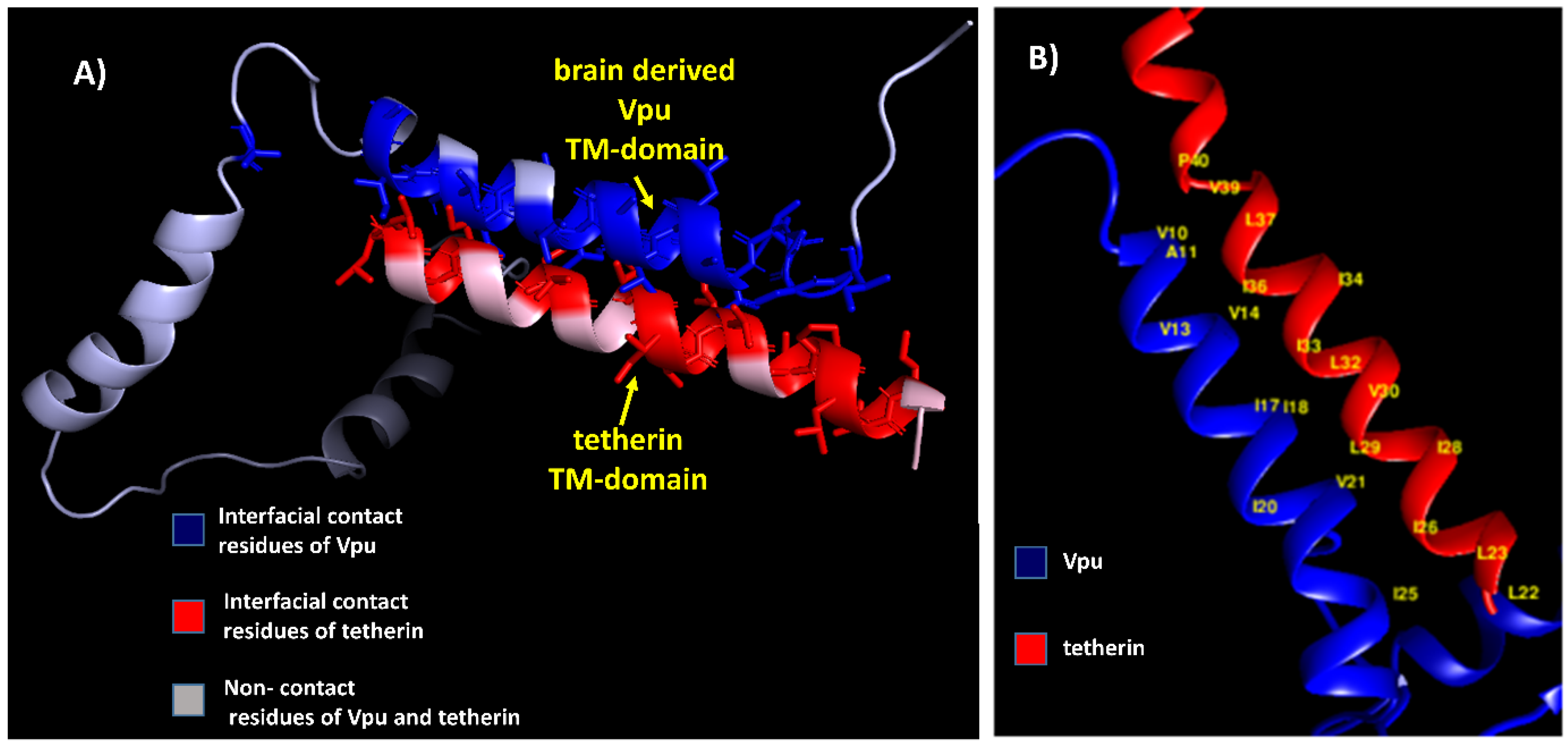
3.2. Docking Studies of Wild-Type Structures
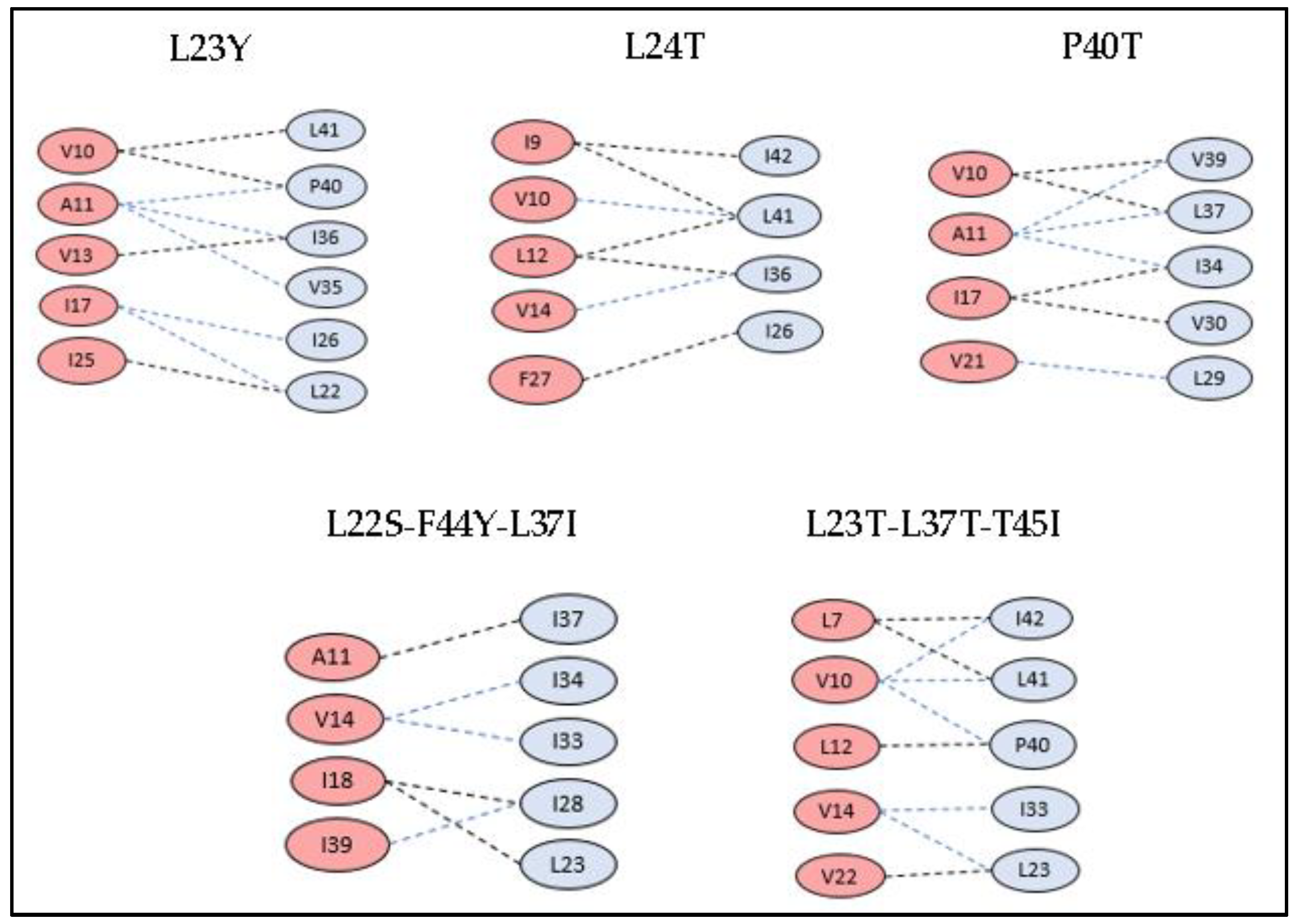
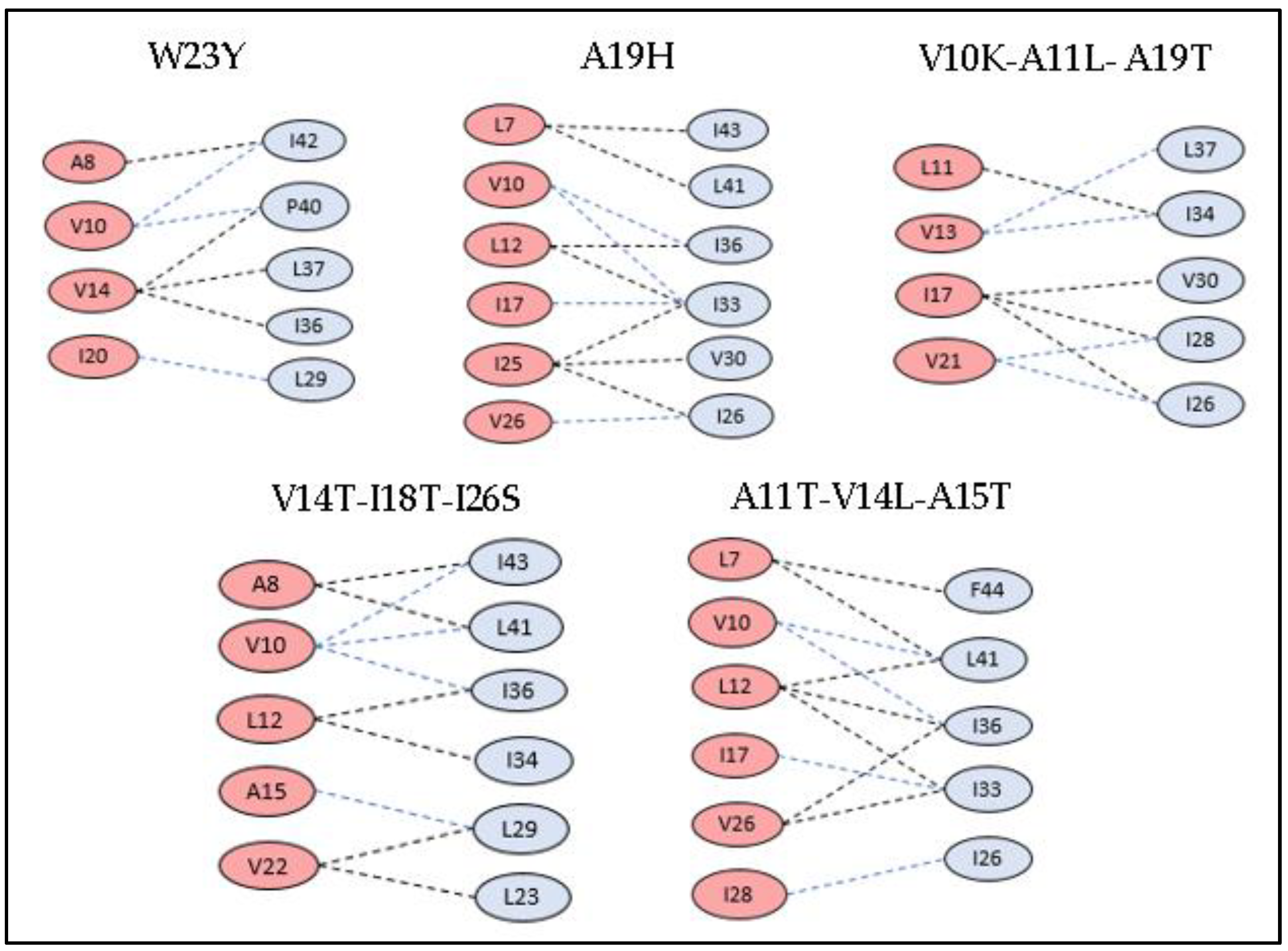
3.3. Docking Studies of Mutant Structures
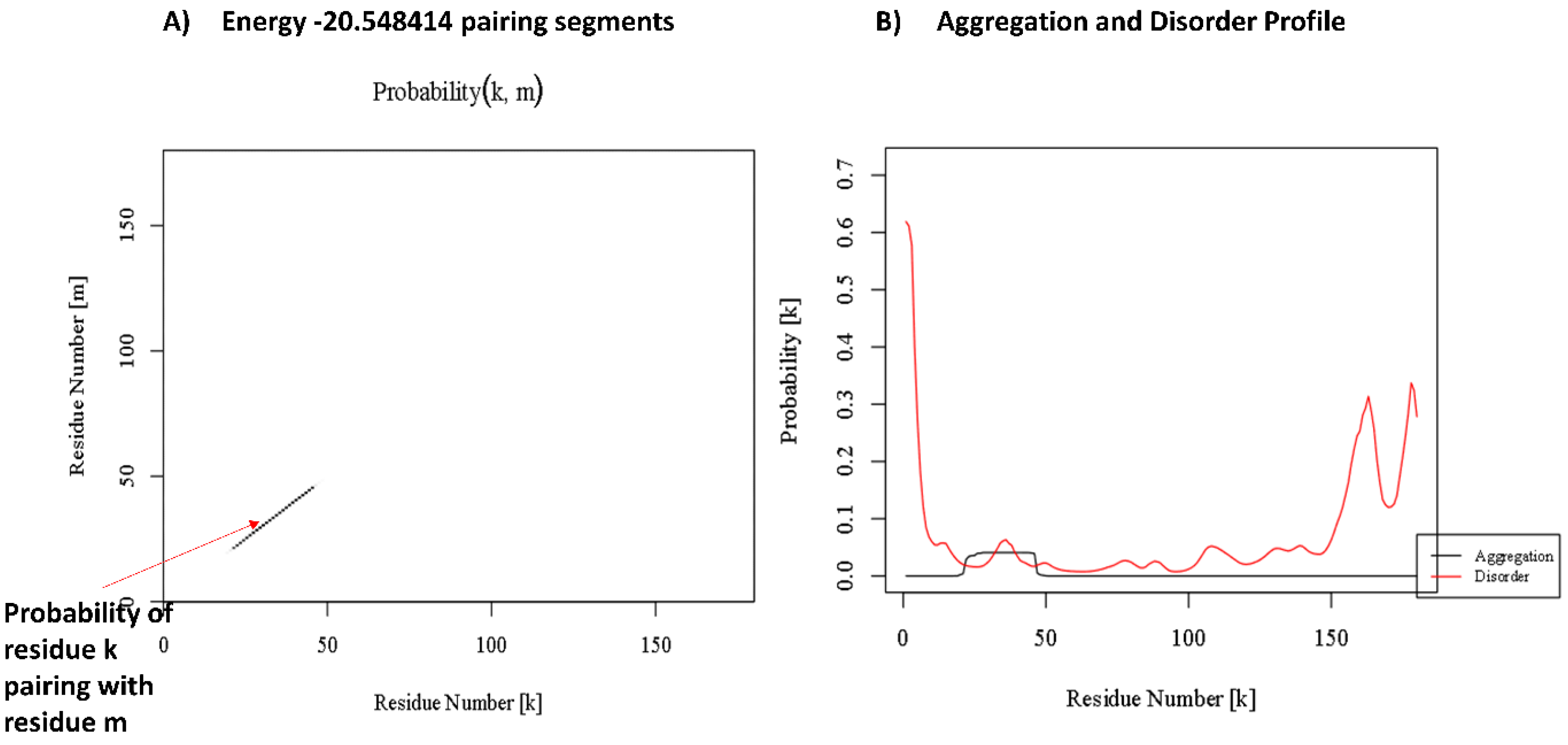
3.4. Amyloidogenicity Prediction of Tetherin
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Le Tortorec, A.; Willey, S.; Neil, S.J. Antiviral Inhibition of Enveloped Virus Release by Tetherin/BST-2: Action and Counteraction. Viruses 2011, 3, 520–540. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.T.; Serra-Moreno, R.; Singh, R.K.; Guatelli, J.C. BST-2/tetherin: A new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010, 18, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Kupzig, S.; Korolchuk, V.; Rollason, R.; Sugden, A.; Wilde, A.; Banting, G. Bst-2/HM1.24 Is a Raft-Associated Apical Membrane Protein with an Unusual Topology. Traffic 2003, 4, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Arias, J.F.; Iwabu, Y.; Tokunaga, K. Structural Basis for the Antiviral Activity of BST-2/Tetherin and Its Viral Antagonism. Front. Microbiol. 2011, 2, 250. [Google Scholar] [CrossRef] [PubMed]
- Bieniasz, P.D. The cell biology of HIV-1 virion genesis. Cell Host Microbe 2009, 5, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef]
- Nomaguchi, M.; Fujita, M.; Adachi, A. Role of HIV-1 Vpu protein for virus spread and pathogenesis. Microbes Infect. 2008, 10, 960–967. [Google Scholar] [CrossRef]
- Mangeat, B.; Gers-Huber, G.; Lehmann, M.; Zufferey, M.; Luban, J.; Piguet, V. HIV-1 Vpu Neutralizes the Antiviral Factor Tetherin/BST-2 by Binding It and Directing Its Beta-TrCP2-Dependent Degradation. PLoS Pathog. 2009, 5, e1000574. [Google Scholar] [CrossRef]
- Iwabu, Y.; Fujita, H.; Kinomoto, M.; Kaneko, K.; Ishizaka, Y.; Tanaka, Y.; Tokunaga, K. HIV-1 Accessory Protein Vpu Internalizes Cell-surface BST-2/Tetherin through Transmembrane Interactions Leading to Lysosomes. J. Biol. Chem. 2009, 284, 35060–35072. [Google Scholar] [CrossRef]
- Douglas, J.L.; Viswanathan, K.; McCarroll, M.N.; Gustin, J.K.; Fruh, K.; Moses, A.V. Vpu Directs the Degradation of the Human Immunodeficiency Virus Restriction Factor BST-2/Tetherin via a TrCP-Dependent Mechanism. J. Virol. 2009, 83, 7931–7947. [Google Scholar] [CrossRef]
- McNatt, M.W.; Zang, T.; Bieniasz, P.D. Vpu binds directly to tetherin and displaces it from nascent virions. PLoS Pathog. 2013, 9, e1003299. [Google Scholar] [CrossRef] [PubMed]
- Skasko, M.; Wang, Y.; Tian, Y.; Tokarev, A.; Munguia, J.; Ruiz, A.; Guatelli, J. HIV-1 Vpu Protein Antagonizes Innate Restriction Factor BST-2 via Lipid-embedded Helix-Helix Interactions. J. Biol. Chem. 2011, 287, 58–67. [Google Scholar] [CrossRef]
- Tokarev, A.; Skasko, M.; Fitzpatrick, K.; Guatelli, J. Antiviral Activity of the Interferon-Induced Cellular Protein BST-2/Tetherin. AIDS Res. Hum. Retrovir. 2009, 25, 1197–1210. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ode, H.; Yoshida, T.; Sato, K.; Gee, P.; Yamamoto, S.P.; Koyanagi, Y. Identification of Amino Acids in the Human Tetherin Transmembrane Domain Responsible for HIV-1 Vpu Interaction and Susceptibility. J. Virol. 2010, 85, 932–945. [Google Scholar] [CrossRef] [PubMed]
- Vigan, R.; Neil, S.J. Determinants of Tetherin Antagonism in the Transmembrane Domain of the Human Immunodeficiency Virus Type 1 Vpu Protein. J. Virol. 2010, 84, 12958–12970. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Hué, S.; Schaller, T.; Verschoor, E.; Pillay, D.; Towers, G.J. Mutation of a Single Residue Renders Human Tetherin Resistant to HIV-1 Vpu-Mediated Depletion. PLoS Pathog. 2009, 5, e1000443. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Hu, S.; Li, J.; Xu, F.; Mei, S.; Zhou, J.; Cen, S.; Jin, Q.; Guo, F. Identification of novel key amino acids at the interface of the transmembrane domains of human BST-2 and HIV-1 Vpu. Retrovirology 2013, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fischer, W.B. Correlation of biological activity with computationally derived structural features from transmembrane hetero-dimers of HIV-1 Vpu with host factors. Biochim. Biophys. Acta Biomembr. 2014, 1838, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Skasko, M.; Tokarev, A.; Chen, C.; Fischer, W.B.; Pillai, S.K.; Guatelli, J. BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu: Evidence for a post-ER mechanism of Vpu-action. Virology 2011, 411, 65–77. [Google Scholar] [CrossRef]
- Blackard, J.T. HIV Compartmentalization: A Review on a Clinically Important Phenomenon. Curr. HIV Res. 2012, 10, 133–142. [Google Scholar] [CrossRef]
- Hasan, Z. Role of host immune responses in sequence variability of HIV-1 Vpu. World J. Immunol. 2014, 4, 107. [Google Scholar] [CrossRef]
- Balaji, S.; Sneha, P.; Rama, M.; Shapshak, P. Global Protein Sequence Variation in HIV-1-B Isolates Derived from Human Blood and Brain. Glob. Virol. Identif. Investig. Viral Dis. 2015, 1, 613–666. [Google Scholar] [CrossRef]
- Sneha, P.; Panda, P.K.; Gharemirshamlu, F.R.; Bamdad, K.; Balaji, S. Structural discordance in HIV-1 Vpu from brain isolate alarms amyloid fibril forming behavior-a computational perspective. J. Theor. Biol. 2018, 451, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.M.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Higgins, D.G. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2014, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, S.; Cowley, A.; Lee, J.; Foix, A.; Lopez, R. Programmatic access to bioinformatics tools from EMBL-EBI update: 2017. Nucleic Acids Res. 2017, 45, W550–W553. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Schwede, T. Swiss-Model: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Sippl, M.J. Recognition of errors in three-dimensional structures of proteins. Proteins Struct. Funct. Genet. 1993, 17, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.W.; Kemp, G.J. Protein docking using spherical polar Fourier correlations. Proteins Struct. Funct. Genet. 2000, 39, 178. [Google Scholar] [CrossRef]
- Ritchie, D.W.; Kozakov, D.; Vajda, S. Accelerating and focusing protein-protein docking correlations using multi-dimensional rotational FFT generating functions. Bioinformatics 2008, 24, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Vangone, A.; Bonvin, A.M. Contacts-based prediction of binding affinity in protein–protein complexes. ELife 2015, 4, e07454. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. Prodigy: A web server for predicting the binding affinity of protein–protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://pymol.org (accessed on 19 May 2019).
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Tina, K.G.; Bhadra, R.; Srinivasan, N. PIC: Protein Interactions Calculator. Nucleic Acids Res. 2007, 35, W473–W476. [Google Scholar] [CrossRef] [PubMed]
- Capriotti, E.; Fariselli, P.; Casadio, R. I-Mutant2.0: Predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005, 33, W306–W310. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Monsellier, E.; Chiti, F. Prevention of amyloid-like aggregation as a driving force of protein evolution. EMBO Rep. 2007, 8, 737–742. [Google Scholar] [CrossRef]
- Chiti, F.; Stefani, M.; Taddei, N.; Ramponi, G.; Dobson, C.M. Rationalization of the effects of mutations on peptide andprotein aggregation rates. Nature 2003, 424, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Serpell, L.C.; Bartlam, M.; Fraser, P.E.; Pepys, M.B.; Blake, C.C. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J. Mol. Biol. 1997, 273, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Garbuzynskiy, S.O.; Lobanov, M.Y.; Galzitskaya, O.V. FoldAmyloid: A method of prediction of amyloidogenic regions from protein sequence. Bioinformatics 2009, 26, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Conchillo-Solé, O.; De Groot, N.S.; Avilés, F.X.; Vendrell, J.; Daura, X.; Ventura, S. AGGRESCAN: A server for the prediction and evaluation of "hot spots" of aggregation in polypeptides. BMC Bioinf. 2007, 8, 65. [Google Scholar] [CrossRef]
- Fernandez-Escamilla, A.; Rousseau, F.; Schymkowitz, J.; Serrano, L. Prediction of sequence-dependent and mutational effects on the aggregation of peptides and proteins. Nat. Biotechnol. 2004, 22, 1302–1306. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Stroh, S.; Debulpaep, M.; Kuemmerer, N.; De la Paz, M.L.; Martins, I.C.; Reumers, J.; Rousseau, F. Exploring the sequence determinants of amyloid structure using position-specific scoring matrices. Nat. Methods 2010, 7, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Emily, M.; Talvas, A.; Delamarche, C. MetAmyl: A METa-Predictor for AMYLoid Proteins. PLoS ONE 2013, 8, e79722. [Google Scholar] [CrossRef] [PubMed]
- Tsolis, A.C.; Papandreou, N.C.; Iconomidou, V.A.; Hamodrakas, S.J. A Consensus Method for the Prediction of ‘Aggregation-Prone’ Peptides in Globular Proteins. PLoS ONE 2013, 8, e54175. [Google Scholar] [CrossRef] [PubMed]
- Walsh, I.; Seno, F.; Tosatto, S.C.; Trovato, A. PASTA 2.0: An improved server for protein aggregation prediction. Nucleic Acids Res. 2014, 42, W301–W307. [Google Scholar] [CrossRef] [PubMed]
- Lopez de la Paz, M.; Serrano, L. Sequence determinants of amyloid fibril formation. Proc. Natl. Acad. Sci. USA 2013, 101, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Zibaee, S.; Makin, O.S.; Goedert, M.; Serpell, L.C. A simple algorithm locates β-strands in the amyloid fibril core of α-synuclein, Aβ, and tau using the amino acid sequence alone. Protein Sci. 2007, 16, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, H.; Lai, L. Identification of amyloid fibril-forming segments based on structure and residue-based statistical potential. Bioinformatics 2007, 23, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Fasman, G.D. Prediction of protein conformation. Biochemistry 1974, 13, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Loh, S.N. Protein Conformational Switches: From Nature to Design. Chem. Eur. J. 2012, 18, 7984–7999. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liang, C. Transmembrane Interactions of HIV-1 Vpu and Tetherin. Curr. HIV Res. 2012, 10, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Burkala, E.J.; He, J.; West, J.T.; Wood, C.; Petito, C.K. Compartmentalization of HIV-1 in the central nervous system: Role of the choroid plexus. AIDS 2005, 19, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri, M.; Amini, S.; Khalili, K.; Sawaya, B.E. HIV-1 associated dementia: Symptoms and causes. Retrovirology 2006, 3, 28. [Google Scholar] [CrossRef]
- Waheed, A.A.; Ablan, S.D.; Soheilian, F.; Nagashima, K.; Ono, A.; Schaffner, C.P.; Freed, E.O. Inhibition of Human Immunodeficiency Virus Type 1 Assembly and Release by the Cholesterol-Binding Compound Amphotericin B Methyl Ester: Evidence for Vpu Dependence. J. Virol. 2008, 82, 9776–9781. [Google Scholar] [CrossRef]
- Ross, C.A.; Poirier, M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef]
- Kallberg, Y.; Gustafsson, M.; Persson, B.; Thyberg, J.; Johansson, J. Prediction of amyloid fibril forming proteins. J. Biol. Chem. 2001, 276, 12945–12950. [Google Scholar] [CrossRef]
- Brew, B.J.; Crowe, S.M.; Landay, A.; Cysique, L.A.; Guillemin, G. Neurodegeneration and Ageing in the HAART Era. J. Neuroimmune Pharmacol. 2008, 4, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Khanlou, N.; Khanlou, N.; Moore, D.J.; Khanlou, N.; Moore, D.J.; Chana, G. The HNRC Group. Increased frequency of α-synuclein in the substantia nigra in human immunodeficiency virus infection. J. Neurovirol. 2009, 15, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Green, D.A.; Masliah, E.; Vinters, H.V.; Beizai, P.; Moore, D.J.; Achim, C.L. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS 2005, 19, 407–411. [Google Scholar] [CrossRef] [PubMed]
- András, I.E.; Toborek, M. Amyloid beta accumulation in HIV-1-infected brain: The role of the blood brain barrier. IUBMB Life 2012, 65, 43–49. [Google Scholar] [CrossRef]
- Rempel, H.C.; Pulliam, L. HIV-1 Tat inhibits neprilysin and elevates amyloid β. AIDS 2005, 19, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hui, L.; Geiger, N.H.; Haughey, N.J.; Geiger, J.D. Endolysosome in-volvement in HIV-1 transactivator protein-induced neuronal amyloid beta pro-duction. Neurobiology 2013, 34, 2370–2378. [Google Scholar]
- Aksenov, M.Y.; Aksenova, M.V.; Mactutus, C.F.; Booze, R.M. HIV-1 protein-me-diated amyloidogenesis in rat hippocampal cell cultures. Neurosci. Lett. 2010, 475, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, W.M.; Farzan, M.; Joyal, J.L.; Carter, K.; Babcock, G.J.; Israel, D.I.; So-droski, J.; Mirzabekov, T. Stimulation of enveloped virus infection by β-amyloid fibrils. J. Biol. Chem. 2002, 277, 35019–35024. [Google Scholar] [CrossRef]
- Kopito, R.R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef]
- Hardy, J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Huang, Y.; Mucke, L. Alzheimer Mechanisms and Therapeutic Strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A.; Kwon, Y.T. Degradation of misfolded proteins in neurodegenerative diseases: Therapeutic targets and strategies. Exp. Mol. Med. 2015, 47, e147. [Google Scholar] [CrossRef] [PubMed]
| Position | Substitution | Characteristic | SIFT Tolerated | SIFT Score | I-Mutant Stability | RI | DDG (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 22 | L → S | Uncharged Polar | No | 0.01 | Decrease | 8 | −1.31 |
| L → M | Nonpolar | Yes | 0.07 | Decrease | 5 | −0.85 | |
| L → K | Basic | No | 0.01 | Decrease | 8 | −1.36 | |
| L → F | Nonpolar | Yes | 0.05 | Decrease | 7 | −0.89 | |
| L →Y | Uncharged Polar | No | 0.01 | Decrease | 2 | −1.07 | |
| 23 | L →T | Uncharged Polar | Yes | 0.07 | Decrease | 8 | −1.52 |
| L → S | Uncharged Polar | No | 0.04 | Decrease | 9 | −1.55 | |
| L → H | Basic | No | 0.03 | Decrease | 9 | −1.72 | |
| L → V | Nonpolar | Yes | 0.43 | Decrease | 6 | −0.91 | |
| L → R | Basic | Yes | 0.11 | Decrease | 5 | −1.15 | |
| L → Y | Uncharged Polar | No | 0.02 | Decrease | 2 | −1.07 | |
| 24 | L → T | Uncharged Polar | No | 0.04 | Decrease | 8 | −1.56 |
| L → R | Basic | No | 0.01 | Decrease | 5 | −1.19 | |
| L →M | Nonpolar | Yes | 0.09 | Decrease | 6 | −0.97 | |
| L→ I | Nonpolar | Yes | 0.63 | Decrease | 7 | −1.10 | |
| L → E | Acidic | No | 0.01 | Decrease | 6 | −1.41 | |
| 25 | G → A | Nonpolar | Yes | 1.00 | Decrease | 1 | −0.57 |
| G → C | Nonpolar | Yes | 0.09 | Decrease | 6 | −0.98 | |
| G → T | Uncharged Polar | Yes | 0.16 | Decrease | 7 | −0.79 | |
| G → L | Nonpolar | Yes | 0.10 | Decrease | 6 | −0.53 | |
| G → Y | Uncharged Polar | No | 0.03 | Decrease | 3 | −0.79 | |
| 26 | I → S | Uncharged Polar | Yes | 0.05 | Decrease | 9 | −1.33 |
| I → L | Nonpolar | Yes | 0.34 | Decrease | 8 | −0.63 | |
| I → D | Acidic | No | 0.02 | Decrease | 8 | −1.29 | |
| I → N | Uncharged Polar | No | 0.03 | Decrease | 8 | −1.26 | |
| 29 | L → D | Acidic | No | 0.04 | Decrease | 8 | −1.82 |
| L → F | Nonpolar | Yes | 0.13 | Decrease | 8 | −1.20 | |
| L → K | Basic | Yes | 0.08 | Decrease | 9 | −1.88 | |
| L → V | Nonpolar | Yes | 0.28 | Decrease | 7 | −1.14 | |
| 30 | V → G | Nonpolar | Yes | 0.55 | Decrease | 10 | −2.06 |
| V → H | Basic | No | 0.03 | Decrease | 10 | −1.87 | |
| V → E | Acidic | Yes | 0.10 | Decrease | 8 | −1.54 | |
| V → Q | Uncharged Polar | Yes | 0.07 | Decrease | 9 | −1.52 | |
| V → A | Nonpolar | Yes | 1.00 | Decrease | 9 | −1.29 | |
| 33 | I → T | Uncharged Polar | No | 0.01 | Decrease | 9 | −1.46 |
| I → F | Nonpolar | Yes | 0.09 | Decrease | 9 | −1.00 | |
| I → K | Basic | No | 0.00 | Decrease | 9 | −1.59 | |
| I → V | Nonpolar | Yes | 1.00 | Decrease | 6 | −0.45 | |
| 34 | I → T | Uncharged Polar | No | 0.04 | Decrease | 9 | −1.50 |
| I → G | Uncharged Polar | Yes | 0.09 | Decrease | 9 | −2.09 | |
| I → F | Nonpolar | Yes | 0.07 | Decrease | 9 | −0.98 | |
| I → L | Nonpolar | Yes | 0.45 | Decrease | 8 | −0.83 | |
| 36 | I → G | Uncharged Polar | Yes | 0.17 | Decrease | 9 | −2.05 |
| I → A | Nonpolar | Yes | 0.38 | Decrease | 9 | −1.71 | |
| I → S | Uncharged Polar | Yes | 0.11 | Decrease | 9 | −1.61 | |
| I → F | Nonpolar | Yes | 0.14 | Decrease | 8 | −0.96 | |
| I → K | Basic | Yes | 0.07 | Decrease | 9 | −1.64 | |
| 37 | L → T | Uncharged Polar | No | 0.00 | Decrease | 9 | −1.86 |
| L → M | Nonpolar | No | 0.05 | Decrease | 8 | −1.13 | |
| L → I | Nonpolar | No | 0.04 | Decrease | 9 | −1.30 | |
| L → V | Nonpolar | Yes | 0.30 | Decrease | 8 | −1.13 | |
| 39 | V → A | Nonpolar | Yes | 0.15 | Decrease | 9 | −1.24 |
| V → D | Acidic | No | 0.01 | Decrease | 9 | −1.57 | |
| V → K | Basic | No | 0.03 | Decrease | 10 | −1.75 | |
| V → T | Uncharged Polar | Yes | 0.12 | Decrease | 10 | −1.26 | |
| 40 | P → T | Uncharged Polar | No | 0.05 | Decrease | 8 | −0.99 |
| P → A | Nonpolar | Yes | 0.09 | Decrease | 8 | −1.10 | |
| P → D | Acidic | No | 0.01 | Decrease | 8 | −1.23 | |
| P → N | Uncharged Polar | No | 0.02 | Decrease | 8 | −1.38 | |
| P → F | Nonpolar | Yes | 0.19 | Decrease | 8 | −0.80 | |
| 41 | L → Y | Uncharged Polar | No | 0.00 | Decrease | 6 | −1.28 |
| L → F | Nonpolar | Yes | 0.06 | Decrease | 8 | −1.10 | |
| L → A | Nonpolar | Yes | 0.06 | Decrease | 9 | −1.75 | |
| L → T | Uncharged Polar | Yes | 0.09 | Decrease | 8 | −1.57 | |
| 44 | F → S | Uncharged Polar | No | 0.03 | Decrease | 8 | −1.15 |
| F → Y | Uncharged Polar | Yes | 1.00 | Decrease | 2 | −0.74 | |
| F → I | Nonpolar | Yes | 0.19 | Decrease | 5 | −0.80 | |
| 45 | T → I | Nonpolar | Yes | 0.62 | Decrease | 6 | −0.77 |
| T → N | Uncharged Polar | No | 0.03 | Increase | 0 | −0.56 | |
| T → L | Nonpolar | Yes | 0.28 | Decrease | 7 | −0.70 | |
| T → Y | Uncharged Polar | No | 0.01 | Decrease | 3 | −0.51 |
| Position | Substitution | Characteristic | SIFT Tolerated | SIFT Score | I-Mutant Stability | RI | DDG (kJ/mol) |
|---|---|---|---|---|---|---|---|
| 3 | S → T | Uncharged Polar | Yes | 0.40 | Increase | 1 | −0.05 |
| S → Y | Uncharged Polar | Yes | 0.27 | Increase | 4 | −0.23 | |
| S → D | Acidic | Yes | 0.49 | Increase | 6 | 0.06 | |
| S → M | Basic | No | 0.04 | Increase | 4 | 0.00 | |
| 5 | Q → V | Nonpolar | Yes | 0.33 | Increase | 0 | 0.11 |
| Q → E | Acidic | Yes | 1.00 | Increase | 3 | −0.40 | |
| Q → L | Nonpolar | Yes | 0.40 | Increase | 2 | 0.01 | |
| Q → G | Nonpolar | Yes | 0.37 | Decrease | 7 | −0.80 | |
| 7 | L → S | Uncharged Polar | Yes | 0.06 | Decrease | 9 | −1.38 |
| L → Y | Uncharged Polar | No | 0.01 | Decrease | 4 | −1.03 | |
| L → K | Basic | No | 0.01 | Decrease | 8 | −1.30 | |
| L → V | Nonpolar | Yes | 0.23 | Decrease | 6 | −0.72 | |
| L → I | Nonpolar | Yes | 0.52 | Decrease | 8 | −0.94 | |
| 8 | A → T | Uncharged Polar | No | 0.04 | Decrease | 9 | −1.22 |
| A → G | Nonpolar | Yes | 0.93 | Decrease | 9 | −1.62 | |
| A → S | Uncharged Polar | Yes | 0.20 | Decrease | 10 | −1.32 | |
| A → N | Uncharged Polar | No | 0.02 | Decrease | 9 | −1.21 | |
| 11 | A → F | Nonpolar | No | 0.00 | Decrease | 9 | −0.77 |
| A → S | Uncharged Polar | Yes | 0.09 | Decrease | 10 | −1.18 | |
| A →G | Nonpolar | Yes | 0.14 | Decrease | 10 | −1.51 | |
| A → E | Acidic | Yes | 0.06 | Decrease | 9 | −1.16 | |
| A → T | Uncharged Polar | No | 0.01 | Decrease | 9 | −1.09 | |
| 14 | V → A | Nonpolar | Yes | 0.10 | Decrease | 4 | −0.82 |
| V → L | Nonpolar | No | 0.02 | Decrease | 6 | −0.96 | |
| V → T | Uncharged Polar | No | 0.01 | Decrease | 10 | −1.08 | |
| V → I | Nonpolar | Yes | 0.14 | Decrease | 8 | −0.69 | |
| 15 | A → T | Uncharged Polar | Yes | 0.16 | Decrease | 9 | −1.20 |
| A → F | Nonpolar | No | 0.00 | Decrease | 9 | −0.77 | |
| A → G | Nonpolar | No | 0.02 | Decrease | 10 | −1.59 | |
| A → S | Uncharged Polar | No | 0.03 | Decrease | 10 | −1.28 | |
| A → E | Acidic | Yes | 0.13 | Decrease | 9 | −1.26 | |
| A → N | Uncharged Polar | No | 0.01 | Decrease | 9 | −1.31 | |
| 17 | I → A | Nonpolar | No | 0.01 | Decrease | 4 | −1.12 |
| I → L | Nonpolar | Yes | 0.17 | Decrease | 1 | −0.34 | |
| I → T | Uncharged Polar | No | 0.01 | Decrease | 8 | −1.12 | |
| I → S | Uncharged Polar | Yes | 0.09 | Decrease | 8 | −1.31 | |
| 19 | A → T | Uncharged Polar | Yes | 0.09 | Decrease | 9 | −1.09 |
| A → N | Uncharged Polar | No | 0.00 | Decrease | 9 | −1.20 | |
| A → G | Nonpolar | No | 0.01 | Decrease | 9 | −1.56 | |
| A → V | Nonpolar | Yes | 0.15 | Decrease | 7 | −0.54 | |
| A → Q | Uncharged Polar | No | 0.01 | Decrease | 9 | −1.10 | |
| 23 | W → K | Basic | No | 0.00 | Decrease | 9 | −1.11 |
| W → Y | Uncharged Polar | No | 0.00 | Decrease | 7 | −0.86 | |
| W → R | Basic | No | 0.00 | Decrease | 7 | −0.69 | |
| W → Q | Uncharged Polar | No | 0.00 | Decrease | 9 | −1.07 | |
| 25 | I → V | Nonpolar | No | 0.04 | Decrease | 7 | −0.32 |
| I → M | Basic | No | 0.01 | Decrease | 8 | −0.81 | |
| I → K | Basic | Yes | 0.05 | Decrease | 9 | −1.52 | |
| I → L | Nonpolar | Yes | 0.24 | Decrease | 5 | −0.39 | |
| 27 | F → S | Uncharged Polar | No | 0.03 | Decrease | 8 | −1.15 |
| F → Y | Uncharged Polar | Yes | 1.00 | Decrease | 2 | −0.74 | |
| F → I | Nonpolar | Yes | 0.19 | Decrease | 5 | −0.80 | |
| F → G | Nonpolar | Yes | 0.08 | Decrease | 7 | −1.35 | |
| 31 | R → T | Uncharged Polar | No | 0.01 | Decrease | 8 | −0.53 |
| R → A | Nonpolar | No | 0.01 | Decrease | 7 | −0.49 | |
| R → K | Basic | Yes | 0.54 | Decrease | 9 | −0.75 | |
| R → L | Nonpolar | No | 0.01 | Decrease | 8 | −0.42 | |
| 32 | K → E | Acidic | Yes | 0.15 | Increase | 2 | −0.22 |
| K→ I | Nonpolar | No | 0.00 | Increase | 1 | −0.23 | |
| K → R | Basic | Yes | 0.15 | Increase | 5 | 0.03 | |
| K → A | Nonpolar | No | 0.01 | Increase | 6 | −0.06 |
| Protein Modeled | Residues in Most Favored Region | Residues in Additional Allowed Region | Residues in Generously Allowed Region | Residues in Disallowed Region | No. of Glycines | No. of Prolines |
|---|---|---|---|---|---|---|
| Blood-derived HIV-1 Vpu | 94.7% | 4.0% | 1.3% | 0.0% | 3 | 1 |
| Brain-derived HIV-1 Vpu | 90.5% | 8.1% | 1.4% | 0.0% | 4 | 2 |
| Consensus HIV-Vpu | 92.0% | 6.7% | 1.3% | 0.0% | 3 | 2 |
| Protein–Protein Complex | Model Selected | Binding Affinity ΔG (kcal/mol) | Dissociation Constant Kd (M) at 37.0 °C | Potential Energy in SPDBV (kcal/mol) |
|---|---|---|---|---|
| Wild-type blood-derived Vpu–tetherin | Model dock0009.pdb | −5.0 | 6.4 × 10−4 | −314.17 |
| Wild-type brain derived Vpu–tetherin | Model dock0001.pdb | −3.8 | 2.0 × 10−3 | −148.65 |
| Wild-type consensus Vpu–tetherin | Model dock0007.pdb | −4.3 | 9.8 × 10−4 | −261.08 |
| Name | Type of Mutation | Best Model Selected | Binding Affinity ΔG (kcal/mol) | Kd (M) at 37.0 °C | Energy (iMutant) (kcal/mol) |
|---|---|---|---|---|---|
| Tetherin | Wild-type | 09 | −5.0 | 6.4 × 10−4 | NA |
| M1 | L22S | 10 | −3.6 | 2.9 × 10−3 | Decrease by −1.31 |
| M2 | L22Y | 06 | −3.9 | 3.5 × 10−1 | Decrease by −2.06 |
| M3 | L23T | 05 | −2.9 | 3.4 × 10−2 | Decrease by −1.52 |
| M4 | L23Y | 01 | −2.6 | 1.2 × 10−3 | Decrease by −2.06 |
| M5 | L24F | 02 | −3.0 | 6.0 × 10−4 | Decrease by −1.00 |
| M6 | L24M | 08 | −3.2 | 3.2 × 10−3 | Decrease by −1.52 |
| M7 | L24T | 04 | −2.2 | 2.9 × 10−3 | Decrease by −2.86 |
| M8 | G25A | 10 | −3.6 | 4.1 × 10−3 | Decrease by −0.57 |
| M9 | I26S | 08 | −3.5 | 3.9 × 10−5 | Decrease by −1.33 |
| M10 | L29Q | 06 | −3.9 | 4.4 × 10−3 | Decrease by −0.87 |
| M11 | V30G | 07 | -4.1 | 3.9 × 10−2 | Decrease by −2.06 |
| M12 | I33T | 07 | −3.5 | 4.2 × 10−3 | Decrease by −1.46 |
| M13 | I34T | 08 | −4.3 | 4.5 × 10−5 | Decrease by −1.50 |
| M14 | I36G | 08 | −4.1 | 3.5×10−1 | Decrease by −2.05 |
| M15 | L37T | 17 | −4.3 | 3.8 × 10−2 | Decrease by -1.86 |
| M16 | V39 | 14 | −3.6 | 4.5 × 10−1 | Decrease by −2.25 |
| M17 | P40T | 11 | −2.7 | 1.9 × 10−3 | Decrease by −0.99 |
| M18 | L41Y | 17 | -3.0 | 3.9×10−6 | Decrease by −1.28 |
| M19 | T45I | 06 | −2.5 | 2.7×10−3 | Decrease by −0.77 |
| M20 | L22S, F44Y, L37I | 01 | −2.2 | 1.8 × 10−2 | NA |
| M21 | L23T, L37T, T45I | 05 | −2.9 | 2.3 × 10−3 | NA |
| Name | Type of Mutation | Best Model Selected | Binding Affinity ΔG (kcal/mol) | Kd (M) at 37.0 °C | Energy (iMutant) (kcal/mol) |
|---|---|---|---|---|---|
| Vpu_blood | Wild-type | 09 | −5.0 | 6.4 × 10−4 | NA |
| M1 | S03Y | 12 | −3.6 | 3.3 × 10−3 | Increase by −0.23 |
| M2 | Q05V | 19 | −2.9 | 3.4 × 10−4 | Increase by −0.11 |
| M3 | L07S | 17 | −3.5 | 3.8 × 10−2 | Decrease by −1.38 |
| M4 | A08T | 07 | −4.9 | 4.4 × 10−4 | Decrease by −1.22 |
| M5 | A11F | 12 | −3.2 | 4.1 × 10−5 | Decrease by −0.77 |
| M6 | A11L | 08 | −3.6 | 3.2 × 10−5 | Decrease by −0.78 |
| M7 | V14K | 04 | −2.9 | 3.4 × 10−4 | Decrease by −1.28 |
| M8 | A15L | 15 | −3.5 | 3.9 × 10−1 | Decrease by −1.20 |
| M9 | A15T | 07 | −3.6 | 3.7 × 10−1 | Decrease by −0.94 |
| M10 | I17A | 08 | −4.5 | 3.5 × 10−4 | Decrease by −1.12 |
| M11 | A19T | 16 | −3.5 | 4.4 × 10−2 | Decrease by −1.09 |
| M12 | A19H | 14 | −2.5 | 2.9 × 10−3 | Decrease by −1.36 |
| M13 | A19F | 06 | −4.7 | 4.1 × 10−2 | Decrease by −0.86 |
| M14 | A19L | 06 | −4.7 | 4.9 × 10−2 | Decrease by −0.92 |
| M15 | W23K | 06 | −4.1 | 5.1 × 10−4 | Decrease by −1.11 |
| M16 | W23L | 05 | −3.4 | 3.7 × 10−1 | Decrease by −0.56 |
| M17 | W23Y | 07 | −2.1 | 2.1 × 10−3 | Decrease by −0.97 |
| M18 | R31A | 17 | −3.3 | 4.8 × 10−4 | Decrease by −0.49 |
| M19 | K32A | 04 | −4.2 | 4.9 × 10−3 | Increase by −0.06 |
| M20 | V10K, A11L, A19T | 01 | −2.6 | 2.1 × 10−5 | NA |
| M21 | V14T, I18T, I26S | 07 | −2.7 | 2.2 × 10−4 | NA |
| M22 | A11T, V14L, A15T | 02 | −2.3 | 2.8 × 10−2 | NA |
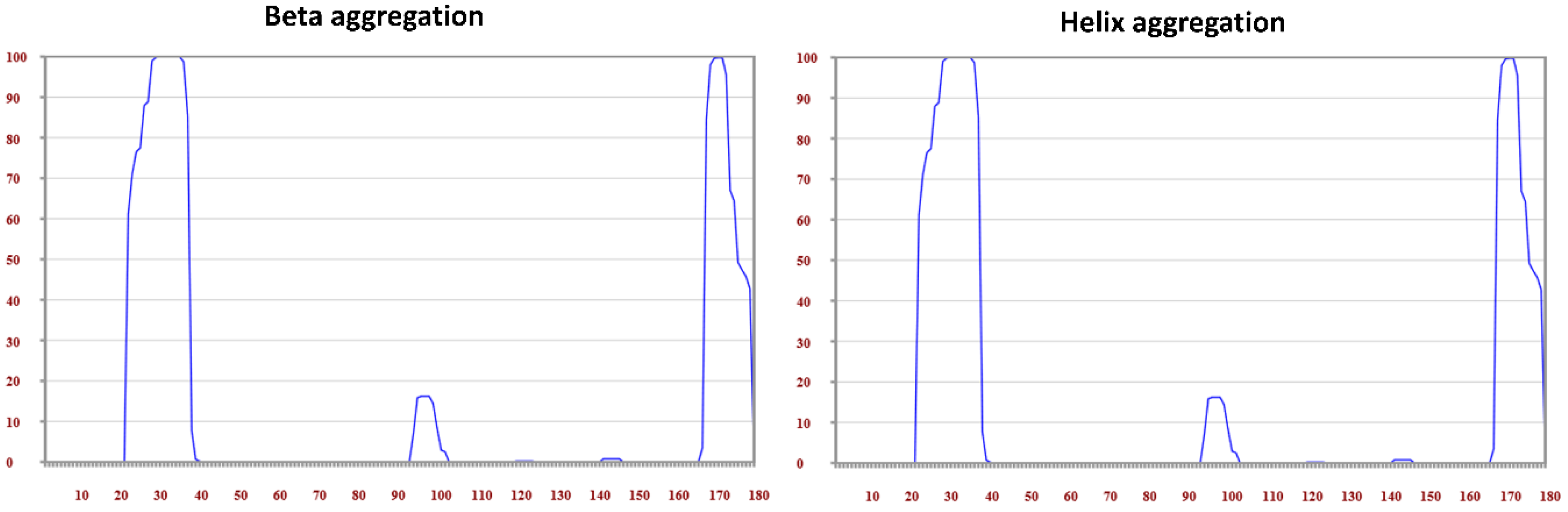
| Protein Name Accession | Position | Consensus Predicted Amyloid Regions | Fold Amyloid | Aggrescan**± | Tango**± | MetAmyl | AMYLPRED2 Consensus | Waltz ** | PASTA |
|---|---|---|---|---|---|---|---|---|---|
| TetherinQ10589 | 21–47 161–176 | >sp|Q10589|BST2_HUMAN Bone marrow stromal antigen 2 OS=Homo sapiens OX=9606 GN=BST2 PE=1 SV=1 MASTSYDYCRVPMEDGDKRCKLLLGIGILVLLIIVILGVPLIIFTIKANSEACRDGLRAVMECRNVTHLLQQELTEAQKGFQDVEAQAATCNHTVMALMASLDAEKAQGQKKVEELEGEITTLNHKLQDASAEVERLRRENQVLSVRIADKKYYPSSQDSSSAAAPQLLIVLLGLSALLQ | 6–11 21–46 58–62 93–100 144–148 167–174 | 22–47 92–101 167–180 | 22–38 168–179 | 25–49 66–71 90–96 120–125 141–149 160–176 | 22–47 93–96 144–148 167–178 | 22–47 70–75 86–104 141–146 166–180 | 19–50 |
| Waltz± 29–47 141–146 166–180 |
| Tetherin | H | E | T |
|---|---|---|---|
| Region 1: 21–47 | 96.3 | 88.9 | 0.0 |
| Region 2: 161–175 | 75.0 | 37.5 | 12.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sneha, P.; Shah, U.; Balaji, S. In Silico Insights into HIV-1 Vpu-Tetherin Interactions and Its Mutational Counterparts. Med. Sci. 2019, 7, 74. https://doi.org/10.3390/medsci7060074
Sneha P, Shah U, Balaji S. In Silico Insights into HIV-1 Vpu-Tetherin Interactions and Its Mutational Counterparts. Medical Sciences. 2019; 7(6):74. https://doi.org/10.3390/medsci7060074
Chicago/Turabian StyleSneha, Patil, Urmi Shah, and Seetharaman Balaji. 2019. "In Silico Insights into HIV-1 Vpu-Tetherin Interactions and Its Mutational Counterparts" Medical Sciences 7, no. 6: 74. https://doi.org/10.3390/medsci7060074
APA StyleSneha, P., Shah, U., & Balaji, S. (2019). In Silico Insights into HIV-1 Vpu-Tetherin Interactions and Its Mutational Counterparts. Medical Sciences, 7(6), 74. https://doi.org/10.3390/medsci7060074






