Assessment of Cardiovascular Disease Risk and Therapeutic Patterns among Urban Black Rheumatoid Arthritis Patients
Abstract
:1. Introduction
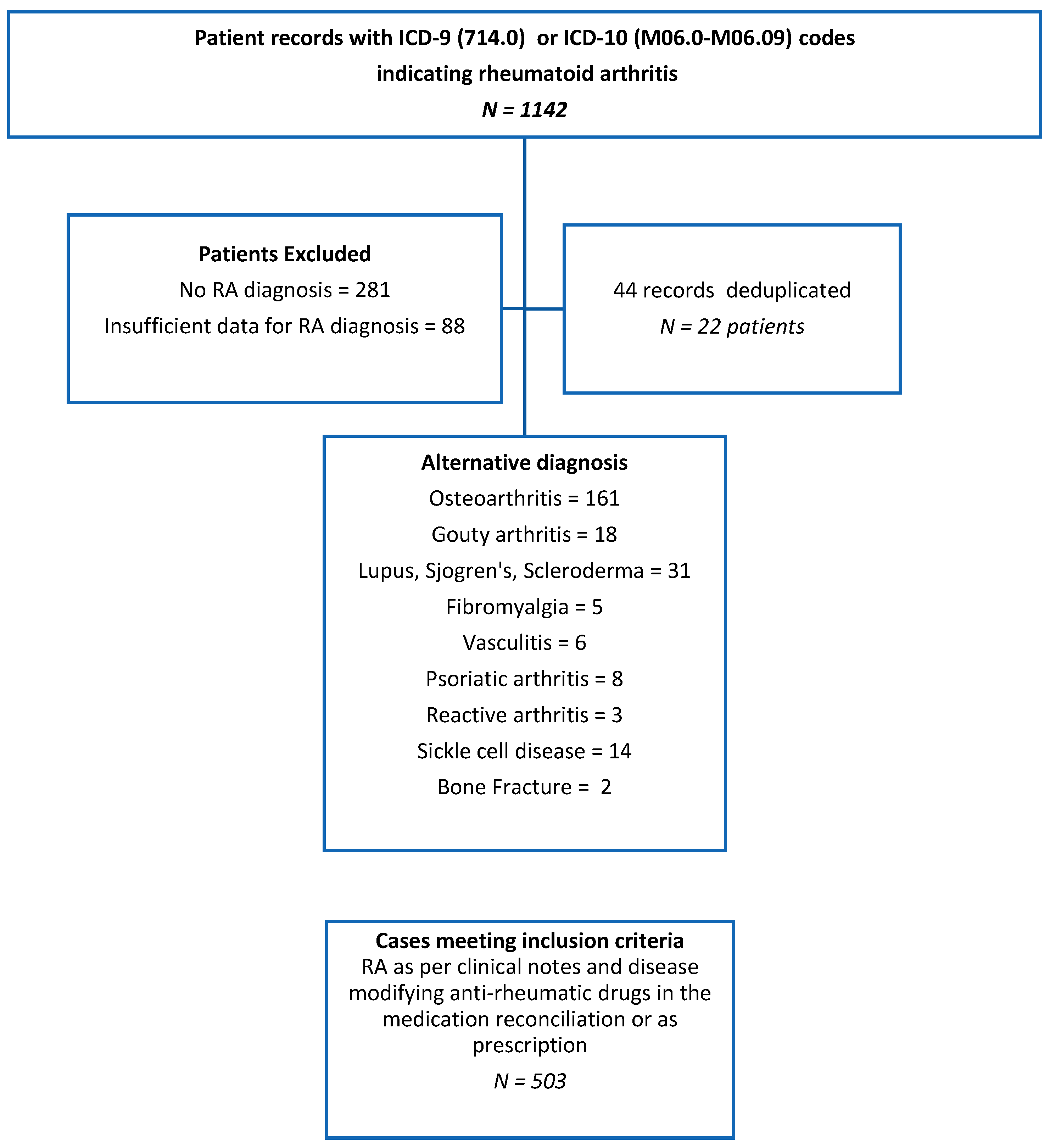
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Peschken, C.A.; Esdaile, J.M. Rheumatic diseases in North America’s indigenous peoples. Semin. Arthritis Rheum. 1999, 28, 368–391. [Google Scholar] [CrossRef]
- England, B.R.; Sayles, H.; Michaud, K.; Caplan, L.; Davis, L.A.; Cannon, G.W.; Sauer, B.C.; Solow, E.B.; Reimold, A.M.; Kerr, G.S.; et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res. 2016, 68, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Avina-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 2008, 59, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
- Sokka, T.; Abelson, B.; Pincus, T. Mortality in rheumatoid arthritis: 2008 update. Clin. Exp. Rheumatol. 2008, 26 (5 Suppl. 51), S35–S61. [Google Scholar] [PubMed]
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003, 107, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Reed, G.W.; Kremer, J.M.; Curtis, J.R.; Farkouh, M.E.; Harrold, L.R.; Hochberg, M.C.; Tsao, P.; Greenberg, J.D. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015, 67, 1449–1455. [Google Scholar] [CrossRef]
- Del Rincon, I.; Williams, K.; Stern, M.P.; Freeman, G.L.; O’Leary, D.H.; Escalante, A. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003, 48, 1833–1840. [Google Scholar] [CrossRef]
- Kurmann, R.D.; Mankad, R. Atherosclerotic Heart Disease in Women With Autoimmune Rheumatologic Inflammatory Conditions. Can. J. Cardiol. 2018, 34, 381–389. [Google Scholar] [CrossRef]
- Sanjadi, M.; Rezvanie Sichanie, Z.; Totonchi, H.; Karami, J.; Rezaei, R.; Aslani, S. Atherosclerosis and autoimmunity: A growing relationship. Int. J. Rheum. Dis. 2018, 21, 908–921. [Google Scholar] [CrossRef]
- Fava, C.; Montagnana, M. Atherosclerosis Is an Inflammatory Disease which Lacks a Common Anti-inflammatory Therapy: How Human Genetics Can Help to This Issue A Narrative Review. Front. Pharmacol. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Herrinton, L.J.; Ray, G.T.; Curtis, J.R.; Wu, J.J.; Fireman, B.; Liu, L.; Goldfien, R. An Observational Study of Cardiovascular Risks Associated with Rheumatoid Arthritis Therapies: A Comparison of Two Analytical Approaches. Perm. J. 2018, 22. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Zinellu, A.; Sotgia, S.; Carru, C.; Piga, M.; Erre, G.L. Protective Effects of Methotrexate against Proatherosclerotic Cytokines: A Review of the Evidence. Mediat. Inflamm. 2017, 2017, 9632846. [Google Scholar] [CrossRef] [PubMed]
- Urman, A.; Taklalsingh, N.; Sorrento, C.; McFarlane, I.M. Inflammation Beyond the Joints: Rheumatoid Arthritis and Cardiovascular Disease. SciFed J. Cardiol. 2018, 2, 1–23. [Google Scholar]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Dias, E.M.; Lukas, C.; Landewe, R.; Fatenejad, S.; van der Heijde, D. Reliability and sensitivity to change of the Simple Erosion Narrowing Score compared with the Sharp-van der Heijde method for scoring radiographs in rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, L.; Delzell, E.; Muntner, P.; Hillegass, W.B.; Safford, M.M.; Millan, I.Y.; Crowson, C.S.; Curtis, J.R. Republished: The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Postgrad. Med. J. 2014, 90, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Kremer, J.; Curtis, J.R.; Hochberg, M.C.; Reed, G.; Tsao, P.; Farkouh, M.E.; Setoguchi, S.; Greenberg, J.D. Explaining the cardiovascular risk associated with rheumatoid arthritis: Traditional risk factors versus markers of rheumatoid arthritis severity. Ann. Rheum. Dis. 2010, 69, 1920–1925. [Google Scholar] [CrossRef]
- Solomon, D.H.; Greenberg, J.; Curtis, J.R.; Liu, M.; Farkouh, M.E.; Tsao, P.; Kremer, J.M.; Etzel, C.J. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol. 2015, 67, 1995–2003. [Google Scholar] [CrossRef]
- Maradit-Kremers, H.; Crowson, C.S.; Nicola, P.J.; Ballman, K.V.; Roger, V.L.; Jacobsen, S.J.; Gabriel, S.E. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2005, 52, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Maradit-Kremers, H.; Nicola, P.J.; Crowson, C.S.; Ballman, K.V.; Gabriel, S.E. Cardiovascular death in rheumatoid arthritis: A population-based study. Arthritis Rheum. 2005, 52, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Goodson, N.J.; Symmons, D.P.; Scott, D.G.; Bunn, D.; Lunt, M.; Silman, A.J. Baseline levels of C-reactive protein and prediction of death from cardiovascular disease in patients with inflammatory polyarthritis: A ten-year followup study of a primary care-based inception cohort. Arthritis Rheum. 2005, 52, 2293–2299. [Google Scholar] [CrossRef] [PubMed]
- Meissner, Y.; Zink, A.; Kekow, J.; Rockwitz, K.; Liebhaber, A.; Zinke, S.; Gerhold, K.; Richter, A.; Listing, J.; Strangfeld, A. Impact of disease activity and treatment of comorbidities on the risk of myocardial infarction in rheumatoid arthritis. Arthritis Res. Ther. 2016, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Grossman, J.; FitzGerald, J.; Dahlin-Lee, E.; Wallace, D.J.; Thong, B.Y.; Badsha, H.; Kalunian, K.; Charles, C.; Navab, M.; et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006, 54, 2541–2549. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Hama, S.Y.; Hough, G.P.; Subbanagounder, G.; Reddy, S.T.; Fogelman, A.M. A cell-free assay for detecting HDL that is dysfunctional in preventing the formation of or inactivating oxidized phospholipids. J. Lipid Res. 2001, 42, 1308–1317. [Google Scholar]
- Navab, M.; Van Lenten, B.J.; Reddy, S.T.; Fogelman, A.M. High-density lipoprotein and the dynamics of atherosclerotic lesions. Circulation 2001, 104, 2386–2387. [Google Scholar] [CrossRef]
- Van Lenten, B.J.; Hama, S.Y.; de Beer, F.C.; Stafforini, D.M.; McIntyre, T.M.; Prescott, S.M.; La Du, B.N.; Fogelman, A.M.; Navab, M. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J. Clin. Investig. 1995, 96, 2758–2767. [Google Scholar] [CrossRef]
- Davis, J.M., 3rd; Maradit Kremers, H.; Crowson, C.S.; Nicola, P.J.; Ballman, K.V.; Therneau, T.M.; Roger, V.L.; Gabriel, S.E. Glucocorticoids and cardiovascular events in rheumatoid arthritis: A population-based cohort study. Arthritis Rheum. 2007, 56, 820–830. [Google Scholar] [CrossRef]
| Total Number of Patients: 503 Mean Age 64.76 ± 0.66 (±SEM) | |
| Number of women | 87.9% |
| Number of men | 12.1% |
| Women’s mean age (±SEM) years | 65 ± 0.68 |
| All patients age in years (±SEM) | 64.7 ± 0.6 |
| Race/Ethnicity | |
| White | 7.2% |
| Black | 88.5% |
| Native American | 0.2% |
| Asian Pacific Islander | 0.6% |
| Hispanics | 9.2% |
| Other Characteristics | |
| Non-smoker | 70.6% |
| Ever smoker | 29.4% |
| BMI (mean ± SEM) | 28.9 ± 0.36 |
| BMI ≥ 30 Kg/m2 | 37.2% |
| Diabetes mellitus | 28.5% |
| Hypertension | 66.6% |
| Hyperlipidemia | 41.3% |
| CAD or AMI | 19.8% |
| Prior cerebrovascular accident | 10.1% |
| Atrial fibrillation | 8.4% |
| Congestive heart failure | 14.8% |
| Women (n = 442) | Men (n = 61) | |
|---|---|---|
| Age in years (mean ± SEM) | 65.2±0.6 | 61 ± 2.1 |
| Ever smoker (current/past) | (28.2%) | (37.9%) |
| BMI Kg/m2 (mean ± SEM) | 29.9 ± 0.382 | 27.51 ± 0.924 |
| BMI >30 Kg/m2 | 157/404 (38.9%) | 12/50 (24%) |
| Diabetes mellitus | 121/419 (28.9% | 15/58 (25.9%) |
| Hypertension | 289/429 (67.4%) | 36/59 (61%) |
| Hyperlipidemia | 172/406 (42.4%) | 20/59 (33.9%) |
| CAD or MI | 77/390 (19.7%) | 11/55 (20%) |
| Prior cerebrovascular accident | 42/398 (10.6%) | 4/57 (7%) |
| Atrial fibrillation | 35/409 (8.6%) | 4/56 (7.1%) |
| Congestive heart failure | 65/417 (15.6%) | 5/57 (8.8%) |
| All Patients | Seropositive RF+ and/or ACPA+ (n = 201) | Seronegative RF- and ACPA- (n = 31) | p-Value | |
|---|---|---|---|---|
| Cardiovascular risk factors | ||||
| Hypertension (HTN) | 325/488 (66.6%) | 65/129 (50.4%) | 20/30 (66.6%) | 0.98 |
| Hyperlipidemia (HLP) | 192/465 (41.3%) | 77/185 (41.6%) | 15/31 (48.4%) | 0.48 |
| Diabetes mellitus (DM) | 136/477 (28.5%) | 28/175 (16%) | 4/29 (13.7%) | 0.76 |
| Traditional risk factors for CVD | ||||
| Any traditional CVD risk factor present | 436/499 (87.4%) | 175/199(87.9%) | 29/31(93.5%) | 0.35 |
| ≥3 traditional risk factors | 157/425 (37%) | 67/194 (34.5%) | 7/29 (24.3%) | 0.27 |
| Rheumatoid arthritis-specific risk factors | ||||
| RA-specific risk factors for CVD | 292/503 (58%) | 180/201 (89.5%) | 21/31 (67.7) | 0.001 * |
| RA duration of disease >10 years | 108/197 (54.8%) | 61/114 (53.5%) | 11/16 (68.5%) | 0.25 |
| BMI < 20 Kg/m2 | 34/454 (7.5%) | 16/189 (8.4%) | 1/27 (3.7%) | 0.39 |
| Joint erosions | 125/188 (66.5%) | 82/120 (68.3%) | 6/13 (46.15%) | 0.11 |
| Joint space narrowing | 130/188 (69.1%) | 84/120 (70%) | 7/13 (53.8%) | 0.23 |
| Extra-articular disease | 38/503 (7.5%) | 12/201 (6.9%) | 0/31 (0%) | 0.13 |
| CRP > 10 mg/L ** | 180/285(63.1%) | 102/171(56.9%) | 12/24 (50%) | 0.36 |
| ESR > 42 mm/h *** | 198/313(63.1%) | 124/177 (70%) | 10/26 (38.4%) | 0.001* |
| Positive Rheumatoid Factor (RF) | 186/247(75.3%) | 186/247 (75.3%) | 0% | NA |
| Positive Anti-citrullinated ab. (ACPA) | 124/178(69.6%) | 124/178 (69.6%) | 0% | NA |
| Either RF+ or ACPA+ | 201/232 (86.6%) | 201/232 (86.6%) | 0% | NA |
| Double seropositive (RF+ and ACPA+) | 109/201(54%) | 109/201(54%) | 0% | NA |
| Cardiovascular outcomes | ||||
| Congestive heart failure | 70/474 (14.8%) | 27/188 (14.3%) | 2/30 (6.6%) | 0.25 |
| CAD or MI | 88/445 (19.8%) | 28/175 (16%) | 4/29 (13.8%) | 0.76 |
| Prior cerebrovascular accident (CVA) | 46/455 (10.1%) | 18/183 (9.8%) | 3/29 (10.3%) | 0.93 |
| Atrial fibrillation | 39/465 (8.4%) | 14/187 (7.4%) | 1/30 (3.3%) | 0.41 |
| Any CVD outcome | 175/503 (34.8%) | 59/201 (29.4%) | 10/31 (32.3%) | 0.29 |
| Glucocorticoids | 238/425 (56%) |
|---|---|
| PDN mean daily dose (±SEM) in mg. | 8.14 ± 0.95 |
| NSAIDs | 89/406 (22.1%) |
| Narcotics | 33/406 (8.1%) |
| Methotrexate (MTX) | 175/434 (40.3%) |
| MTX mean weekly dose (±SEM) in mg. | 6.6 ± 0.47 |
| Other DMARDs | 153/356 (43%) |
| Biologics | 68/420 (16.2%) |
| Only Steroids | Steroids and DMARDs/Biologics | Only DMARDs/Biologics | |
|---|---|---|---|
| Any CVD risk factor | 205/436 (47%) | 152/436 (34.9%) | 119/436 (27.3%) |
| ≥3 CVD risk factors | 69/157 (44%) | 52/157 (33.1%) | 46/157 (29.3%) |
| Any CVD outcome | 81/175 (46.3%) | 62/175 (35.4%) | 45/175 (25.7%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McFarlane, I.M.; Leon, S.Y.Z.; Bhamra, M.S.; Burza, A.; Waite, S.A.; Rodriguez Alvarez, M.; Koci, K.; Taklalsingh, N.; Kaplan, I.; Pathiparampil, J.; et al. Assessment of Cardiovascular Disease Risk and Therapeutic Patterns among Urban Black Rheumatoid Arthritis Patients. Med. Sci. 2019, 7, 31. https://doi.org/10.3390/medsci7020031
McFarlane IM, Leon SYZ, Bhamra MS, Burza A, Waite SA, Rodriguez Alvarez M, Koci K, Taklalsingh N, Kaplan I, Pathiparampil J, et al. Assessment of Cardiovascular Disease Risk and Therapeutic Patterns among Urban Black Rheumatoid Arthritis Patients. Medical Sciences. 2019; 7(2):31. https://doi.org/10.3390/medsci7020031
Chicago/Turabian StyleMcFarlane, Isabel M., Su Yien Zhaz Leon, Manjeet S. Bhamra, Aaliya Burza, Stephen Anthony Waite, Milena Rodriguez Alvarez, Kristaq Koci, Nicholas Taklalsingh, Ian Kaplan, Joshy Pathiparampil, and et al. 2019. "Assessment of Cardiovascular Disease Risk and Therapeutic Patterns among Urban Black Rheumatoid Arthritis Patients" Medical Sciences 7, no. 2: 31. https://doi.org/10.3390/medsci7020031
APA StyleMcFarlane, I. M., Leon, S. Y. Z., Bhamra, M. S., Burza, A., Waite, S. A., Rodriguez Alvarez, M., Koci, K., Taklalsingh, N., Kaplan, I., Pathiparampil, J., Kabani, N., Watler, E., Sorrento, C. S., Frefer, M., Vaitkus, V., Green, J., Matthew, K., Arroyo-Mercado, F., Lyo, H., ... Kolla, S. (2019). Assessment of Cardiovascular Disease Risk and Therapeutic Patterns among Urban Black Rheumatoid Arthritis Patients. Medical Sciences, 7(2), 31. https://doi.org/10.3390/medsci7020031





