Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents and Antibodies
2.2. Culturing of Osteosarcoma Cells
2.3. Cell Viability
2.4. Western Blot Analysis
2.5. Measurement of Polyamines
2.6. Flow Cytometry Cell Cycle Analysis
2.7. Statistical Analyses
3. Results
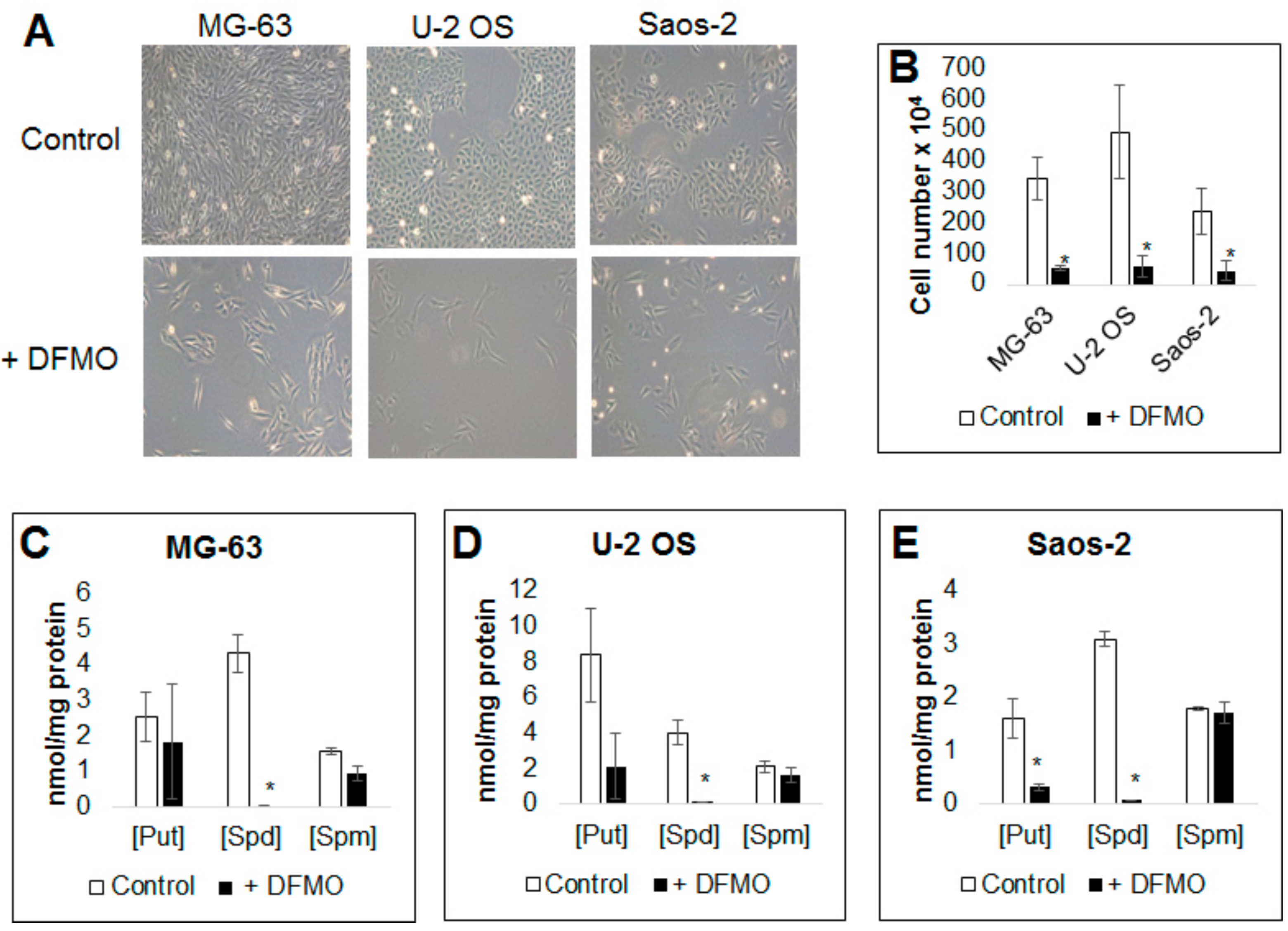
3.1. Alpha-difluoromethylornithine Treatment Decreases Osteosarcoma Cell Proliferation
3.2. Alpha-difluoromethylornithine Treatment Decreases Intracellular Polyamine Levels
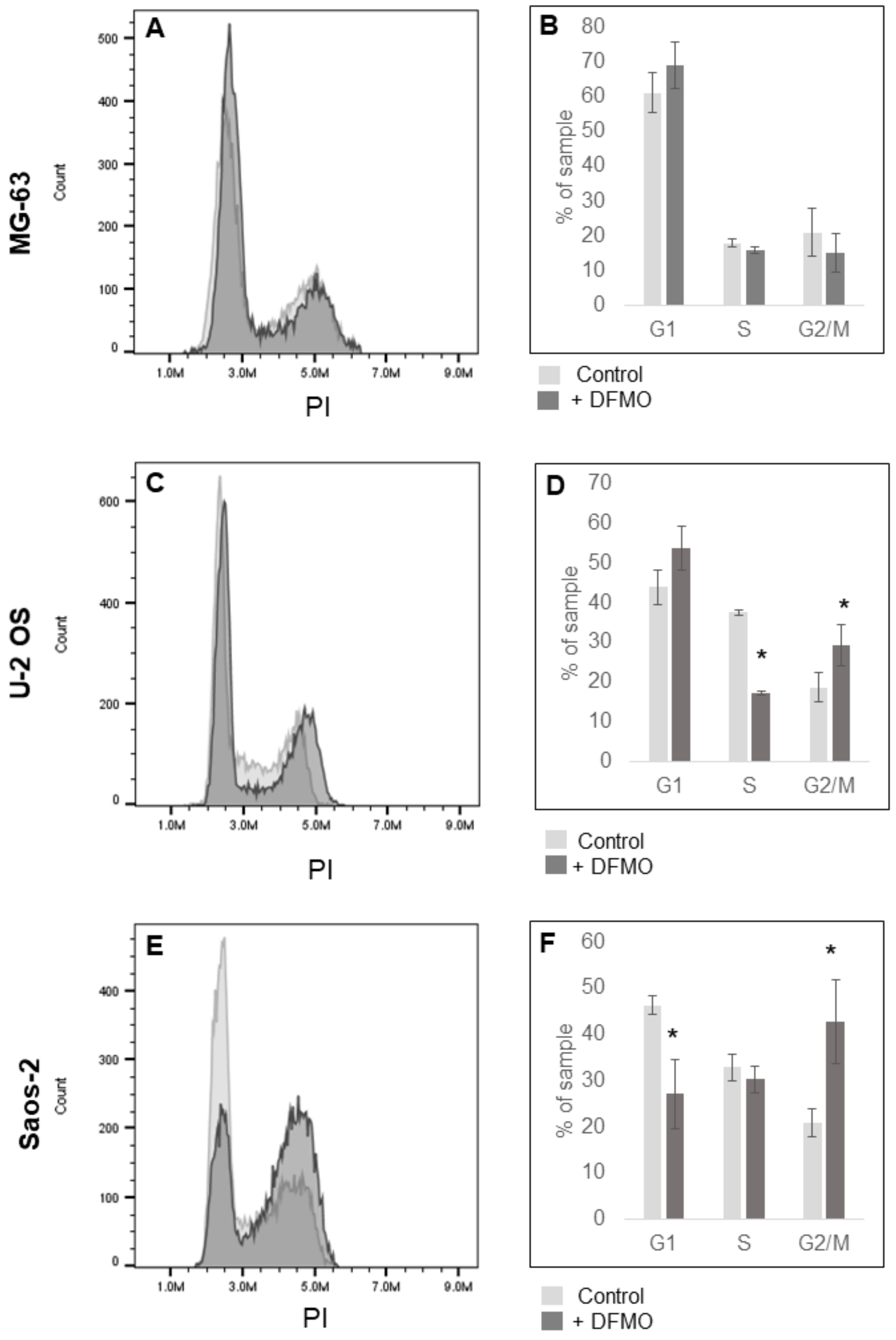
3.3. Alpha-difluoromethylornithine Induces Cell Cycle Arrest
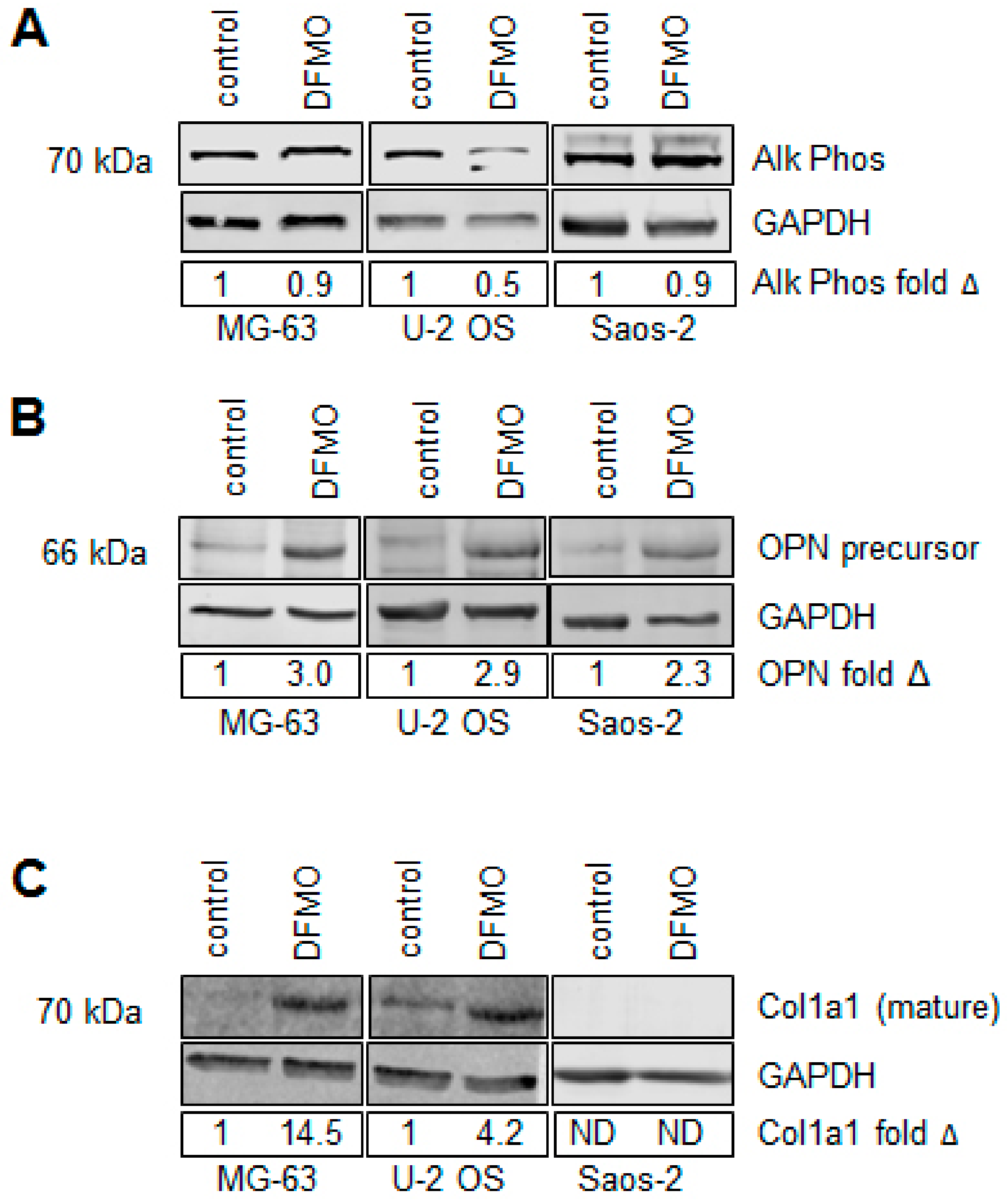
3.4. Alpha-difluoromethylornithine Induces Differentiation
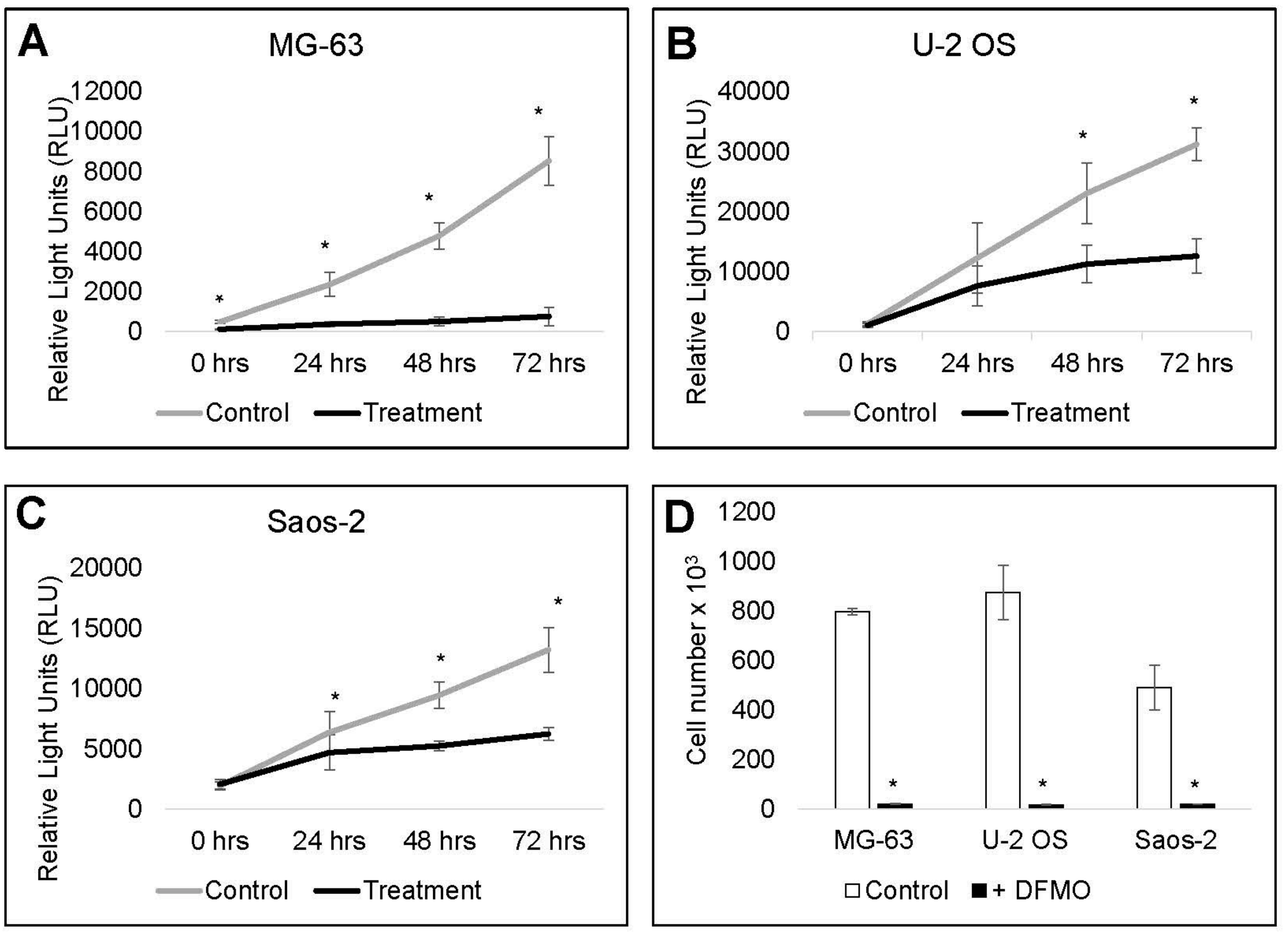
3.5. Cell Recovery is Delayed by Alpha-difluoromethylornithine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moore, D.D.; Luu, H.H. Osteosarcoma. Cancer Treat. Res. 2014, 162, 65–92. [Google Scholar] [PubMed]
- Ottaviani, G.; Jaffe, N. The epidemiology of osteosarcoma. Cancer Treat. Res. 2009, 152, 3–13. [Google Scholar] [PubMed]
- Roberts, R.D.; Wedekind, M.F.; Setty, B.A. Chemotherapy regimens for patients with newly diagnosed malignant bone tumors. In Malignant Pediatric Bone—Tumorstreatment & Management; Cripe, T.P., Yeager, N.D., Eds.; Springer International Publishing: Berlin, Germany, 2015; pp. 83–107. [Google Scholar]
- Pegg, A.E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem. J. 1986, 234, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016, 291, 14904–14912. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E.; Feith, D.J. Polyamines and neoplastic growth. Biochem. Soc. Trans. 2007, 35, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Wallace, H.M.; Fraser, A.V.; Hughes, A. A perspective of polyamine metabolism. Biochem. J. 2003, 376, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.S. The role of polyamines in human cancer: Prospects for drug combination therapies. Hawaii Med. J. 2004, 63, 371–374. [Google Scholar] [PubMed]
- Casero, R.A., Jr.; Marton, L.J. Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discov. 2007, 6, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Geerts, D.; Koster, J.; Albert, D.; Koomoa, D.L.; Feith, D.J.; Pegg, A.E.; Volckmann, R.; Caron, H.; Versteeg, R.; Bachmann, A.S. The polyamine metabolism genes ornithine decarboxylase and antizyme 2 predict aggressive behavior in neuroblastomas with and without MYCN amplification. Int. J. Cancer 2010, 126, 2012–2024. [Google Scholar] [PubMed]
- Gerner, E.W.; Meyskens, F.L., Jr. Polyamines and cancer: Old molecules, new understanding. Nat. Rev. Cancer 2004, 4, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Koomoa, D.L.; Geerts, D.; Lange, I.; Koster, J.; Pegg, A.E.; Feith, D.J.; Bachmann, A.S. DFMO/eflornithine inhibits migration and invasion downstream of MYCN and involves p27kip1 activity in neuroblastoma. Int. J. Oncol. 2013, 42, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Murray-Stewart, T.R.; Woster, P.M.; Casero, R.A., Jr. Targeting polyamine metabolism for cancer therapy and prevention. Biochem. J. 2016, 473, 2937–2953. [Google Scholar] [CrossRef] [PubMed]
- Wallick, C.J.; Gamper, I.; Thorne, M.; Feith, D.J.; Takasaki, K.Y.; Wilson, S.M.; Seki, J.A.; Pegg, A.E.; Byus, C.V.; Bachmann, A.S. Key role for p27kip1, retinoblastoma protein RB, and MYCN in polyamine inhibitor-induced g1 cell cycle arrest in MYCN-amplified human neuroblastoma cells. Oncogene 2005, 24, 5606–5618. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Regulation of ornithine decarboxylase. J. Biol. Chem. 2006, 281, 14529–14532. [Google Scholar] [CrossRef] [PubMed]
- Seiler, N. Thirty years of polyamine-related approaches to cancer therapy. Retrospect and prospect. Parts 1&2. Selective enzyme inhibitor & structural analogues and derivatives. Curr. Drug Targets 2003, 4, 537–585. [Google Scholar] [PubMed]
- Meyskens, F.L., Jr.; Gerner, E.W. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin. Cancer Res. 1999, 5, 945–951. [Google Scholar] [PubMed]
- Meyskens, F.L., Jr.; McLaren, C.E. Chemoprevention, risk reduction, therapeutic prevention, or preventive therapy? J. Natl. Cancer Inst. 2010, 102, 1815–1817. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; McLaren, C.E.; Pelot, D.; Fujikawa-Brooks, S.; Carpenter, P.M.; Hawk, E.; Kelloff, G.; Lawson, M.J.; Kidao, J.; McCracken, J.; et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: A randomized placebo-controlled, double-blind trial. Cancer Prev. Res. 2008, 1, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L., Jr.; Simoneau, A.R.; Gerner, E.W. Chemoprevention of prostate cancer with the polyamine synthesis inhibitor difluoromethylornithine. Recent Res. Cancer Res. 2014, 202, 115–120. [Google Scholar]
- Pegg, A.E.; Shantz, L.M.; Coleman, C.S. Ornithine decarboxylase as a target for chemoprevention. J. Cell Biochem. Suppl. 1995, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, A.S.; Levin, V.A. Clinical applications of polyamine-based therapeutics. In Polyamine Drug Discovery; Woster, P.M., Casero, R.A., Jr., Eds.; Royal Society of Chemistry: London, UK, 2012; pp. 257–276. [Google Scholar]
- Bachmann, A.S.; Geerts, D.; Sholler, G. Neuroblastoma: Ornithine decarboxylase and polyamines are novel targets for therapeutic intervention. In Pediatric Cancer, Neuroblastoma: Diagnosis, Therapy, and Prognosis; Hayat, M.A., Ed.; Springer: Berlin, Germany, 2012; Volume 1, pp. 91–103. [Google Scholar]
- Koomoa, D.L.; Yco, L.P.; Borsics, T.; Wallick, C.J.; Bachmann, A.S. Ornithine decarboxylase inhibition by {alpha}-difluoromethylornithine activates opposing signaling pathways via phosphorylation of both Akt/protein kinase B and P27kip1 in neuroblastoma. Cancer Res. 2008, 68, 9825–9831. [Google Scholar] [CrossRef] [PubMed]
- Rounbehler, R.J.; Li, W.; Hall, M.A.; Yang, C.; Fallahi, M.; Cleveland, J.L. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res. 2009, 69, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Saulnier Sholler, G.L.; Gerner, E.W.; Bergendahl, G.; MacArthur, R.B.; VanderWerff, A.; Ashikaga, T.; Bond, J.P.; Ferguson, W.; Roberts, W.; Wada, R.K.; et al. A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS ONE 2015, 10, e0127246. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Chen, Y.; Huang, Y.P.; Hsu, Y.C.; Chiang, L.H.; Chen, T.Y.; Wang, G.J. Exogenous polyamines promote osteogenic differentiation by reciprocally regulating osteogenic and adipogenic gene expression. J. Cell Biochem. 2013, 114, 2718–2728. [Google Scholar] [CrossRef] [PubMed]
- Tjabringa, G.S.; Zandieh-Doulabi, B.; Helder, M.N.; Knippenberg, M.; Wuisman, P.I.; Klein-Nulend, J. The polyamine spermine regulates osteogenic differentiation in adipose stem cells. J. Cell Mol. Med. 2008, 12, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.H.; Lin, K.L.; Huang, Y.P.; Hsu, Y.C.; Chen, C.H.; Chen, Y.; Sie, M.H.; Wang, G.J.; Lee, M.J. Suppression of ornithine decarboxylase promotes osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2015, 589, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Schultz, C.R.; Buras, A.L.; Friedman, E.; Fedorko, A.; Seamon, L.; Chandramouli, G.V.R.; Maxwell, G.L.; Bachmann, A.S.; Risinger, J.I. Ornithine decarboxylase as a therapeutic target for endometrial cancer. PLoS ONE 2017, 12, e0189044. [Google Scholar] [CrossRef] [PubMed]
- Minocha, S.C.; Minocha, R.; Robie, C.A. High-performance liquid chromatographic method for the determination of dansyl-polyamines. J. Chromatogr. 1990, 511, 177–183. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Sharma, D.; Dixit, A. D,l-alpha-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase, induces differentiation in mel cells. Cell Biol. Int. 1999, 23, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, J.; Song, W.X.; Tang, N.; Luo, J.; Deng, Z.L.; Sharff, K.A.; He, G.; Bi, Y.; He, B.C.; et al. Osteogenic BMPS promote tumor growth of human osteosarcomas that harbor differentiation defects. Lab. Invest. 2008, 88, 1264–1277. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weicht, R.R.; Schultz, C.R.; Geerts, D.; Uhl, K.L.; Bachmann, A.S. Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy. Med. Sci. 2018, 6, 65. https://doi.org/10.3390/medsci6030065
Weicht RR, Schultz CR, Geerts D, Uhl KL, Bachmann AS. Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy. Medical Sciences. 2018; 6(3):65. https://doi.org/10.3390/medsci6030065
Chicago/Turabian StyleWeicht, Rebecca R., Chad R. Schultz, Dirk Geerts, Katie L. Uhl, and André S. Bachmann. 2018. "Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy" Medical Sciences 6, no. 3: 65. https://doi.org/10.3390/medsci6030065
APA StyleWeicht, R. R., Schultz, C. R., Geerts, D., Uhl, K. L., & Bachmann, A. S. (2018). Polyamine Biosynthetic Pathway as a Drug Target for Osteosarcoma Therapy. Medical Sciences, 6(3), 65. https://doi.org/10.3390/medsci6030065





