The Use of Biochemical and Biophysical Markers in Early Screening for Preeclampsia in Mongolia
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
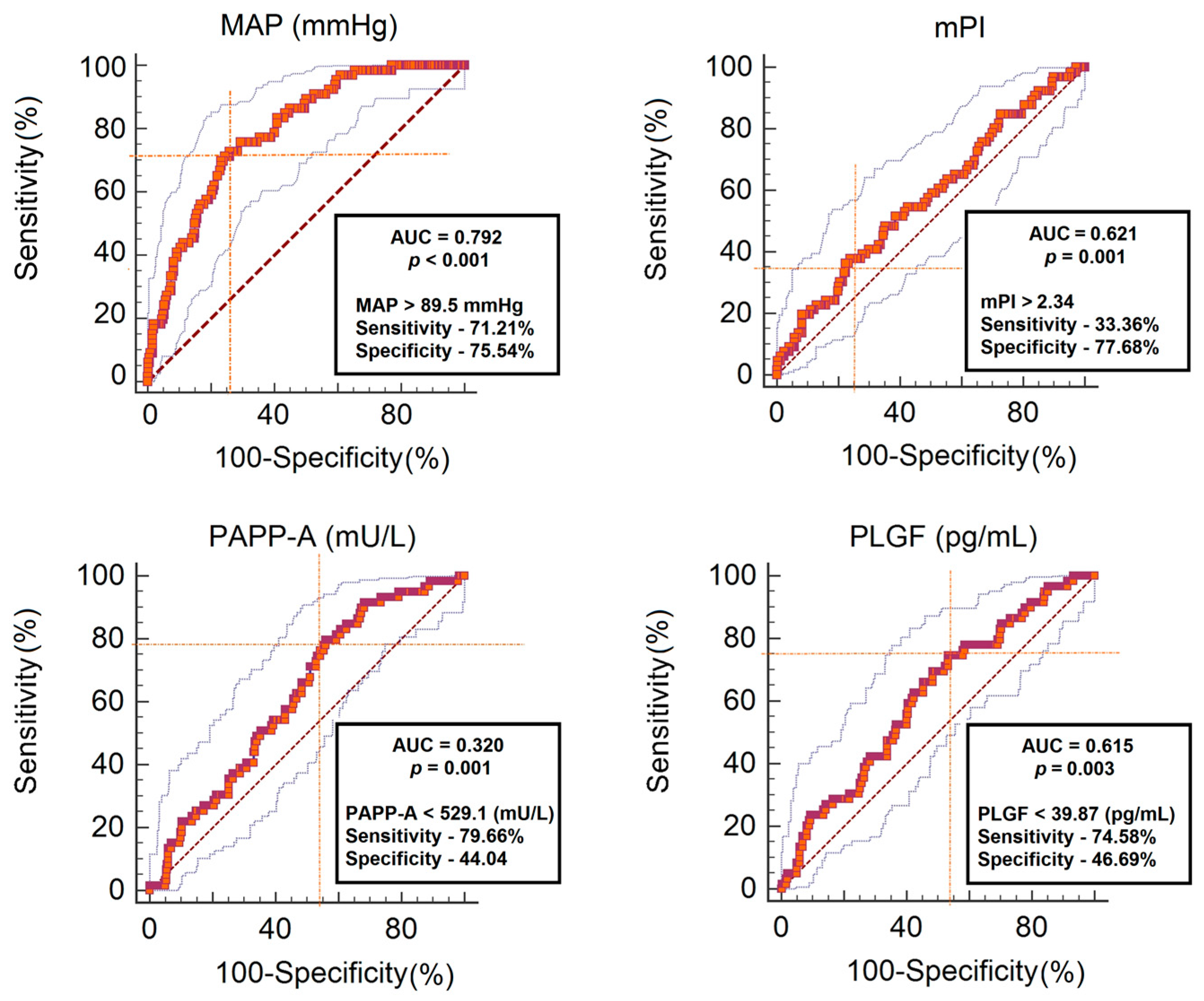
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Disclosure
References
- Khan, K.S.; Wojdyla, D.; Say, L.; Gulmezoglu, A.M.; Van Look, P.F. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Center for Health Development. Health Indicators. 2016. Available online: http://hdc.gov.mn/2017/smta/2016%20Health%20indicator.pdf (accessed on 20 May 2018).
- Enkhtur, S.H.; Battulga, B.; Bayalag, M.; Khishgee, S. (Eds.) Why Mother Died, 3rd ed.; Munkhiin Useg Group: Ulaanbaatar, Mongolia, 2016; pp. 84–86. [Google Scholar]
- Nicolaides, K.H. Turning the pyramid of prenatal care. Fetal Diagn. Ther. 2011, 29, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Syngelaki, A.; Sarquis, R.; Zvanca, M.; Nicolaides, K.H. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat. Diagn. 2011, 31, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Nicolaides, K.H. First-trimester maternal factors and biomarker screening for preeclampsia. Prenat. Diagn. 2014, 34, 618–627. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, N.; Wright, D.; Rolnik, D.L.; Nicolaides, K.H.; Poon, L.C. Study Protocol for the randomized controlled trial: Combined multimarker screening and randomized patient treatment with ASpirin for evidence based PREeclampsia prevention (ASPRE). BMJ Open 2016, 6, e011801. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Zaragoza, E.; Poon, L.C.; Pepes, S.; Nicolaides, K.H. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 2008, 32, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Akolekar, R.; Lachmann, R.; Beta, J.; Nicolaides, K.H. Hypertensive disorders in pregnancy: Screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet. Gynecol. 2010, 35, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Audibert, F.; Boucoiran, I.; An, N.; Aleksandrov, N.; Delvin, E.; Bujold, E.; Rey, E. Screening for preeclampsia using first-trimester serum markers and uterine artery Doppler in nulliparous women. Am. J. Obstet. Gynecol. 2010, 203, 383.e1–383.e8. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Kametas, N.A.; Pandeva, I.; Valencia, C.; Nicolaides, K.H. Mean arterial pressure at 11 (+0) to 13 (+6) weeks in the prediction of preeclampsia. Hypertension 2008, 51, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Villar, M.A.; Sibai, B.M. Clinical significance of elevated mean arterial blood pressure in second trimester and threshold increase and systolic or diastolic blood pressure during third trimester. Am. J. Obstet. Gynecol. 1989, 160, 419–423. [Google Scholar] [CrossRef]
- Oney, T.; Kaulhausen, H. The value of the mean arterial blood pressure in the second trimester (MAP-2 value) as a predictor of pregnancy induced-hypertension and preeclampsia. A. preliminary report. Clin. Exp. Hypertens. B 1983, 2, 211–216. [Google Scholar] [PubMed]
- The Fetal Medicine Foundation. Certificate of Competence in the Preeclampsia Screening and Fetal Doppler Ultrasound. Available online: http://www.fetalmedicine.org/ (accessed on 11 July 2018).
- World Health Organization. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia; World Health Organization: Geneva, Switzerland, 2011; Available online: http://apps.who.int/iris/bitstream/handle/10665/44703/9789241548335_eng.pdf?sequence=1 (accessed on 8 July 2018).
- Von Dadelszen, P.; Magee, L.A. Pre-eclampsia: An update. Curr. Hypertens. Rep. 2014, 16, 454. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef]
- Anderson, U.D.; Olsson, M.G.; Kristensen, K.H.; Akerstrom, B.; Hansson, S.R. Review: Biochemical markers to predict preeclampsia. Placenta 2012, 33, S42–S47. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.C.; Stenhouse, E.J.; Crossley, J.A.; Aitken, D.A.; Cameron, A.D.; Connor, J.M. Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 2002, 87, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Yaron, Y.; Heifetz, S.; Ochshorn, Y.; Lehavi, O.; Orr-Urtreger, A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat. Diagn. 2002, 22, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Syngelaki, A.; Akolekar, R.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 2015, 213, 62.e1–62.e10. [Google Scholar] [CrossRef] [PubMed]
- Spencer, K.; Yu, C.K.; Cowans, N.J.; Otigbah, C.; Nicolaides, K.H. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free β-hCG and with second-trimester uterine artery Doppler. Prenat. Diagn. 2005, 25, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Bindra, R.; Curcio, P.; Cicero, S.; Nicolaides, K.H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11–14 weeks of gestation. Ultrasound Obstet. Gynecol. 2001, 18, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Parra, M.; Rodrigo, R.; Barja, P.; Bosco, C.; Fernandez, V.; Munoz, H.; Soto-Chacon, E. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am. J. Obstet. Gynecol. 2005, 193, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Gomez, O.; Martinez, J.M.; Figueras, F.; Del Rio, M.; Borobio, V.; Puerto, B.; Coll, O.; Cararach, V.; Vanrell, J.A. Uterine artery Doppler at 11–14 weeks of gestation to screen for hypertensive disorders and associated complications in an unselected population. Ultrasound Obstet. Gynecol. 2005, 26, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Pilalis, A.; Souka, A.P.; Antsaklis, P.; Daskalakis, G.; Papantoniou, N.; Mesogitis, S.; Antsaklis, A. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler and PAPP-A at 11–14 weeks’ gestation. Ultrasound Obstet. Gynecol. 2007, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, J.S.; Vollebregt, K.C.; de Vrieze, N.; ter Riet, G.; Mol, B.W.; Franx, A.; Khan, K.S.; van der Post, J.A. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: Systematic review and meta-analysis. BMJ 2008, 336, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.C.; Kametas, N.A.; Chelemen, T.; Leal, A.; Nicolaides, K.H. Maternal risk factors for hypertensive disorders in pregnancy: A multivariate approach. J. Hum. Hypertens. 2010, 24, 104–110. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total | Preeclampsia | Unaffected PE |
|---|---|---|---|
| Maternal age (years) | 33.4 ± 6.0 | 36.1 ± 5.6 | 32.8 ± 5.9 |
| Gestational age (weeks) | 12.3 ± 0.6 | - | - |
| Maternal weight (kg) | 63.2 ± 10.8 | 69.2 ± 10.7 | 62.12 ± 10.6 |
| Fetal crown-rump length (mm) | 60.4 ± 8.6 | - | - |
| BMI (kg/m2) | 24.3 ± 3.9 | 26.8 ± 4.1 | 23.8 ± 3.7 |
| <18.5 | 14 (3.6) | 1 (1.5) | 13 (3.9) |
| 18.5–24.99 | 232 (59.0) | 21 (31.8) | 211 (64.5) |
| 25.00–29.9 | 107 (27.2) | 29 (43.9) | 78 (23.8) |
| >30 | 40 (10.2) | 15 (22.7) | 25 (7.6) |
| Medical history | |||
| Chronic hypertension | 20 (5.1) | 11 (16.7) | 9 (2.8) |
| Renal disease | 40 (10.2) | 10 (15.2) | 30 (9.2) |
| Diabetes II type | 3 (0.8) | 2 (3.0) | 1 (0.3) |
| Normal | 294 (74.8) | 35 (53.0) | 259 (79.2) |
| Other | 34 (8.7) | 8 (12.1) | 28 (8.5) |
| Previous preeclampsia | |||
| Yes | 71 (25.9) | 29 (58.0) | 42 (19.1) |
| No | 203 (74.1) | 21 (42.0) | 182 (80.9) |
| Family history of PE | |||
| Yes | 33 (8.4) | 5 (7.6) | 28 (8.5) |
| No | 299 (76.1) | 47 (71.2) | 252 (77.1) |
| Unknown | 61 (15.5) | 14 (21.2) | 47 (14.4) |
| Parity (N %) | |||
| Nulliparous | 119 (30.3) | 16 (24.2) | 103 (31.5) |
| Multiparous | 274 (69.7) | 50 (75.8) | 224 (68.5) |
| Birth weight (grams) | 3441.1 ± 503.5 | 3283.6 ± 633.2 | 3472.9 ± 467.8 |
| GA at delivery (weeks) | 39.0 ± 1.5 | 38.0 ± 1.8 | 39.2 ± 1.4 |
| Weeks | PAPP-A (mU/L) | PlGF (pg/mL) | MAP (mmHg) | mPI |
|---|---|---|---|---|
| 11–11+6 | 494.5 ± 443.4 | 37.8 ± 22.0 | 86.4 ± 9.9 | 2.1 ± 0.4 |
| 12–12+6 | 579.9 ± 497.1 | 45.8 ± 50.2 | 85.4 ± 8.2 | 2.0 ± 0.5 |
| 13–13+6 | 629.4 ± 519.8 | 63.2 ± 67.3 | 87.4 ± 9.6 | 1.9 ± 0.5 |
| Characteristics and Markers | Preeclampsia | Unaffected PE | p-Value |
|---|---|---|---|
| Maternal age (year) | 36.1 ± 5.6 | 32.8 ± 5.9 | <0.001 |
| BMI (Kg/m2) | 26.7 ± 4.1 | 23.8 ± 3.7 | <0.001 |
| Parity (nulliparous/multiparous) | 16/50 | 103/224 | <0.001 |
| Smoking (n) (yes/no) | 11/55 | 39/288 | <0.001 |
| PAPP-A (mU/L) | 366.1 ± 195.3 | 633.6 ± 496.9 | 0.003 |
| PlGF (pg/mL) | 38.6 ± 19.6 | 45.1 ± 24.0 | 0.01 |
| MAP (mm Hg) | 94.05 ± 9.05 | 84.55 ± 8.15 | <0.001 |
| mPI | 2.16 ± 0.55 | 2.0 ± 0.50 | 0.019 |
| Delivery age (weeks) | 38.0 ± 1.8 | 39.0 ± 1.4 | <0.001 |
| Birth weight (grams) | 3283.63 ± 633.19 | 3472 ± 467.86 | 0.005 |
| Risk Factors | Frequency n (%) | RR (95% CI) | p | |
|---|---|---|---|---|
| PE | Unaffected PE | |||
| Previous PE | 29 (58.0) | 42 (19.1) | 5.81 (3.27–11.16) | <0.001 |
| Chronic hypertension | 11 (16.7) | 9 (2.9) | 7.06 (2.79–17.84) | <0.001 |
| Obesity | 15 (22.7) | 25 (7.3) | 3.87 (1.89–7.92) | <0.001 |
| Birth interval > 10 years | 5 (7.8) | 28 (8.6) | 2.08 (1.03–4.19) | 0.033 |
| Kidney diseases (non glomerulonephritis) | 10 (15.2) | 30 (9.2) | 1.76 (0.81–3.82) | 0.143 |
| Smoking | 11 (16.9) | 39 (11.9) | 1.05 (0.72–3.12) | 0.270 |
| Detection Rate * (Sensitivity) | ||
|---|---|---|
| FPR (5%) | FPR (10%) | |
| With History | ||
| MAP | 62.7 (40.0–80.8) | 68.3 (46.3–86.5) |
| MAP + mPI | 69.5 (48.7–83.1) | 73.7 (54,7–89.6) |
| MAP + PAPP-A | 79.3 (56.6–89.2) | 81.1 (60.4–91.5) |
| MAP + PlGF | 86.5 (73.4–90.2) | 90.9 (78.5–92.3) |
| MAP + PlGF + PAPP-A + mPI | 91.4 (78.0–96.0) | 95.3 (80.1–96.5) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tserensambuu, U.; Chuluun-Erdene, A.; Janlav, M.; Tudevdorj, E. The Use of Biochemical and Biophysical Markers in Early Screening for Preeclampsia in Mongolia. Med. Sci. 2018, 6, 57. https://doi.org/10.3390/medsci6030057
Tserensambuu U, Chuluun-Erdene A, Janlav M, Tudevdorj E. The Use of Biochemical and Biophysical Markers in Early Screening for Preeclampsia in Mongolia. Medical Sciences. 2018; 6(3):57. https://doi.org/10.3390/medsci6030057
Chicago/Turabian StyleTserensambuu, Urjindelger, Ariunbold Chuluun-Erdene, Munkhtsetseg Janlav, and Erkhembaatar Tudevdorj. 2018. "The Use of Biochemical and Biophysical Markers in Early Screening for Preeclampsia in Mongolia" Medical Sciences 6, no. 3: 57. https://doi.org/10.3390/medsci6030057
APA StyleTserensambuu, U., Chuluun-Erdene, A., Janlav, M., & Tudevdorj, E. (2018). The Use of Biochemical and Biophysical Markers in Early Screening for Preeclampsia in Mongolia. Medical Sciences, 6(3), 57. https://doi.org/10.3390/medsci6030057





