Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Pharmacokinetic Modeling
2.3. Dose Finding Simulations
2.4. Clinical Trial Simulations
3. Results
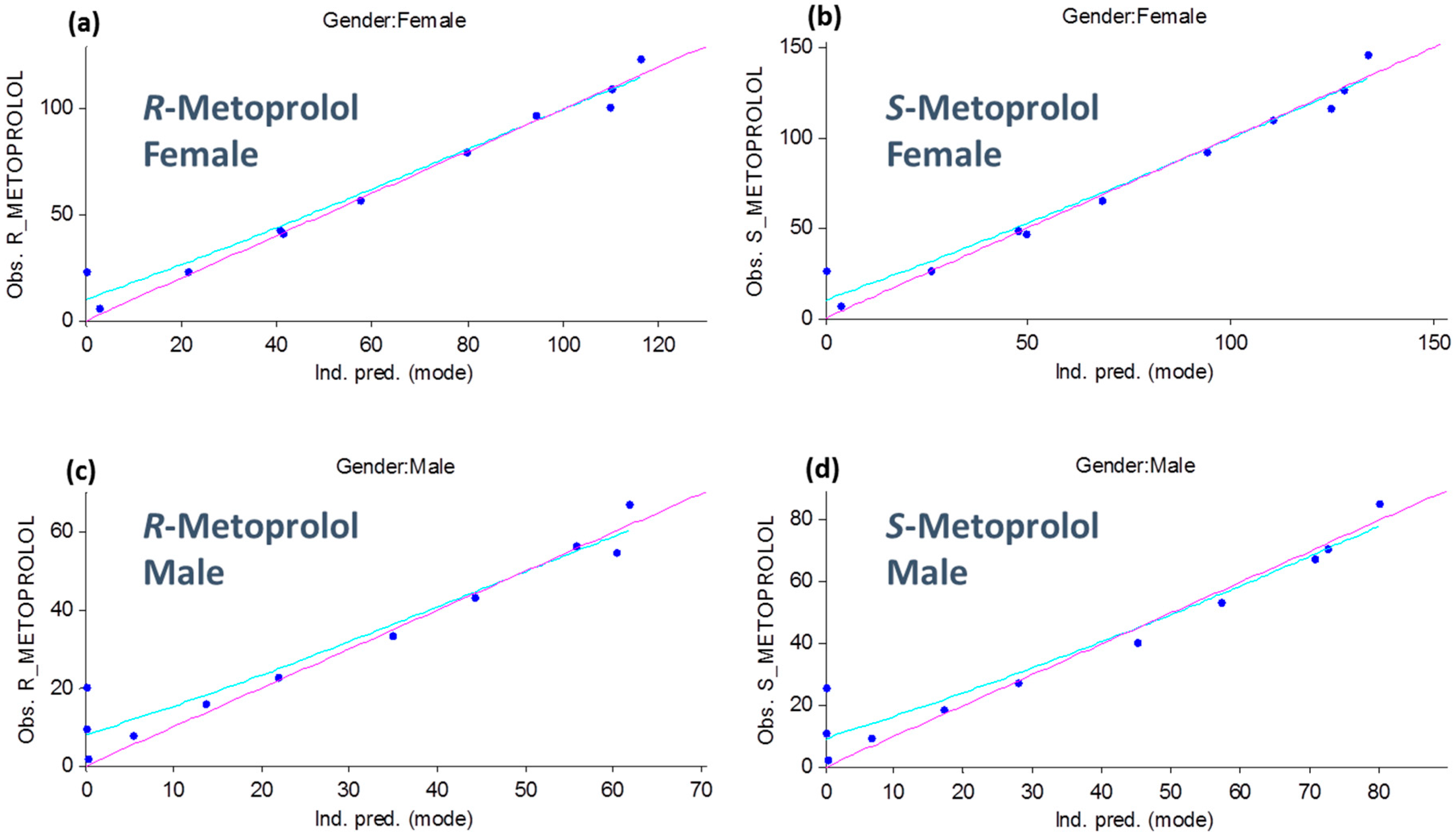
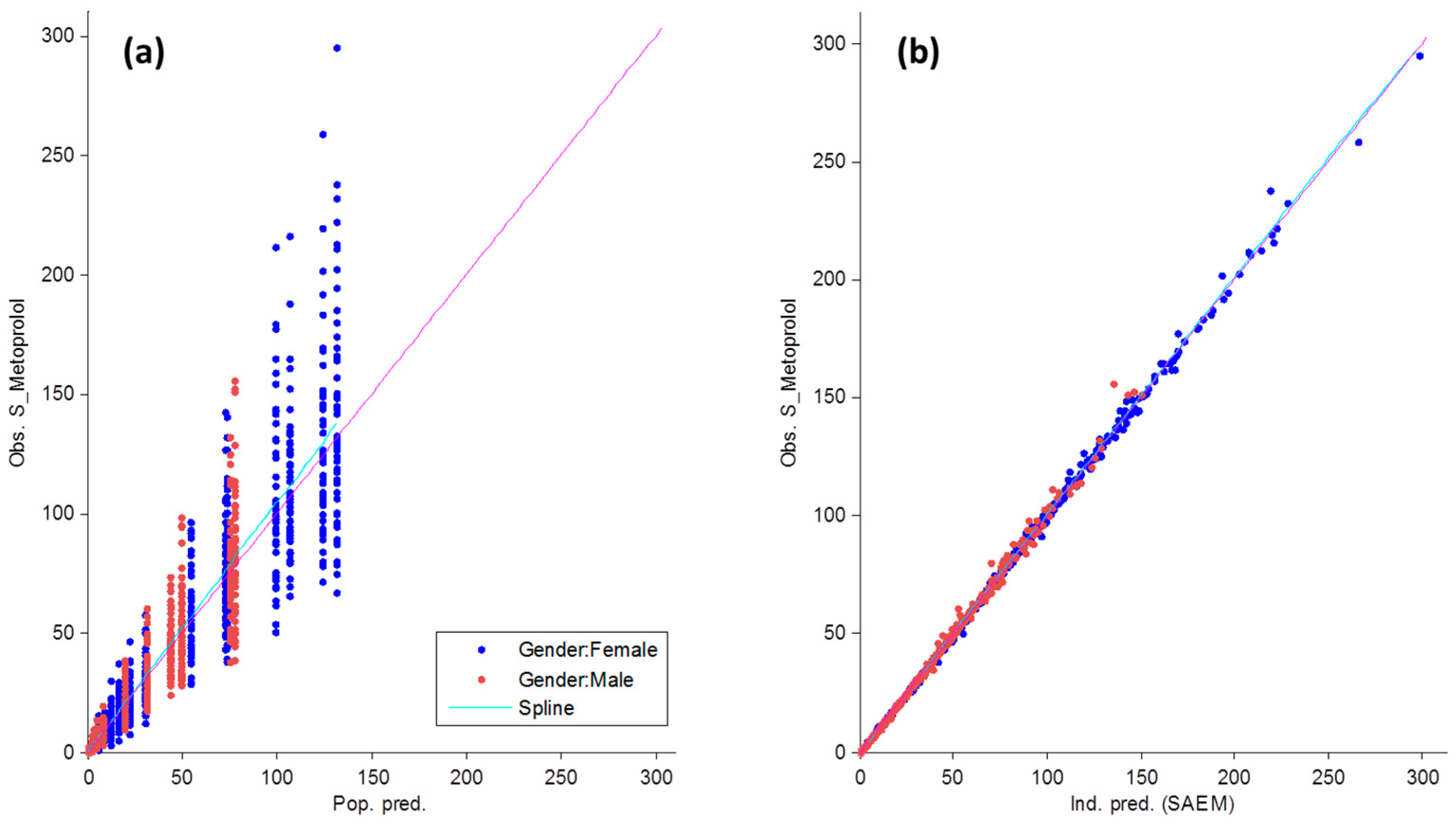
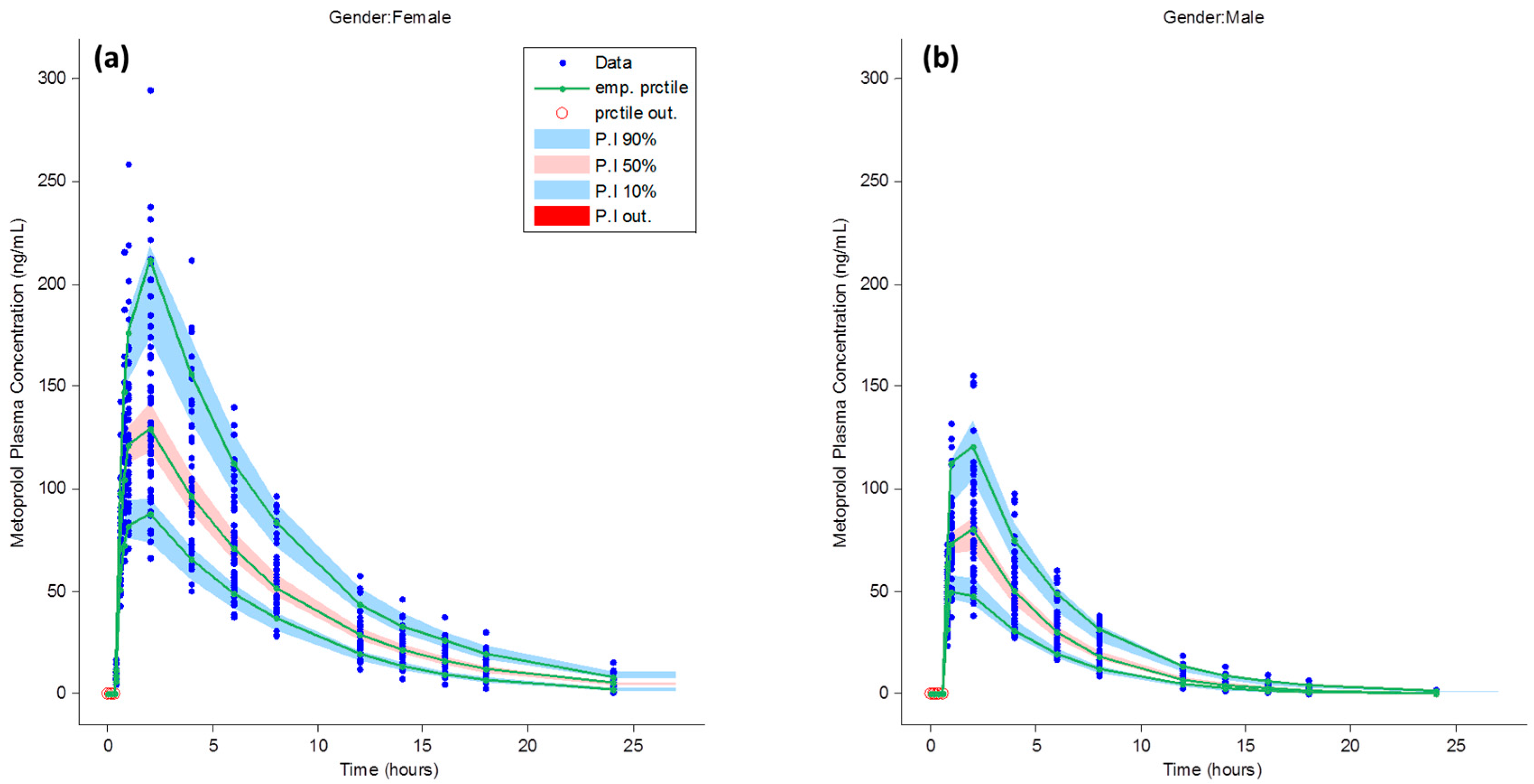
3.1. Metoprolol Pharmacokinetics
3.2. Dose-Finding Simulations
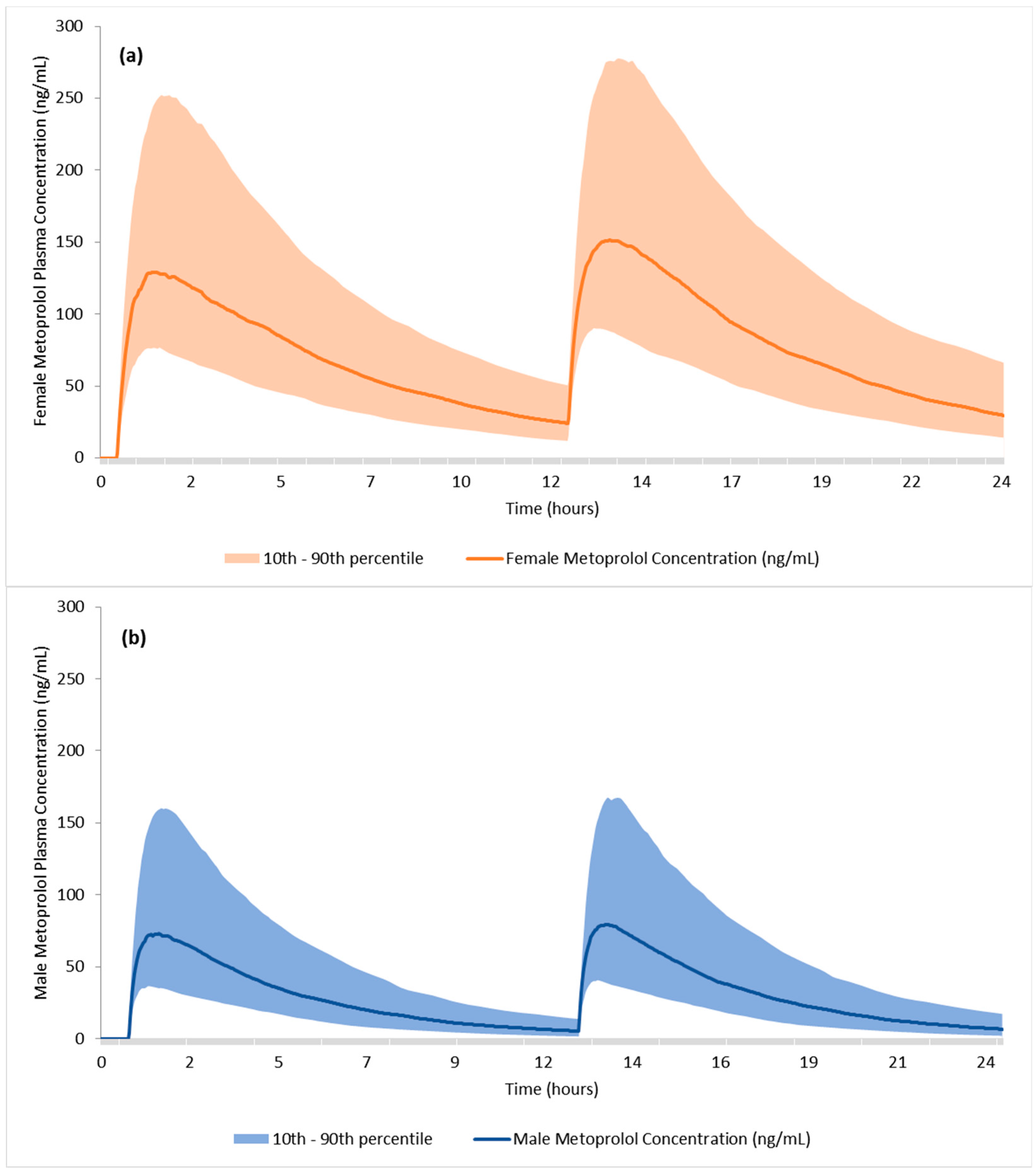
3.3. Clinical Trial Simulations
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Eugene, A.R. Gender based dosing of metoprolol in the elderly using population pharmacokinetic modeling and simulations. Int. J. Clin. Pharmacol. Toxicol. 2016, 5, 209–215. [Google Scholar] [PubMed]
- Luzier, A.B.; Killian, A.; Wilton, J.H.; Wilson, M.F.; Forrest, A.; Kazierad, D.J. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Pharmacol. Ther. 1999, 66, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Manzano, L.; Krum, H.; Rosano, G.; Holmes, J.; Altman, D.G.; Collins, P.D.; Packer, M.; Wikstrand, J.; Coats, A.J.S.; et al. Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: Individual patient data meta-analysis. BMJ 2016, 353, i1855. [Google Scholar] [CrossRef] [PubMed]
- Whitley, H.P.; Lindsey, W. Sex-based differences in drug activity. Am. Fam. Physician 2009, 80, 1254–1258. [Google Scholar] [PubMed]
- Bebia, Z.; Buch, S.C.; Wilson, J.W.; Frye, R.F.; Romkes, M.; Cecchetti, A.; Chaves-Gnecco, D.; Branch, R.A. Bioequivalence revisited: Influence of age and sex on CYP enzymes. Clin. Pharmacol. Ther. 2004, 76, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, J.; Li, D.; Wang, L.; Wang, W.; Liu, H. Systematic analysis of adverse event reports for sex differences in adverse drug events. Sci. Rep. 2016, 6, 24955. [Google Scholar] [CrossRef] [PubMed]
- Delyon, B.; Lavielle, M.; Moulines, E. Convergence of a stochastic approximation version of the EM algorithm. Ann. Stat. 1999, 27, 94–128. [Google Scholar]
- Kuhn, E.; Lavielle, M. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 2005, 49, 1020–1038. [Google Scholar] [CrossRef]
- Germani, M.; Del Bene, F.; Rocchetti, M.; Van Der Graaf, P.H. A4S: A user-friendly graphical tool for pharmacokinetic and pharmacodynamic (PK/PD) simulation. Comput. Methods Programs Biomed. 2013, 110, 203–214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and environment for statistical computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; ISBN 3-900051-07-0. Available online: http://www.R-project.org/ (accessed on 15 August 2016).
- Kaila, N.; Straka, R.J.; Brundage, R.C. Mixture models and subpopulation classification: A pharmacokinetic simulation study and application to metoprolol CYP2D6 phenotype. J. Pharmacokinet. Pharmacodyn. 2007, 34, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Nozawa, T.; Mizumaki, K.; Inoue, H.; Tahara, K.; Takesono, C.; Hashimoto, Y. Nonlinear mixed effects model analysis of the pharmacokinetics of metoprolol in routinely treated Japanese patients. Biol. Pharm. Bull. 2004, 27, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pibarot, P.; Pilote, S.; Dumesnil, J.G.; Arsenault, M.; Bélanger, P.M.; Meibohm, B.; Hamelin, B.A. Modulation of metoprolol pharmacokinetics and hemodynamics by diphenhydramine coadministration during exercise testing in healthy premenopausal women. J. Pharmacol. Exp. Ther. 2005, 313, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- FACT SHEET: President Obama's Precision Medicine Initiative. Available online: https://www.whitehouse.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative (accessed on 15 August 2016).
- Snyder, E.M.; Beck, K.C.; Dietz, N.M.; Eisenach, J.H.; Joyner, M.J.; Turner, S.T.; Johnson, B.D. Arg16Gly polymorphism of the β 2-adrenergic receptor is associated with differences in cardiovascular function at rest and during exercise in humans. J. Physiol. 2006, 571, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rodenburg, E.M.; Stricker, B.H.; Visser, L.E. Sex differences in cardiovascular drug-induced adverse reactions causing hospital admissions. Br. J. Clin. Pharmacol. 2012, 74, 1045–1052. [Google Scholar] [CrossRef] [PubMed]

| S-Metoprolol | R-Metoprolol | |||
|---|---|---|---|---|
| Female | Male | Female | Male | |
| V (L): Volume of distribution | 34.9 | 55.3 | 38.1 | 63.9 |
| CL (L/h): Clearance Rate | 101 | 253 | 120 | 316 |
| Ka (h−1): Absorption rate constant | 0.161 | 0.241 | 0.165 | 0.234 |
| Tlag (h): Absorption lag time | 0.38 | 0.67 | 0.39 | 0.59 |
| Parameters | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Value | SE | RSE (%) | CV (%) | Value | SE | RSE (%) | CV (%) | |
| Tlag (h) | 0.677 | 0.0021 | 0 | 0.20% | 0.38 | 0.00013 | 0 | 0.20% |
| Ka (1/h): Absorption Rate | 0.233 | 0.0058 | 2 | 42% | 0.149 | 0.0037 | 2 | 42% |
| V (L): Volume of Distribution | 49 | 1.5 | 3 | 43% | 33.3 | 0.88 | 3 | 43% |
| CL (L/h): Clearance Rate | 231 | 10 | 4 | 55% | 92.9 | 4 | 4 | 55% |
| Interindividual variability | ||||||||
| ωTlag, variance for Tlag | 0.0003 | 0.0021 | 809 | |||||
| ωKa, variance for Ka | 0.176 | 0.012 | 7 | |||||
| ωVd, variance for V | 0.182 | 0.013 | 7 | |||||
| ωCL, variance for CL | 0.305 | 0.022 | 7 | |||||
| Proportional error model | 0.0281 | 0.0007 | 2 | |||||
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eugene, A.R. Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations. Med. Sci. 2016, 4, 18. https://doi.org/10.3390/medsci4040018
Eugene AR. Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations. Medical Sciences. 2016; 4(4):18. https://doi.org/10.3390/medsci4040018
Chicago/Turabian StyleEugene, Andy R. 2016. "Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations" Medical Sciences 4, no. 4: 18. https://doi.org/10.3390/medsci4040018
APA StyleEugene, A. R. (2016). Metoprolol Dose Equivalence in Adult Men and Women Based on Gender Differences: Pharmacokinetic Modeling and Simulations. Medical Sciences, 4(4), 18. https://doi.org/10.3390/medsci4040018





