Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats
Abstract
:1. Introduction

2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Treatments
2.3. Experimental Design
2.4. Collection of Blood Samples for Plasma Preparation and Animal Sacrifice
2.5. Preparation of Cytosolic Fractions
2.6. Renal and Liver Functions Test
2.7. Determination of Plasma AST, ALT, ALP, and GGT Activities
2.8. Assay of Nonenzymatic Antioxidants and Lipid Peroxidation
2.9. Determination of Antioxidant Enzymes
2.10. Protein Determination
2.11. Statistical Analysis
3. Results
3.1. Protective Effects of Gallic Acid on Cyclophosphamide Induced Changes in the Levels of Plasma Creatinine, Urea and Bilirubin in Rats
| Treatment | Creatinine (mg/dL) | Urea (mg/dL) | Bilirubin (mg/dL) |
|---|---|---|---|
| Control | 0.98 ± 0.13 | 51.4 ± 3.2 | 0.36 ± 0.03 |
| CP | 2.34 ± 0.11 (139%) * | 63.8 ± 3.7 * (24%) | 0.62 ± 0.02 (72.2%) * |
| CP + GA (Pre-treated) | 1.56 ± 0.1 *,a | 54.2 ± 2.4 *,a | 0.55 ± 0.02 *,a |
| CP + GA (Co-treated) | 1.48 ± 0.2 *,a | 53.6 ± 2.9 *,a | 0.52 ± 0.02 *,a |
| GA | 1.0 ± 0.1 | 50.3 ± 2.8 * | 0.38 ± 0.01 |
3.2. Protective Effects of Gallic Acid on Cyclophosphamide-Induced Changes in the Activities of Plasma Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Alkaline Phosphatase (ALP), and Gamma Glutamyl Transferase (GGT) in Rats
| Treatment | ALT (U/L) | AST (U/L) | ALP (U/L) | GGT (U/L) |
|---|---|---|---|---|
| Control | 21.1 ± 1.4 | 174 ± 2.30 | 257 ± 2.41 | 7.1 ± 0.3 |
| CP | 43.4 ± 2.5 (106%) * | 228 ± 8.7 (31%) * | 746.4 ± 8.05 (190%) * | 17.2 ± 1.3 (142%) * |
| CP + GA (Pre-treated) | 30.2 ± 1.8 *,a | 192.8 ± 5.9*,a | 444 ± 18.2 *,a | 11.4 ± 0.84 *,a |
| CP + GA (Co-treated) | 29.1 ± 1.6 *,a | 188.4 ± 7.4 *,a | 404.4 ± 8.26 *,a | 11.2 ± 1.3 *,a |
| GA | 20.3 ± 2.6 | 177.6 ± 5.4 | 263.6 ± 2.61 | 6.8 ± 0.9 |
3.3. Protective Effects of Gallic Acid on Cyclophosphamide-Induced Changes in the Activities of Hepatic Enzymatic Antioxidants in Rats
| Treatment | SOD (units) | CAT (µmol H2O2 consumed/min/mg protein) |
|---|---|---|
| Control | 4.8 ± 0.3 | 0.23 ± 0.03 |
| CP | 2.34 ± 0.2 (51%) * | 0.11 ± 0.01 (52%) * |
| CP + GA (Pre-treated) | 4.06 ± 0.2 *,a | 0.17 ± 0.01 *,a |
| CP + GA (Co-treated) | 3.9 ± 0.1 *,a | 0.17 ± 0.02 *,a |
| GA | 4.65 ± 0.1 | 0.22 ± 0.01 |
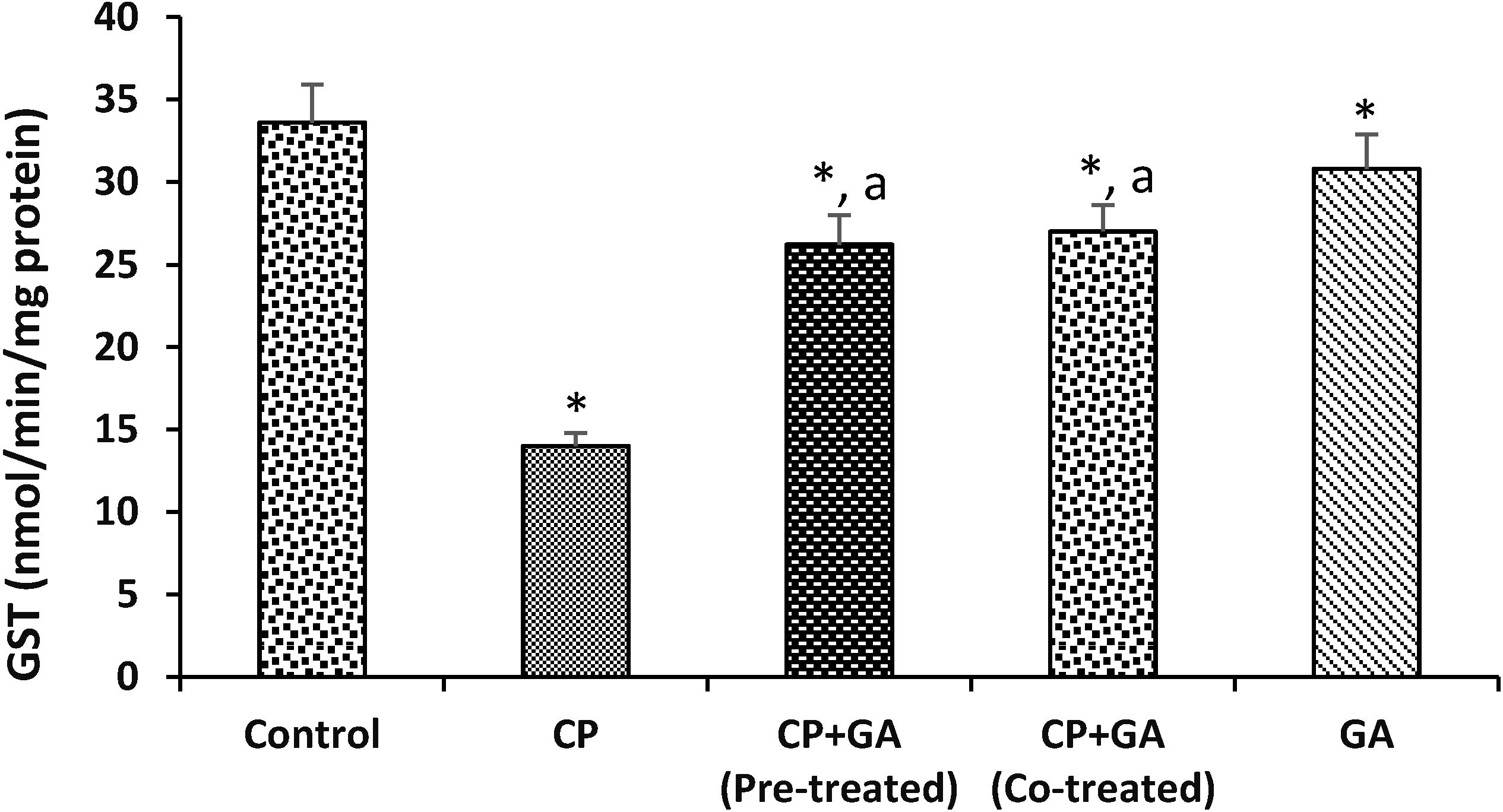
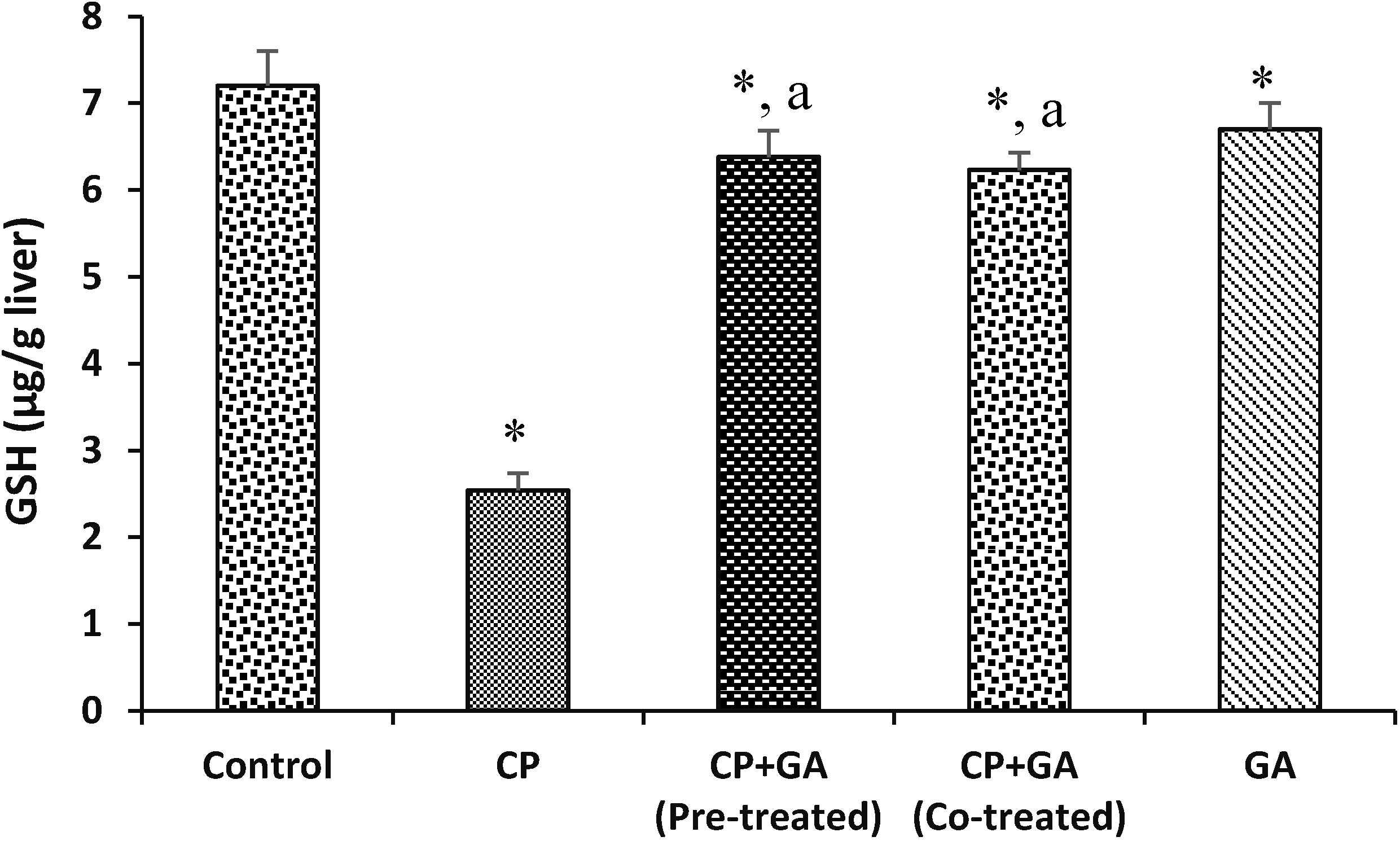
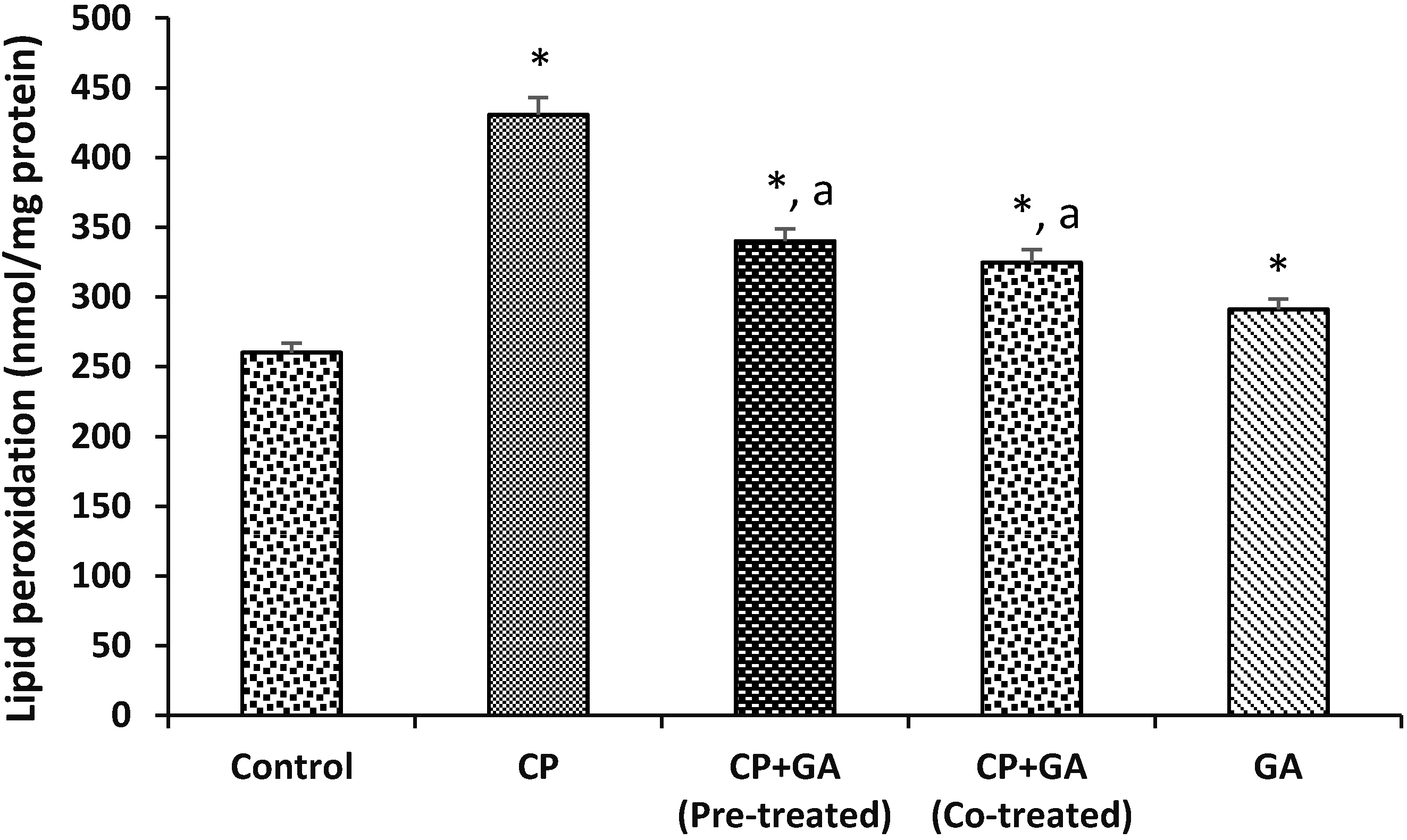
3.4. Protective Effects of Gallic Acid on Cyclophosphamide Induced Changes in the Levels of Hepatic Nonenzymatic Antioxidants and Lipid Peroxidation

4. Discussion
5. Conclusions
Author Contributions
Conflict of Interest
References
- Takimoto, C.H.; Calvo, E. Principles of oncologic pharmacotherapy. In Cancer Management: A Multidisciplinary Approach, 9th ed.; Pazdur, R., Coia, L.R., Hoskins, W.J., Wagman, L.D., Eds.; PRR Inc.: Seattle, WA, USA, 2005; pp. 23–42. [Google Scholar]
- Clern, M.; Bickers, D.R. Dermatological pharmacology. In The Pharmacological Basis of Therapeutics; Goodman, G.A., Rall, T.W., Nies, A.S., Taylor, P., Eds.; Pergamon Press: New York, NY, USA, 1991; pp. 1572–1591. [Google Scholar]
- Paul, C.; Bruce, A.C. Antineoplastic agents. In The Pharmacological Basis of Therapeutics; Goodman, G.A., Rall, T.W., Nies, A.S., Taylor, P., Eds.; Pergamon Press: New York, NY, USA, 1991; pp. 1209–1263. [Google Scholar]
- Kirkland, R.T.; Bongiovanni, A.M.; Cornfield, D.; McCormic, J.B.; Parks, J.S.; Tenore, A. Gonadotropin responses to leutinizing releasing factor in boys treated with cyclophosphamide for nephrotic syndrome. J. Pediatr. 1976, 89, 941–944. [Google Scholar] [CrossRef]
- Etteldorf, J.N.; West, C.D.; Pitcock, J.A.; Williams, D.L. Gonadal function, testicular histology, and meiosis following cyclophosphamide therapy in patients with nephrotic syndrome. J. Pediatr. 1976, 88, 206–212. [Google Scholar] [CrossRef]
- Khan, T.S.; Sundin, A.; Juhlin, C.; Wilander, E.; Oberg, K.; Eriksson, B. Vincristine, cisplatin, teniposide, and cyclophosphamide combination in the treatment of recurrent or metostatic adrenocortical cancer. Med. Oncol. 2004, 21, 167–177. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Lin, T.; Geyer, S.M.; Zent, C.S.; Leung, N.; Kabat, B.; Bowen, D.; Grever, M.R.; Byrd, J.C.; Kay, N.E. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 2007, 109, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, M.; Huitema, A.; Holtkamp, M.; van Dam, S.; Beijnea, J.; Rodenhuis, S. Aprepitant inhibits cyclophosphamide bioactivation and thiotepa metabolism. Cancer Chemother. Pharmacol 2005, 56, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, G.; Slattery, J.; Bouvier, E.; Song, R.; Batchelder, A.; Kalhorn, T.; Schoch, H.; Anasetti, C.; Goley, T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003, 101, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Hanane, A.; Claire, L.; Caroline, A.; Fabrice, M. Anticancer Drug Metabolism: Chemotherapy Resistance and New Therapeutic Approaches. In Topics on Drug Metabolism; Paxton, J., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Maiti, A.K. Genetic determinants of oxidative stress-mediated sensitization of drug resistant cancer cells. Int. J. Cancer 2012, 130, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stankiewicz, A.; Skrzydlewska, E.; Makieła, M. Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats. Drug Metabol. Drug Interact. 2002, 19, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.C.; Kehrer, J.P. Acrolein-induced cell death. A caspase-influenced decision between apoptosis and oncosis/necrosis. Chem Biol Interact. 2002, 139, 79–95. [Google Scholar] [CrossRef]
- Premkumar, K.; Pachiappan, A.; Abraham, S.K.; Santhiya, S.T.; Gopinath, P.M.; Ramesh, A. Effect of Spirulina fusiformis on cyclophosphamide and mitomycin-C induced genotoxicity and oxidative stress in mice. Fitoterapia 2001, 72, 906–911. [Google Scholar] [CrossRef]
- Venkatesan, N.; Chandrakasan, G. In vivo administration of taurine and niacin modulate cyclophosphamide-induced lung injury. Eur. J. Pharmacol. 1994, 292, 75–80. [Google Scholar] [CrossRef]
- Abariku, S.O.; Otuechere, C.A.; Eko, M.; Monwuba, K.; Osobu, D. Rutin Ameliorates Cyclophosphamide-induced Reproductive Toxicity in Male Rats. Toxicol. Int. 2012, 19, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Premila, A.; Indirani, K.; Preethi, K. Alterations in antioxidant enzyme activities and increased oxidative stress in cyclophosphamide-induced hemorrhagic cystitis in the rat. Cancer Ther. 2008, 6, 563–570. [Google Scholar]
- Selvakumar, E.; Prahalathan, C.; Mythili, Y.; Varalakshmi, P. Protective effect of dl-β-lipoic acid in cyclophosphamide induced oxidative injury in rat testis. Reprod. Toxicol. 2004, 19, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Das, U.B.; Mallick, M.; Debnath, J.M.; Ghosh, D. Protective effect of ascorbic acid on cyclophosphamide-induced testicular gametogenic and androgenic disorders in male rats. Asian J. Androl. 2002, 4, 201–207. [Google Scholar] [PubMed]
- McDermott, E.M.; Powell, R.J. Incidence of ovarian failure in systemic lupus erythematosus after treatment with pulse cyclophosphamide. Ann. Rheum. Dis. 1996, 55, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, D.; Chattopadhyay, S.; Debnath, J. Effect of ascorbic acid supplementation on liver and kidney toxicity in cyclophosphamide-treated female albino rats. J. Toxicol. Sci. 1999, 24, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Schmitt, C.A. Chemotherapy response and resistance. Curr. Opin. Genet. Dev. 2003, 13, 63–69. [Google Scholar] [CrossRef]
- Weiji, N.I.; Cleton, F.J.; Osanto, S. Free radicals and antioxidants in chemotherapy-induced toxicity. Cancer Treat. Rev. 1997, 23, 209–240. [Google Scholar]
- McCall, M.R.; Frei, B. Can antioxidant vitamins materially reduce oxidative damage in humans? Free Radic. Biol. Med. 1999, 26, 1034–1053. [Google Scholar] [CrossRef]
- Singleton, V.L. Naturally occurring food toxicants: Phenolic substances of plant origin common in foods. Adv. Food Res. 1981, 27, 149–242. [Google Scholar] [PubMed]
- Niu, X.; Fan, X.; Sun, J.; Ting, P.; Narula, S.; Lundell, D. Inhibition of Fucosyltransferase VII by gallic acid and its derivative. Arch. Biochem. Biophys. 2004, 425, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, B.K.; Lee, K.W.; Lee, H.J. Resveratrol Counteracts gallic acid-induced down regulation of gap-junction intercellular communication. J. Nutr. Biochem. 2009, 20, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Stanely, P.; Prince, M.; Priscilla, H.; Devika, P.T. Gallic acid prevents lysosomal damage in isoproterenol induced cardiotoxicity in Wistar Rats. Eur. J. Pharmacol. 2009, 27, 230–235. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food Sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [PubMed]
- Priscilla, D.H.; Prince, P.S. Cardioprotective effect of gallic acid on cardiac troponin-T, Cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009, 179, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Leiro, I.M.; Alvarez, E.; Arranz, J.A.; Siso, I.G.; Orallo, F. In vitro effect of magniferin on superoxide concentration and expression of the inducible nitric oxide synthase, tumuor necrosis factor-alpha and transforming growth factor-beta genes. Biochem. Pharmacol. 2014, 9, 901–937. [Google Scholar]
- Mahmoud, R.H.; Barakat, H. The protective effect of gallic acid and caffeine against CCl4-induced oxidative hepatotoxicity and mitochondrial DNA depletion in male albino rats. Egypt. J. Biochem. Mol. Biol. 2010, 28, 543–562. [Google Scholar]
- Padma, V.V.; Castro, S.A.; Felix, T.A.; Rathinasamy, B.; Paramasivan, P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem. Toxicol. 2011, 49, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, J.M.; Viswanatha Swamy, A.H.M. Cardioprotective effect of gallic acid against doxorubicin-induced myocardial toxicity in albino rats. Indian J. Health Sci. 2015, 8, 28–35. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Research; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Punithavathi, V.R.; Prince, P.S.; Kumar, R.; Selvakumari, J. Antihyperglycaemic, antilipid peroxidative and antioxidant effects of gallic acid on streptozotocin induced diabetic Wistar rats. Eur. J. Pharmacol. 2011, 650, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, B. What made the radical break. N. Engl. J. Med. 1972, 286, 156–157. [Google Scholar] [PubMed]
- Tietz, N.W.; Pruden, E.L.; Siggaard-Andersen, O. Liver function. In Tietz Textbook of Clinical Chemistry; Burtis, A.C., Ashwood, E.R., Eds.; Saunders: London, UK, 1994; pp. 1354–1374. [Google Scholar]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; Tietz, N.W., Ed.; Saunders: Philadelphia, PA, USA, 1995. [Google Scholar]
- Horder, M.; Magid, E.; Pitkanen, E.; Härkönen, M.; Strömme, J.H.; Theodorsen, L.; Gerhardt, W.; Waldenström, J. Recommended method for the determination of creatine kinase in blood modified by the inclusion of EDTA. The committee on enzymes of the Scandinavian Society for Clinical Chemistry and Clinical Physiology (SCE). Scand. J. Clin. Lab. Investig. 1979, 39, 1–5. [Google Scholar] [CrossRef]
- Erel, O.; Kocyigit, A.; Avci, S.; Aktepe, N.; Bulut, V. Oxidative stress and antioxidative status of plasma and erythrocytes in patients with vivax malaria. Clin. Biochem. 1997, 30, 631–639. [Google Scholar] [CrossRef]
- Jollow, D.J.; Mitchell, J.R.; Zampaglione, N.; Gillette, J.R. Bromobenzene induced liver necrosis: Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Kale, R.K. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Magwere, T.; Naik, Y.S.; Hasler, J.A. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic. Biol. Med. 1996, 22, 321–327. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione transferases, the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- De Jonge, M.E.; Huitema, A.D.R.; Rodenhuis, S.; Beijnen, J.H. Clinical Pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005, 44, 1135–1164. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, P.; Das, U.N.; Koratkar, R.; Suryaprabha, P. Increase in free radical generation and lipid peroxidation following chemotherapy in patients with cancer. Free Radic. Biol. Med. 1990, 8, 15–19. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Kuttan, G. Cardiospermum halicacabum inhibits cyclophosphamide induced immunosupression and oxidative stress in mice and also regulates iNOS and COX-2 gene expression in LPS stimulated macrophages. Asian Pac. J. Cancer Prev. 2010, 11, 1245–1252. [Google Scholar] [PubMed]
- Jakopič, J.; Veberič, R.; Štampar, F. Extraction of phenolic compounds from green walnut fruits in different solvents. Acta agriculturae Slovenica 2009, 93, 11–15. [Google Scholar] [CrossRef]
- Amacher, D.E. Serum Transaminase Elevations as Indicators of Hepatic Injury Following the Administration of Drugs. Regulat. Toxicol. Pharmacol. 1998, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, S.G.; Kannan, V.; Pandian, R.M. Antihepatotoxic effect of β-carotene on paracetamol induced hepatic damage in rats. Ind. J. Exp. Biol. 2005, 43, 351–355. [Google Scholar]
- Kaur, G.; Alam, M.S.; Jabbar, Z.; Javed, K.; Athar, M. Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol. 2006, 108, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Sreetha, S.; Padma, P.R.; Umadevi, M. Effect of Coriandrum sativum extracts on carbon tetrachloride-induced hepatotoxicity in rats. Food Chem. Toxicol. 2009, 47, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Habibi, E.; Shokrzadeh, M.; Chabra, A.; Naghshvar, F.; Keshavarz-Maleki, R.; Ahmadi, A. Protective effects of Origanum vulgare ethanol extract against cyclophosphamide-induced liver toxicity in mice. Pharm. Bio. 2015, 53, 1–6. [Google Scholar]
- Nyblom, H.; Bjornsson, E.; Simren, M.; Aldenborg, F.; Almer, S.; Olsson, R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006, 26, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical Biochemistry of Hepatotoxicity. J. Clin. Toxicol. 2011, 4, 1–19. [Google Scholar]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Hepatoprotective and antioxidant effect of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Pharmacol. 2010, 62, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Daugirdas, J. CKD Screening and Management Overview. In Handbook of Chronic Kidney Disease Management; Lippincolt Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Ferguson, M.A.; Waikar, S.S. Established and Emerging Markers of Kidney Function. Clin. Chem. 2012, 58, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.L.; de Azevedo, M.J.; Silveiro, S.P.; Canani, L.H.; Caramori, M.L.; Zelmanovitz, T. Diabetic nephropathy: Diagnosis, prevention and treatment. Diabetes Care 2005, 28, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Mouton, R.; Holder, K. Laboratory tests of renal function. Anaesth. Intensive Care Med. 2006, 7, 240–243. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kikkawa, R.; Yamada, H.; Horii, I. Identification of oxidative stress related proteins for predictive screening of hepatotoxicity using a proteomic approach. J. Toxicol. Sci. 2005, 30, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Jung, Y.S.; Jeon, B.S.; Yoon, B.I.I.; Lee, K.S.; Kim, B.H.; Oh, S.J.; Kim, S.K. Evaluation of hepatotoxicity and oxidative stress in rats treated with tert-butyl hydroperoxide. Food Chem. Toxicol. 2012, 50, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- King, P.D.; Perry, M.C. Hepatotoxicity of Chemotherapy. Oncolgist 2001, 6, 162–176. [Google Scholar] [CrossRef]
- Yost, G.S.; Horstman, M.G.; Walily, A.F.; Gordon, W.P.; Nelson, S.D. Procarbazine spermatogenesis toxicity: Deuterium isotope effect point to regioselective metabolism in mice. Toxicol. Appl. Pharmacol. 1985, 80, 316–322. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncola, J.; Cronin, M.T.D.; Mazura, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Han, W.K.; Bonventre, J.V. Biological markers for the early detection of acute kidney injury. Curr. Opin. Crit. Care 2004, 10, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Ali-Osman, F. Quenching of DNA cross-link precursors of chloroethylnitrosoureas and attenuation of DNA interstrand cross-linking by glutathione. Cancer Res. 1989, 49, 5258–5261. [Google Scholar] [PubMed]
- Masella, R.; Di Benedetto, R.; Vari, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Touliatos, J.S.; Neitzel, L.; Whitworth, C.; Rybak, L.P.; Malafa, M. Effect of cisplatin on the expression of glutathione-S-transferase in the cochlea of the rat. Eur. Arch. Otorhinolaryngol. 2000, 25, 6–9. [Google Scholar] [CrossRef]
- Pramita, C.; Ugir, H.S.K.; Nabendu, M.; Jayanta, K.D.; Smarajit, P.; Sudin, B. Modulation of Cyclophosphamide-Induced Cellular Toxicity by Diphenylmethyl Selenocyanate in vivo, an Enzymatic Study. J. Cancer Mol. 2009, 4, 183–189. [Google Scholar]
- Senthil-Kumar, M.; Udayakumar, M. High-throughput virus-induced gene-silencing approach to assess the functional relevance of a moisture stress-induced cDNA homologous to lea4. J. Exp. Bot. 2006, 57, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin. Chem. 1995, 41, 1819–1828. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olayinka, E.T.; Ore, A.; Ola, O.S.; Adeyemo, O.A. Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats. Med. Sci. 2015, 3, 78-92. https://doi.org/10.3390/medsci3030078
Olayinka ET, Ore A, Ola OS, Adeyemo OA. Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats. Medical Sciences. 2015; 3(3):78-92. https://doi.org/10.3390/medsci3030078
Chicago/Turabian StyleOlayinka, Ebenezer Tunde, Ayokanmi Ore, Olaniyi Solomon Ola, and Oluwatobi Adewumi Adeyemo. 2015. "Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats" Medical Sciences 3, no. 3: 78-92. https://doi.org/10.3390/medsci3030078
APA StyleOlayinka, E. T., Ore, A., Ola, O. S., & Adeyemo, O. A. (2015). Ameliorative Effect of Gallic Acid on Cyclophosphamide-Induced Oxidative Injury and Hepatic Dysfunction in Rats. Medical Sciences, 3(3), 78-92. https://doi.org/10.3390/medsci3030078