Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Preparation of the Methanolic Extract
2.2. Chemicals
2.3. Animals
2.4. Determination of the Effect of Rutin on DMBA Croton Oil Induced Skin Carcinogenesis
2.5. Determination of the Effect of Rutin on Enzyme Involved in Oxidative Stress
2.6. Determination of the Effect of Rutin on Serum Enzyme Analysis
2.7. Histopathology
2.8. Statistical Analysis
3. Results
| Treatment | Body Weight (g) | Number of Papilloma | Tumor Size | Average Latent Period a | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| Group I (Control) | 26.26 ± 2.16 | 22.75 ± 11.24 | 10.16 ± 5.03 | 2.06 ± 0.37 | 10.10 ± 5.17 |
| Treatment | GSH µmole/mg Protein | SOD µmole/mg Protein | Catalase U/mg Protein | LPO nmole/mg Protein |
|---|---|---|---|---|
| Group I (Control) | 8.02 ± 0.96 | 59.59 ± 2.15 | 5.97 ± 1.07 | 4.82 ± 1.71 |
| Group II (Treated) | 27.24 ± 1.16 * | 77.50 ± 0.84 * | 33.73 ± 10.93 * | 3.02 ± 0.37 * |
| Group III (Treated) | 21.22 ± 0.49 * | 80.05 ± 10.54 * | 38.84 ± 20.18 * | 2.7 ± 0.36 * |
| Treatment | SGOTIU/L | SGPTIU/L | SALPIU/L | Bilurubbin mg/dL |
|---|---|---|---|---|
| Group I (Control) | 162.73 ± 12.33 | 149.06 ± 17.00 | 231.52 ± 13.93 | 5.56 ± 2.53 |
| Group II (Treated) | 101.37 ± 6.36 * | 69.91 ± 23.02 * | 86.35 ± 47.16 * | 2.21 ± 0.86 * |
| Group III (Treated) | 85.26 ± 22.07 * | 49.02 ± 24.06 * | 75.41 ± 24.64 * | 1.95 ± 0.76 * |
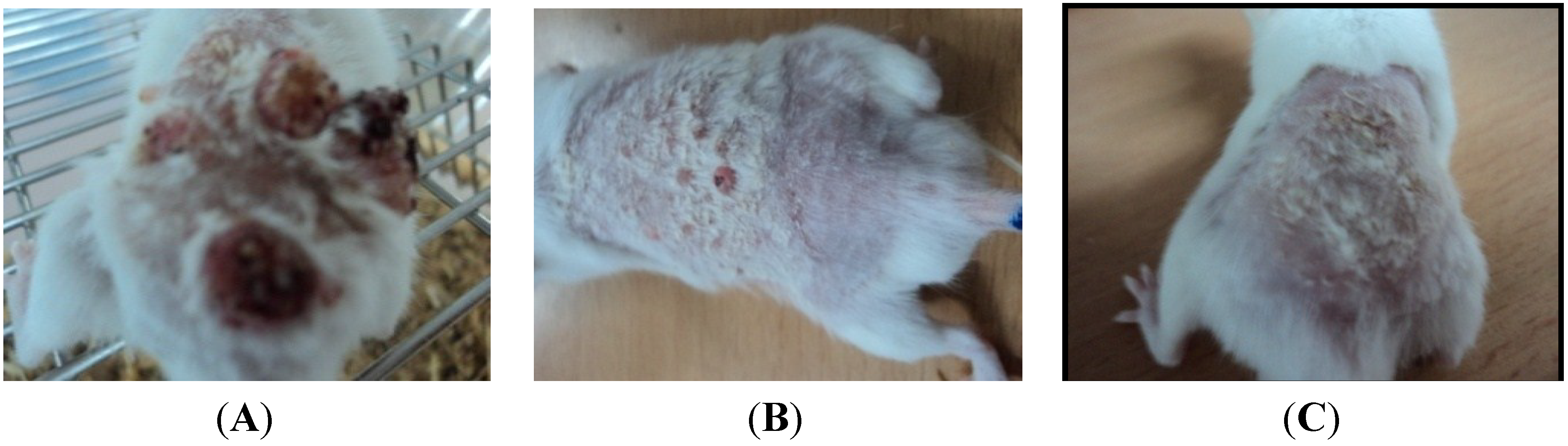
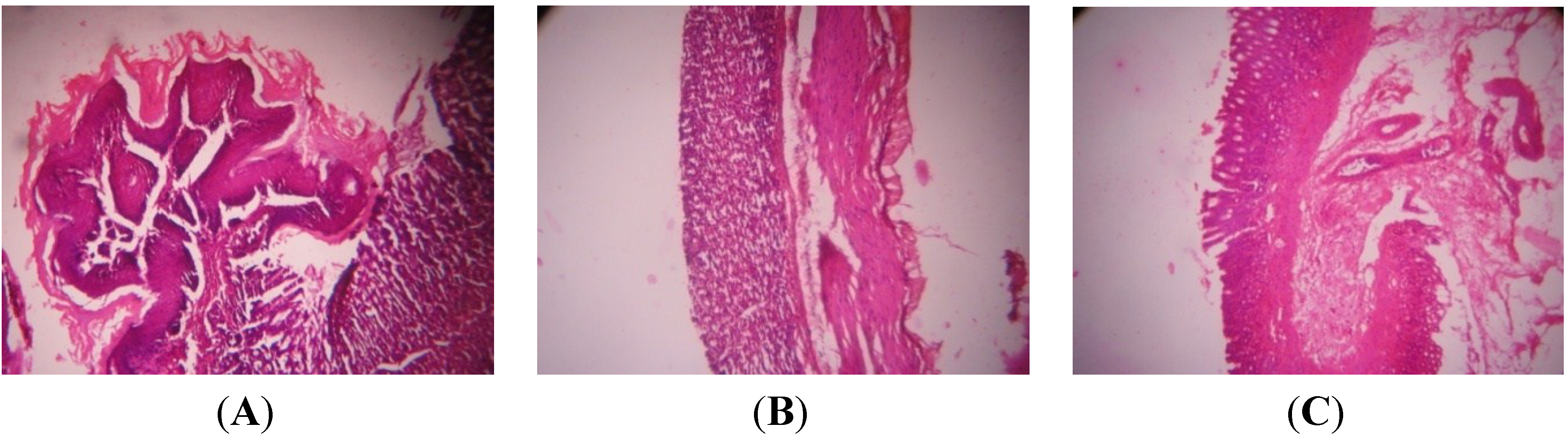
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Singh, N.; Verma, B.P.; Pandey, B.R. Therapeutic potential of organic Triticum aestivum linn. (wheat grass) in prevention and treatment of chronic diseases: An overview. Int. J. Pharm. Sci. Drug Res. 2012, 4, 10–14. [Google Scholar]
- Nenonen, M.T.; Helve, T.A.; Rauma, A.L.; Hanninen, O.O. Uncooked, lactobacilli-rich, vegan food and rheumatoid arthritis. Br. J. Rheumatol. 1998, 37, 274–281. [Google Scholar] [CrossRef]
- Mates, M.J.; Jimenez, S.; Fransisca, M. Role of reactive oxygen species in apoptosis: Implication for cancer therapy. Int. J. Biochem. Cell Biol. 2000, 32, 157–170. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, U.S.; Sharma, U.K.; Mishra, V.; Ranjan, S. In vitro antimicrobial activity of the Triticum aestivum straw extracts. Int. J. Drug Discov. 2010, 2, 1–4. [Google Scholar]
- Ahmad, M.; Qureshi, R.; Arshad, M.; Khan, M.A.; Zafar, M. Traditional herbal remedies used for the treatment of diabetes from district Attock (Pakistan). Pak. J. Bot. 2009, 41, 2777–2782. [Google Scholar]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef]
- Arima, H.; Ashida, H.; Danno, G. Rutin-enhanced antibacterial activities of flavonoids against Bacillus cereus and Salmonella enteritidis. Biosci. Biotechnol. Biochem. 2002, 66, 1009–1014. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effect of rutin, quercetin and hesperidin on adjuvant arthritis in rat. II Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Afanas’ev, I.B.; Ostrakhovitcha, E.A.; Mikhalchik, E.V.; Ibragimova, G.A.; Korkina, L.G. Enhancement of antioxidant and anti-inflammatory activities of bioflavonoid rutin by complexation with transition metals. Biochem. Pharmacol. 2001, 61, 677–684. [Google Scholar] [CrossRef]
- Ostrakhovitcha, E.A.; Afanas’ev, I.B. Oxidative stress in rheumatoid arthritis leukocytes: Suppression by rutin and other antioxidants and chelators. Biochem. Pharmacol. 2001, 62, 743–746. [Google Scholar] [CrossRef]
- Gupta, R.; Singh, M.; Sharma, A. Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol. Res. 2003, 48, 209–215. [Google Scholar] [CrossRef]
- Middleton, J.; Kandaswami, E.; Theoharides, C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- La Casa, C.; Villegas, I.; de la Lastra, C.A.; Motilva, V.; Calero, M.J.M. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Davis, L.; Kuttan, G. Effect of Withania somnifera on DMBA induced carcinogenesis. J. Ethnopharmacol. 2001, 75, 165–168. [Google Scholar] [CrossRef]
- Atanassova, M.; Bagdassarian, V. Rutin content in plant products. J. Univ. Chem. Technol. Metal. 2009, 44, 201–203. [Google Scholar]
- Ando, S.; Kon, K.; Aino, K.; Totani, Y. Increased levels of lipid peroxides in aged rat brain as revealed by direct assay of peroxide values. Neurosci. Lett. 1990, 113, 199–204. [Google Scholar] [CrossRef]
- Moron, M.A.; Depierre, J.W.; Mannervick, B. Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat liver. J. Biochim. Biophys. Acta 1979, 582, 67–68. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A. Handbook of Methods in Oxygen Radical Research; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Sengupta, A.; Ghosh, S.; Bhattacharjee, S.; Das, S. Indianfood ingredients and cancer prevention—An experimental evaluation of anticarcinogenic effects of garlicinrat colon. Asian Pac. J. Cancer Prev. 2004, 5, 126–132. [Google Scholar] [PubMed]
- Nishino, H.; Murakoshi, M.; Mou, X.Y.; Wada, S.; Masuda, M.; Ohsaka, Y. Cancer prevention by phytochemicals. Oncology 2005, 69, 38–40. [Google Scholar] [CrossRef]
- Slaga, T.J.; Fischer, S.M.; Nelson, K.; Gleason, G.L. Studies on the mechanism of skin tumor promotion: Evidence for several stages in promotion. Proc. Natl. Acad. Sci. USA 1980, 77, 3659–3663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, Y.M.; Wang, W.M.; Liu, Y.Q. Experimental study on anti-tumor effect of extractive from Celastrus orbiculatus in vivo. Zhongguo Zhong Yao Za Zhi 2006, 31, 1514–1516. [Google Scholar] [PubMed]
- Blessy, D.; Suresh, K.; Manoharan, S.; Vijayaanand, M.A.; Sugunadevi, G. Evaluation of Chemopreventive Potential of Zingiber Officinale Roscoe Ethanolic Root Extract on 7,12-dimethyl Benz[a]anthracene Induced Oral Carcinogenesis. Res. J. Agric. Biol. Sci. 2009, 5, 775–781. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixit, S. Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice. Med. Sci. 2014, 2, 153-160. https://doi.org/10.3390/medsci2040153
Dixit S. Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice. Medical Sciences. 2014; 2(4):153-160. https://doi.org/10.3390/medsci2040153
Chicago/Turabian StyleDixit, Savita. 2014. "Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice" Medical Sciences 2, no. 4: 153-160. https://doi.org/10.3390/medsci2040153
APA StyleDixit, S. (2014). Anticancer Effect of Rutin Isolated from the Methanolic Extract of Triticum aestivum Straw in Mice. Medical Sciences, 2(4), 153-160. https://doi.org/10.3390/medsci2040153




