Impact of Elexacaftor–Tezacaftor–Ivacaftor on Muscle Composition in Cystic Fibrosis: An AI-Assisted Chest CT-Based Body Composition Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
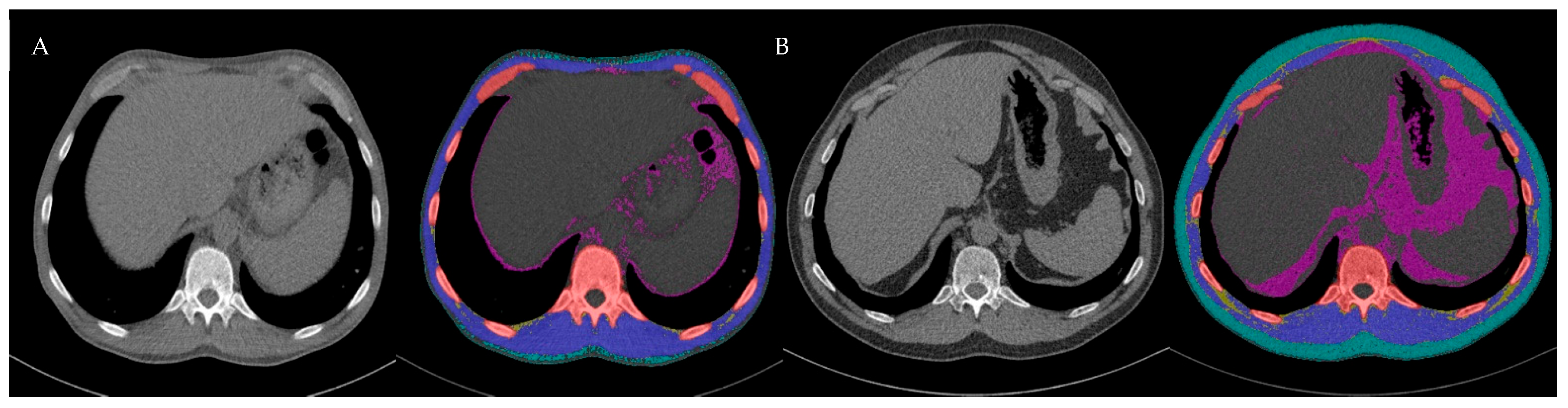
2.2. CT Image Acquisition and Body Composition Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Course of Body Composition at T12 Level Under ETI
3.3. Sex-Specific Changes and Differences During Treatment with ETI
4. Discussion
4.1. Exercise and Body Composition
4.2. Nutritional Intake/Interventions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CT | computed tomography |
| ETI | elexacaftor/tezacaftor/ivacaftor |
| pwCF | people with cystic fibrosis |
| IMAT | inter- and intramuscular adipose tissue |
| SMA | skeletal muscle area |
| SMI | skeletal muscle indices |
| LAMA | low-attenuation muscle area |
| ppFEV1 | percent predicted forced expiratory volume in 1 s |
References
- Grasemann, H.; Ratjen, F. Cystic Fibrosis. N. Engl. J. Med. 2023, 389, 1693–1707. [Google Scholar] [CrossRef]
- Gruet, M.; Troosters, T.; Verges, S. Peripheral muscle abnormalities in cystic fibrosis: Etiology, clinical implications and response to therapeutic interventions. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2017, 16, 538–552. [Google Scholar] [CrossRef]
- Calella, P.; Valerio, G.; Brodlie, M.; Donini, L.M.; Siervo, M. Cystic fibrosis, body composition, and health outcomes: A systematic review. Nutrition 2018, 55–56, 131–139. [Google Scholar] [CrossRef]
- Lamhonwah, A.-M.; Bear, C.E.; Huan, L.J.; Kim Chiaw, P.; Ackerley, C.A.; Tein, I. Cystic fibrosis transmembrane conductance regulator in human muscle: Dysfunction causes abnormal metabolic recovery in exercise. Ann. Neurol. 2010, 67, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Divangahi, M.; Balghi, H.; Danialou, G.; Comtois, A.S.; Demoule, A.; Ernest, S.; Haston, C.; Robert, R.; Hanrahan, J.W.; Radzioch, D.; et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009, 5, e1000586. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, D.W.; Ko, Y.; Ha, J.; Shin, Y.B.; Lee, J.; Sung, Y.S.; Kim, K.W. Updated systematic review and meta-analysis on diagnostic issues and the prognostic impact of myosteatosis: A new paradigm beyond sarcopenia. Ageing Res. Rev. 2021, 70, 101398. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Valencak, T.G.; Shan, T. Fat infiltration in skeletal muscle: Influential triggers and regulatory mechanism. iScience 2024, 27, 109221. [Google Scholar] [CrossRef]
- Correa-de-Araujo, R.; Addison, O.; Miljkovic, I.; Goodpaster, B.H.; Bergman, B.C.; Clark, R.V.; Elena, J.W.; Esser, K.A.; Ferrucci, L.; Harris-Love, M.O.; et al. Myosteatosis in the Context of Skeletal Muscle Function Deficit: An Interdisciplinary Workshop at the National Institute on Aging. Front. Physiol. 2020, 11, 963. [Google Scholar] [CrossRef]
- Ebadi, M.; Tsien, C.; Bhanji, R.A.; Dunichand-Hoedl, A.R.; Rider, E.; Motamedrad, M.; Mazurak, V.C.; Baracos, V.; Montano-Loza, A.J. Myosteatosis in Cirrhosis: A Review of Diagnosis, Pathophysiological Mechanisms and Potential Interventions. Cells 2022, 11, 1216. [Google Scholar] [CrossRef]
- Kim, H.K.; Kim, C.H. Quality Matters as Much as Quantity of Skeletal Muscle: Clinical Implications of Myosteatosis in Cardiometabolic Health. Endocrinol. Metab. 2021, 36, 1161–1174. [Google Scholar] [CrossRef]
- Nachit, M.; Horsmans, Y.; Summers, R.M.; Leclercq, I.A.; Pickhardt, P.J. AI-based CT Body Composition Identifies Myosteatosis as Key Mortality Predictor in Asymptomatic Adults. Radiology 2023, 307, e222008. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Garcia-Diez, A.I.; Porta-Vilaro, M.; Isern-Kebschull, J.; Naude, N.; Guggenberger, R.; Brugnara, L.; Milinkovic, A.; Bartolome-Solanas, A.; Soler-Perromat, J.C.; Del Amo, M.; et al. Myosteatosis: Diagnostic significance and assessment by imaging approaches. Quant. Imaging Med. Surg. 2024, 14, 7937–7957. [Google Scholar] [CrossRef]
- Tan, L.; Ji, G.; Bao, T.; Fu, H.; Yang, L.; Yang, M. Diagnosing sarcopenia and myosteatosis based on chest computed tomography images in healthy Chinese adults. Insights Into Imaging 2021, 12, 163. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Mall, M.A. The future of cystic fibrosis treatment: From disease mechanisms to novel therapeutic approaches. Lancet 2023, 402, 1185–1198. [Google Scholar] [CrossRef]
- Sutharsan, S.; Dillenhoefer, S.; Welsner, M.; Stehling, F.; Brinkmann, F.; Burkhart, M.; Ellemunter, H.; Dittrich, A.M.; Smaczny, C.; Eickmeier, O.; et al. Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: Real-world evidence from the German CF Registry. Lancet Reg. Health Eur. 2023, 32, 100690. [Google Scholar] [CrossRef]
- Petersen, M.C.; Begnel, L.; Wallendorf, M.; Litvin, M. Effect of elexacaftor-tezacaftor-ivacaftor on body weight and metabolic parameters in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 265–271. [Google Scholar] [CrossRef]
- Mouzaki, M.; Dupuis, A.; Avolio, J.; Griffin, K.; Ratjen, F.; Tullis, E.; Gonska, T. Weight increase in people with cystic fibrosis on CFTR modulator therapy is mainly due to increase in fat mass. Front. Pharmacol. 2023, 14, 1157459. [Google Scholar] [CrossRef] [PubMed]
- Navas-Moreno, V.; Sebastian-Valles, F.; Rodriguez-Laval, V.; Knott-Torcal, C.; Marazuela, M.; de la Blanca, N.S.; Arranz Martin, J.A.; Giron, R.M.; Sampedro-Nunez, M.A. Impact of CFTR modulator therapy on body composition as assessed by thoracic computed tomography: A follow-up study. Nutrition 2024, 123, 112425. [Google Scholar] [CrossRef] [PubMed]
- Westholter, D.; Haubold, J.; Welsner, M.; Salhofer, L.; Wienker, J.; Sutharsan, S.; Strassburg, S.; Taube, C.; Umutlu, L.; Schaarschmidt, B.M.; et al. Elexacaftor/tezacaftor/ivacaftor influences body composition in adults with cystic fibrosis: A fully automated CT-based analysis. Sci. Rep. 2024, 14, 9465. [Google Scholar] [CrossRef]
- Ratti, G.A.; Smith, H.; Mirfakhraee, S.; Reisch, J.; Cohen, L.; Jain, R.; Finklea, J.D. Development of metabolic syndrome in people with Cystic Fibrosis one year after exposure to elexacaftor-tezacaftor-ivacaftor. J. Cyst. Fibros. 2025, 24, 47–52. [Google Scholar] [CrossRef]
- Salvatore, D.; Padoan, R.; Amato, A.; Salvatore, M.; Campagna, G.; On Behalf of The Italian Cf Registry Working, G. Nutritional Trends in Cystic Fibrosis: Insights from the Italian Cystic Fibrosis Patient Registry. J. Clin. Med. 2024, 13, 3652. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Haubold, J.; Baldini, G.; Parmar, V.; Schaarschmidt, B.M.; Koitka, S.; Kroll, L.; van Landeghem, N.; Umutlu, L.; Forsting, M.; Nensa, F.; et al. BOA: A CT-Based Body and Organ Analysis for Radiologists at the Point of Care. Investig. Radiol. 2023, 59, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Keyl, J.; Keyl, P.; Montavon, G.; Hosch, R.; Brehmer, A.; Mochmann, L.; Jurmeister, P.; Dernbach, G.; Kim, M.; Koitka, S.; et al. Decoding pan-cancer treatment outcomes using multimodal real-world data and explainable artificial intelligence. Nat. Cancer 2025, 6, 307–322. [Google Scholar] [CrossRef]
- Salhofer, L.; Jost, G.; Meetschen, M.; van Landeghem, D.; Forsting, M.; Bos, D.; Bojahr, C.; Hosch, R.; Nensa, F.; Pietsch, H.; et al. The Impact of Radiation Dose on CT-Based Body Composition Analysis: A Large-Animal Study. J. Cachexia Sarcopenia Muscle 2025, 16, e13741. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, A.; Pacchiarini, M.C.; Regolisti, G.; Ciuni, A.; Sverzellati, N.; Lesignoli, M.; Picetti, E.; Fiaccadori, E.; Di Mario, F. The impact of muscle mass and myosteatosis on mortality in critically ill patients with Sars-Cov2-related pneumonia. Clin. Nutr. ESPEN 2023, 58, 409–415. [Google Scholar] [CrossRef]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Su, G.L.; Wang, S.C. Skeletal muscle cutoff values for sarcopenia diagnosis using T10 to L5 measurements in a healthy US population. Sci. Rep. 2018, 8, 11369. [Google Scholar] [CrossRef]
- Jennerich, A.L.; Downey, L.; Goss, C.H.; Kapnadak, S.G.; Pryor, J.B.; Ramos, K.J. Computed tomography body composition and clinical outcomes following lung transplantation in cystic fibrosis. BMC Pulm. Med. 2023, 23, 105. [Google Scholar] [CrossRef]
- Delmonico, M.J.; Harris, T.B.; Visser, M.; Park, S.W.; Conroy, M.B.; Velasquez-Mieyer, P.; Boudreau, R.; Manini, T.M.; Nevitt, M.; Newman, A.B.; et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am. J. Clin. Nutr. 2009, 90, 1579–1585. [Google Scholar] [CrossRef]
- Ramirez-Velez, R.; Ezzatvar, Y.; Izquierdo, M.; Garcia-Hermoso, A. Effect of exercise on myosteatosis in adults: A systematic review and meta-analysis. J. Appl. Physiol. (1985) 2021, 130, 245–255. [Google Scholar] [CrossRef]
- Wewege, M.A.; Desai, I.; Honey, C.; Coorie, B.; Jones, M.D.; Clifford, B.K.; Leake, H.B.; Hagstrom, A.D. The Effect of Resistance Training in Healthy Adults on Body Fat Percentage, Fat Mass and Visceral Fat: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 287–300. [Google Scholar] [CrossRef]
- Khodadadi, F.; Bagheri, R.; Negaresh, R.; Moradi, S.; Nordvall, M.; Camera, D.M.; Wong, A.; Suzuki, K. The Effect of High-Intensity Interval Training Type on Body Fat Percentage, Fat and Fat-Free Mass: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Clin. Med. 2023, 12, 2291. [Google Scholar] [CrossRef]
- Scully, K.J.; Jay, L.T.; Freedman, S.; Sawicki, G.S.; Uluer, A.; Finkelstein, J.S.; Putman, M.S. The Relationship between Body Composition, Dietary Intake, Physical Activity, and Pulmonary Status in Adolescents and Adults with Cystic Fibrosis. Nutrients 2022, 14, 310. [Google Scholar] [CrossRef]
- Nicolson, W.B.; Bailey, J.; Alotaibi, N.Z.; Krick, S.; Lowman, J.D. Effects of Exercise on Nutritional Status in People with Cystic Fibrosis: A Systematic Review. Nutrients 2022, 14, 933. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef]
- Szentpetery, S.; Fernandez, G.S.; Schechter, M.S.; Jain, R.; Flume, P.A.; Fink, A.K. Obesity in Cystic fibrosis: Prevalence, trends and associated factors data from the US cystic fibrosis foundation patient registry. J. Cyst. Fibros. 2022, 21, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Caley, L.R.; Jarosz-Griffiths, H.H.; Smith, L.; Gale, L.; Barrett, J.; Kinsey, L.; Davey, V.; Nash, M.; Jones, A.M.; Whitehouse, J.L.; et al. Body mass index and nutritional intake following Elexacaftor/Tezacaftor/Ivacaftor modulator therapy in adults with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Hevilla, F.; Porras, N.; Giron, M.V.; Garcia-Olivares, M.; Padial, M.; Sanchez-Torralvo, F.J.; Olveira, C.; Olveira, G. Impact of Elexacaftor-Tezacaftor-Ivacaftor Therapy on Body Composition, Dietary Intake, Biomarkers, and Quality of Life in People with Cystic Fibrosis: A Prospective Observational Study. Nutrients 2024, 16, 3293. [Google Scholar] [CrossRef]
- Blankenship, S.; Landis, A.R.; Harrison Williams, E.; Peabody Lever, J.E.; Garcia, B.; Solomon, G.; Krick, S. What the future holds: Cystic fibrosis and aging. Front. Med. 2023, 10, 1340388. [Google Scholar] [CrossRef] [PubMed]
- Molwitz, I.; Ozga, A.K.; Gerdes, L.; Ungerer, A.; Kohler, D.; Ristow, I.; Leiderer, M.; Adam, G.; Yamamura, J. Prediction of abdominal CT body composition parameters by thoracic measurements as a new approach to detect sarcopenia in a COVID-19 cohort. Sci. Rep. 2022, 12, 6443. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All (n = 102) | ETI (n = 80) | No-ETI (n = 22) | p-Value ETI vs. No-ETI |
|---|---|---|---|---|
| Age, years | 33.9 ± 11.1 [31.7–36.0] | 33.9 ± 11.2 [31.4–36.4] | 33.6 ± 11.1 [28.7–38.6] | 0.918 |
| Female sex, n (%) | 42 (41) | 37 (46) | 5 (23) | 0.047 |
| Genotype, n (%) | <0.001 | |||
| F508del homozygous | 45 (44) | 45 (56) | 0 | |
| F508del heterozygous | 32 (31) | 32 (40) | 0 | |
| Other | 25 (25) | 3 (4) | 22 (100) | |
| Pancreatic insufficiency, n (%) | 93 (91) | 75 (94) | 18 (82) | 0.035 |
| Cystic fibrosis-related diabetes, n (%) | 21 (21) | 18 (23) | 3 (14) | 0.003 |
| Pseudomonas aeruginosa | 52 (51) | 46 (58) | 6 (27) | 0.016 |
| Body mass index [kg/m2] | 0.433 | |||
| Underweight (<18.5) | 26 (25) | 22 (28) | 4 (18) | |
| Normal/healthy weigt (18.5–24.9) | 61 (60) | 48 (60) | 13 (59) | |
| Overweight/Obese (>25) | 15 (15) | 10 (12) | 5 (23) | |
| ppFEV1 | 0.125 | |||
| Normal (>90) | 6 (6) | 4 (5) | 2 (9) | |
| Mild obstruction (70–90) | 11 (11) | 7 (9) | 4 (18) | |
| Moderate obstruction (40.70) | 38 (37) | 30 (38) | 8 (36) | |
| Severe obstruction (<40) | 47 (46) | 39 (49) | 8 (36) |
| Characteristics | All (n = 102) | ETI Group (n = 80) | No ETI Group (n = 22) | p-Value ETI vs. No ETI |
|---|---|---|---|---|
| Time between T0 and T1 [days] | 1057.8 ± 399.8 [979.3–1136.3] | 1024.3 ± 401.3 [935.0–1113.6] | 1179.7 ± 378.3 [1012.0–1347.5] | 0.107 |
| ppFEV1 | ||||
| T0 | 46.4 ± 21.4 [42.2–50.6] | 44.3 ± 20.1 [39.8–48.8] | 54.0 ± 24.5 [43.1–64.8] | 0.082 |
| T1 | 52.8 ± 22.1 [48.5–57.2] | 52.5 ± 21.0 [47.8–57.2] | 54.1 ± 26.2 [42.5–65.7] | 0.756 |
| Mean difference | 6.4 ± 10 [4.4–8.4] | 8.1 ± 10.0 [5.9–10.4] | 0.2 ± 7.4 [−3.1–3.5] | |
| p-value | <0.001 | <0.001 | 0.909 | |
| FEV1 [L] | ||||
| T0 | 1.8 ± 1.0 [1.6–2.0] | 1.7 ± 1.0 [1.5–1.9] | 2.1 ± 1.0 [1.7–2.6] | 0.108 |
| T1 | 2.0 ± 1.0 [1.8–2.2] | 2.0 ± 1.0 [1.8–2.2] | 2.1 ± 1.1 [1.6–2.6] | 0.789 |
| Mean difference | 0.2 ± 0.4 [0.1–0.3] | 0.3 ± 0.4 [0.2–0.4] | −0.03 ± 0.3 [−0.2–0.1] | |
| p-value | <0.001 | <0.001 | 0.603 | |
| Body weight [kg] | ||||
| T0 | 62.3 ± 13.5 [59.7–64.9] | 61.3 ± 13.0 [58.4–64.2] | 65.9 ± 14.9 [59.3–72.5] | 0.160 |
| T1 | 66.4 ± 14.2 [63.6–69.2] | 66.4 ± 14.0 [63.3–69.6] | 66.3 ± 15.1 [59.7–73.0] | 0.890 |
| Mean difference | 4.1 ± 6.5 [2.8–5.4] | 5.1 ± 6.1 [3.8–6.5] | 0.5 ± 6.7 [−2.5–3.4] | |
| p-value | <0.001 | <0.001 | 0.883 | |
| BMI [kg/m2] | ||||
| T0 | 21.1 ± 3.6 [20.4–21.8] | 20.7 ± 3.2 [20.0–21.4] | 22.6 ± 4.9 [20.4–24.7] | 0.125 |
| T1 | 22.5 ± 3.7 [21.7–23.2] | 22.4 ± 3.3 [21.6–23.1] | 22.9 ± 5.1 [20.6–25.1] | 0.974 |
| Mean difference | 1.4 ± 2.1 [1.0–1.8] | 1.7 ± 1.9 [1.3–2.1] | 0.3 ± 2.4 [0.8–1.3] | |
| p-value | <0.001 | <0.001 | 0.917 |
| Characteristics | All (n = 102) | ETI Group (n = 80) | No ETI Group (n = 22) | p-Value ETI vs. No ETI |
|---|---|---|---|---|
| SMA [cm2] | ||||
| T0 | 90.5 ± 22.6 [86.0–94.9] | 89.2 ± 22.2 [84.2–94.1] | 95.2 ± 23.9 [84.6–105.7] | 0.274 |
| T1 | 95.1 ± 25.1 [90.2–100] | 94.6 ± 24.3 [89.2–100.0] | 96.9 ± 28.4 [84.3–109.5] | 0.705 |
| Mean difference | 4.6 ± 10.5 [2.6–6.7] | 5.4 ± 10.3 [3.1–7.7] | 1.8 ± 11.1 [−3.2–6.7] | |
| p-value | <0.001 | <0.001 | 0.464 | |
| IMAT [cm2] | ||||
| T0 | 7.4 ± 5.5 [6.3–8.5] | 7.0 ± 4.6 [6.0–8.0] | 8.8 ± 7.9 [5.3–12.3] | 0.855 |
| T1 | 11.2 ± 7.1 [9.8–12.6] | 11.3 ± 6.8 [9.7–12.8] | 11.0 ± 8.2 [7.3–14.6] | 0.472 |
| Mean difference | 3.8 ± 4.5 [2.9–4.7] | 4.2 ± 0.9 [3.2–5.3] | 2.1 ± 4.1 [0.3–4.0] | |
| p-value | <0.001 | <0.001 | 0.025 | |
| %IMAT | ||||
| T0 | 7.2 ± 4.1 [6.4–8.0] | 7.1 ± 3.8 [6.2–7.9] | 7.6 ± 5.1 [5.3–9.8] | 0.611 |
| T1 | 10.0 ± 4.4 [9.2–10.9] | 10.3 ± 4.4 [9.3–11.2] | 9.2 ± 4.5 [7.2–11.2] | 0.289 |
| Mean difference | 2.9 ± 2.9 [2.3–3.4] | 3.2 ± 3.0 [2.5–3.9] | 1.7 ± 2.2 [0.7–2.6] | |
| p-value | <0.001 | <0.001 | 0.005 | |
| LAMA [cm2] | ||||
| T0 | 26.5 ± 10.0 [24.5–28.5] | 26.5 ± 9.5 [24.4–28.6] | 26.4 ± 12.0 [21.1–31.7] | 0.961 |
| T1 | 31.1 ± 10.3 [29.0–33.1] | 31.1 ± 9.9 [28.9–33.3] | 30.9 ± 11.7 [25.7–36.1] | 0.921 |
| Mean difference | 4.6 ± 5.9 [3.4–5.7] | 4.6 ± 5.8 [3.3–5.9] | 4.5 ± 6.5 [1.6–7.3] | |
| p-value | <0.001 | <0.001 | 0.004 | |
| %LAMA | ||||
| T0 | 29.0 ± 7.1 [27.6–30.3] | 29.5 ± 6.6 [28.0–31.0] | 27.0 ± 8.6 [23.2–30.9] | 0.150 |
| T1 | 32.4 ± 5.4 [31.4–33.5] | 32.7 ± 5.3 [31.6–33.9] | 31.3 ± 5.5 [28.8–33.7] | 0.258 |
| Mean difference | 3.5 ± 5.5 [2.4–4.5] | 3.2 ± 5.5 [2.0–4.5] | 4.2 ± 5.5 [1.8–6.7] | |
| p-value | <0.001 | <0.001 | 0.002 | |
| IMAT/SMA ratio | ||||
| T0 | 8.0 ± 5.0 [7.0–8.9] | 7.8 ± 4.6 [6.8–8.8] | 8.5 ± 6.2 [5.8–11.3] | 0.977 |
| T1 | 11.4 ± 5.6 [10.3–12.5] | 11.7 ± 5.7 [10.5–13.0] | 10.4 ± 5.6 [7.95–13.0] | 0.289 |
| Mean difference | 3.5 ± 3.7 [2.8–4.2] | 3.9 ± 3.8 [3.1–4.8] | 1.9 ± 2.8 [0.7–3.1] | |
| p-value | <0.001 | <0.001 | 0.007 | |
| skeletal muscle indices (SMI) | ||||
| SMA/height [m2] | ||||
| T0 | 30.6 ± 6.4 [29.3–31.8] | 30.0 ± 5.8 [28.7–31.3] | 32.7 ± 8.1 [29.1–36.3] | 0.080 |
| T1 | 32.1 ± 7.2 [30.7–33.6] | 31.8 ± 6.3 [30.4–33.2] | 33.4 ± 9.9 [29.0–37.8] | 0.368 |
| Mean difference | 1.6 ± 3.4 [0.9–2.2] | 1.8 ± 3.3 [1.1–2.6] | 0.7 ± 3.9 [−1.0–2.4] | |
| p-value | <0.001 | <0.001 | 0.416 | |
| SMA/kg | ||||
| T0 | 1.5 ± 0.2 [1.4–1.5] | 1.5 ± 0.2 [1.4–1.5] | 1.5 ± 0.2 [1.4–1.5] | 0.905 |
| T1 | 1.4 ± 0.2 [1.4–1.5] | 1.4 ± 0.2 [1.4–1.5] | 1.5 ± 0.2 [1.4–1.5] | 0.488 |
| Mean difference | −0.02 ± 0.1 [−0.05–0] | −0.03 ± 0.1 [−0.1–0.01] | 0 ± 0.15 [−0.1–0.1] | |
| p-value | 0.016 | 0.003 | 0.931 | |
| SMA/BMI [kg/m2] | ||||
| T0 | 4.3 ± 0.7 [4.1–4.4] | 4.3 ± 0.7 [4.1–4.4] | 4.2 ± 0.7 [3.9–4.6] | 0.752 |
| T1 | 4.2 ± 0.7 [4.1–4.3] | 4.2 ± 0.7 [4.0–4.4] | 4.2 ± 0.7 [3.9–4.6] | 0.878 |
| Mean difference | −0.1 ± 0.3 [−0.1–0] | −0.1 ± 0.3 [−0.2–0] | 0 ± 0.4 [−0.2–0.2] | |
| p-value | 0.032 | 0.006 | 0.941 |
| Characteristics | Male (n = 43) | Female (n = 37) | p-Value Male vs. Female |
|---|---|---|---|
| Time between start ETI and T1 [days] | 897.4 ± 302.3 [804.4–990.5] | 792.5 ± 318.9 [686.2–898.9] | 0.135 |
| Age [years] | |||
| T0 | 35.8 ± 12.2 [32.1–39.6] | 31.7 ± 9.5 [28.5–34.9] | 0.096 |
| T1 | 38.7 ± 12.2 [35.0–42.5] | 34.4 ± 9.6 [31.2–37.6] | 0.087 |
| Mean difference | 2.9 ± 1.1 [2.6–3.3] | 2.8 ± 1.3 [2.3–3.2] | |
| p-value | <0.001 | <0.001 | |
| ppFEV1 | |||
| T0 | 48.9 ± 22.5 [42.0–55.9] | 38.8 ± 15.3 [33.7–44.0] | 0.056 |
| T1 | 57.0 ± 24.0 [49.6–64.4] | 47.1 ± 15.6 [41.8–52.4] | 0.100 |
| Mean difference | 8.1 ± 10.9 [4.7–11.4] | 8.3 ± 8.8 [5.3–11.2] | |
| p-value | <0.001 | <0.001 | |
| Body weight [kg] | |||
| T0 | 68.1 ± 11.9 [64.4–71.7] | 53.5 ± 9.2 [50.4–56.5] | <0.001 |
| T1 | 73.6 ± 12.9 [69.6–77.5] | 58.2 ± 10.4 [54.7–61.7] | <0.001 |
| Mean difference | 5.5 ± 7.1 [3.3–7.7] | 4.7 ± 4.8 [3.1–6.4] | |
| p-value | <0.001 | <0.001 | |
| BMI [kg/m2] | |||
| T0 | 21.5 ± 3.0 [20.6–22.5] | 19.7 ± 3.1 [18.7–20.7] | 0.009 |
| T1 | 23.2 ± 3.2 [22.2–24.2] | 21.4 ± 3.2 [20.4–22.5] | 0.016 |
| Mean difference | 1.7 ± 2.1 [1.0–2.3] | 1.7 ± 1.7 [1.1–2.3] | |
| p-value | <0.001 | <0.001 | |
| SMA [cm2] | |||
| T0 | 103.6 ± 19.0 [97.7–109.4] | 72.4 ± 11.3 [68.6–76.2] | <0.001 |
| T1 | 109.7 ± 20.9 [103.3–116.1] | 77.1 ± 13.9 [72.4–81.7] | <0.001 |
| Mean difference | 6.1 ± 12.0 [2.5–9.8] | 4.6 ± 8.2 [1.9–7.4] | |
| p-value | 0.002 | 0.002 | |
| IMAT [cm2] | |||
| T0 | 7.2 ± 4.8 [5.8–8.7] | 6.7 ± 4.5 [5.3–8.2] | 0.642 |
| T1 | 11.8 ± 7.1 [9.7–14.0] | 10.6 ± 6.5 [8.4–12.7] | 0.401 |
| Mean difference | 4.6 ± 4.9 [3.1–6.1] | 3.8 ± 4.1 [2.4–5.2] | |
| p-value | <0.001 | <0.001 | |
| %IMAT | |||
| T0 | 6.2 ± 3.4 [5.2–7.3] | 8.1 ± 4.1 [6.7–9.4] | 0.028 |
| T1 | 9.3 ± 4.1 [8.0–10.5] | 11.4 ± 4.4 [10.0–12.9] | 0.025 |
| Mean difference | 3.0 ± 2.9 [2.2–3.9] | 3.4 ± 3.2 [2.3–4.5] | |
| p-value | <0.001 | <0.001 | |
| LAMA [cm2] | |||
| T0 | 30.5 ± 10.0 [27.4–33.6] | 21.9 ± 6.5 [19.7–24.1] | <0.001 |
| T1 | 35.9 ± 9.8 [32.9–38.9] | 25.6 ± 6.7 [23.3–27.8] | <0.001 |
| Mean difference | 5.4 ± 6.1 [3.5–7.3] | 3.7 ± 5.4 [1.9–5.4] | |
| p-value | <0.001 | <0.001 | |
| %LAMA | |||
| T0 | 29.1 ± 6.8 [27.0–31.2] | 30.0 ± 6.4 [27.9–32.1] | 0.542 |
| T1 | 32.5 ± 4.9 [31.0–34.0] | 33.0 ± 5.8 [31.1–35.0] | 0.637 |
| Mean difference | 3.4 ± 4.9 [1.9–4.9] | 3.1 ± 6.2 [1.0–5.1] | |
| p-value | <0.001 | 0.005 | |
| IMAT/SMA ratio | |||
| T0 | 6.8 ± 4.0 [5.5–8.0] | 9.0 ± 5.0 [7.3–10.7] | 0.032 |
| T1 | 10.4 ± 5.2 [8.8–12.0] | 13.2 ± 5.9 [11.2–15.2] | 0.028 |
| Mean difference | 3.7 ± 3.6 [2.6–4.8] | 4.2 ± 4.0 [2.9 ± 5.6] | |
| p-value | <0.001 | <0.001 | |
| skeletal muscle indices (SMI) | |||
| SMA/height [m2] | |||
| T0 | 32.7 ± 5.6 [31.0–34.5] | 26.8 ± 4.1 [25.4–28.1] | <0.001 |
| T1 | 34.7 ± 6.2 [32.8–36.6] | 28.4 ± 4.6 [26.9–29.9] | <0.001 |
| Mean difference | 2.0 ± 3.6 [0.9–3.1] | 1.6 ± 2.9 [0.7–2.6] | |
| p-value | <0.001 | 0.004 | |
| SMA/kg | |||
| T0 | 1.5 ± 0.2 [1.5–1.6] | 1.4 ± 0.1 [1.3–1.4] | <0.001 |
| T1 | 1.5 ± 0.2 [1.4–1.6] | 1.3 ± 0.1 [1.3–1.4] | <0.001 |
| Mean difference | 0 ± 0.1 [−0.1–0] | 0 ± 0.1 [−0.1–0] | |
| p-value | 0.054 | 0.015 | |
| SMA/BMI [kg/m2] | |||
| T0 | 4.8 ± 0.5 [4.7–4.9] | 3.7 ± 0.4 [3.6–3.8] | <0.001 |
| T1 | 4.7 ± 0.5 [4.6–4.9] | 3.6 ± 0.4 [3.5–3.7] | <0.001 |
| Mean difference | −0.1 ± 0.3 [−0.2–0.02] | −0.09 ± 0.2 [−0.2–−0.02] | |
| p-value | 0.096 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welsner, M.; Stehling, F.; Gruber, W.; Westhölter, D.; Sutharsan, S.; Taube, C.; Büscher, E.; Nensa, F.; Zensen, S.; Umutlu, L.; et al. Impact of Elexacaftor–Tezacaftor–Ivacaftor on Muscle Composition in Cystic Fibrosis: An AI-Assisted Chest CT-Based Body Composition Analysis. Med. Sci. 2025, 13, 284. https://doi.org/10.3390/medsci13040284
Welsner M, Stehling F, Gruber W, Westhölter D, Sutharsan S, Taube C, Büscher E, Nensa F, Zensen S, Umutlu L, et al. Impact of Elexacaftor–Tezacaftor–Ivacaftor on Muscle Composition in Cystic Fibrosis: An AI-Assisted Chest CT-Based Body Composition Analysis. Medical Sciences. 2025; 13(4):284. https://doi.org/10.3390/medsci13040284
Chicago/Turabian StyleWelsner, Matthias, Florian Stehling, Wolfgang Gruber, Dirk Westhölter, Sivagurunathan Sutharsan, Christian Taube, Erik Büscher, Felix Nensa, Sebastian Zensen, Lale Umutlu, and et al. 2025. "Impact of Elexacaftor–Tezacaftor–Ivacaftor on Muscle Composition in Cystic Fibrosis: An AI-Assisted Chest CT-Based Body Composition Analysis" Medical Sciences 13, no. 4: 284. https://doi.org/10.3390/medsci13040284
APA StyleWelsner, M., Stehling, F., Gruber, W., Westhölter, D., Sutharsan, S., Taube, C., Büscher, E., Nensa, F., Zensen, S., Umutlu, L., Forsting, M., Haubold, J., Salhöfer, L., Holtkamp, M., Kohnke, J., Hosch, R., & Opitz, M. (2025). Impact of Elexacaftor–Tezacaftor–Ivacaftor on Muscle Composition in Cystic Fibrosis: An AI-Assisted Chest CT-Based Body Composition Analysis. Medical Sciences, 13(4), 284. https://doi.org/10.3390/medsci13040284






