Should We Fear the Frail? A Review on the Impact of Frailty on Liver Surgery
Abstract
1. Introduction
2. Methods
3. How Is Frailty Measured?
4. Prevalence of Frailty in Hepatectomy Patients
5. Postoperative Morbidity, Mortality, and Length of Stay
6. Long-Term Survival Outcomes
7. Independent Predictive Value of Frailty and Clinical Implications
7.1. Frailty in Specific Patient Populations Undergoing Liver Resection
7.2. How Frailty Influences Surgical Decision-Making and Patient Selection in Liver Resection
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACG | Adjusted Clinical Groups |
| ALBI | Albumin–Bilirubin |
| BMI | Body Mass Index |
| CFS | Clinical Frailty Scale |
| CHF | Congestive Heart Failure |
| CI | Confidence Interval |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRLM | Colorectal Liver Metastases |
| CSS | Cancer-Specific Survival |
| DFS | Disease-Free Survival |
| DVT | Deep Vein Thrombosis |
| FFI | Fried Frailty Index (Fried Frailty Scale) |
| G8 | Geriatric-8 (oncogeriatric screening tool) |
| HCC | Hepatocellular Carcinoma |
| HR | Hazard Ratio |
| ICC | Intrahepatic Cholangiocarcinoma |
| ICU | Intensive Care Unit |
| JHACG | Johns Hopkins Adjusted Clinical Groups |
| KCL | Kihon Checklist |
| LOS | Length of Stay |
| mFI | Modified Frailty Index |
| NRD | National Readmissions Database |
| NSQIP | National Surgical Quality Improvement Program |
| OR | Odds Ratio |
| OS | Overall Survival |
| PHLF | Post-Hepatectomy Liver Failure |
| PVD | Peripheral Vascular Disease |
| RFS | Recurrence-Free Survival |
| RR | Relative Risk |
| TIA | Transient Ischemic Attack |
References
- Ofori-Asenso, R.; Chin, K.L.; Mazidi, M.; Zomer, E.; Ilomaki, J.; Zullo, A.R.; Gasevic, D.; Ademi, Z.; Korhonen, M.J.; LoGiudice, D.; et al. Global Incidence of Frailty and Prefrailty Among Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e198398. [Google Scholar] [CrossRef]
- Kim, D.H.; Rockwood, K. Frailty in Older Adults. N. Engl. J. Med. 2024, 391, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Gill, T.M.; Wyk, B.V.; Leo-Summers, L.; Murphy, T.E.; Becher, R.D. Population-Based Estimates of 1-Year Mortality After Major Surgery Among Community-Living Older US Adults. JAMA Surg. 2022, 157, e225155. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.F.; Budiansky, D.; Sharif, F.; McIsaac, D.I. The Association of Frailty with Outcomes After Cancer Surgery: A Systematic Review and Metaanalysis. Ann. Surg. Oncol. 2022, 29, 4690–4704. [Google Scholar] [CrossRef] [PubMed]
- Shinall, M.C., Jr.; Arya, S.; Youk, A.; Varley, P.; Shah, R.; Massarweh, N.N.; Shireman, P.K.; Johanning, J.M.; Brown, A.J.; Christie, N.A.; et al. Association of Preoperative Patient Frailty and Operative Stress with Postoperative Mortality. JAMA Surg. 2020, 155, e194620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kotsifa, E.; Vergadis, C.; Vailas, M.; Machairas, N.; Kykalos, S.; Damaskos, C.; Garmpis, N.; Lianos, G.D.; Schizas, D. Transarterial Chemoembolization for Hepatocellular Carcinoma: Why, When, How? J. Pers. Med. 2022, 12, 436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shah, D.R.; Green, S.; Elliot, A.; McGahan, J.P.; Khatri, V.P. Current oncologic applications of radiofrequency ablation therapies. World J. Gastrointest. Oncol. 2013, 5, 71–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDermott, S.; Gervais, D.A. Radiofrequency ablation of liver tumors. Semin. Interv. Radiol. 2013, 30, 49–55. [Google Scholar] [CrossRef]
- Hu, L.; Lin, J.; Wang, A.; Shi, X.; Qiao, Y. Comparison of liver resection and radiofrequency ablation in long-term survival among patients with early-stage hepatocellular carcinoma: A meta-analysis of randomized trials and high-quality propensity score-matched studies. World J. Surg. Oncol. 2024, 22, 56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Howell, S.J.; Nair, S. Measuring frailty in the older surgical patient: The case for evidence synthesis. Br. J. Anaesth. 2021, 126, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Theou, O.; Walston, J.; Rockwood, K. Operationalizing Frailty Using the Frailty Phenotype and Deficit Accumulation Approaches. In Interdisciplinary Topics in Gerontology and Geriatrics; Karger International: Basel, Switzerland, 2015; Volume 41, pp. 66–73. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bieniek, J.; Wilczyński, K.; Szewieczek, J. Fried frailty phenotype assessment components as applied to geriatric inpatients. Clin. Interv. Aging 2016, 11, 453–459. [Google Scholar] [CrossRef]
- Op het Veld, L.P.; van Rossum, E.; Kempen, G.I.; de Vet, H.C.; Hajema, K.; Beurskens, A.J. Fried phenotype of frailty: Cross-sectional comparison of three frailty stages on various health domains. BMC Geriatr. 2015, 15, 77. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Di Lena, L.; D’urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; et al. Different Cognitive Frailty Models and Health- and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J. Alzheimers Dis. 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Velanovich, V.; Antoine, H.; Swartz, A.; Peters, D.; Rubinfeld, I. Accumulating deficits model of frailty and postoperative mortality and morbidity: Its application to a national database. J. Surg. Res. 2013, 183, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-Factor Modified Frailty Index Using American College of Surgeons NSQIP Data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Okabe, H.; Hayashi, H.; Higashi, T.; Nitta, H.; Ikuta, Y.; Yusa, T.; Takeyama, H.; Ogawa, K.; Ozaki, N.; Akahoshi, S.; et al. Frailty Predicts Severe Postoperative Complication after Elective Hepatic Resection. Gastrointest. Tumors 2019, 6, 28–35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tokuda, K.; Morine, Y.; Miyazaki, K.; Yamada, S.; Saito, Y.; Nishi, M.; Ikemoto, T.; Shimada, M. Frailty Can Predict Prognosis After Hepatectomy in Patients with Colorectal Liver Metastasis. Anticancer. Res. 2021, 41, 4637–4644. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ueno, M.; Iida, H.; Kaibori, M.; Nomi, T.; Hirokawa, F.; Ikoma, H.; Nakai, T.; Eguchi, H.; Kubo, S. Preoperative assessment of frailty predicts age-related events after hepatic resection: A prospective multicenter study. J. Hepatobiliary Pancreat. Sci. 2018, 25, 377–387. [Google Scholar] [CrossRef]
- Okada, T.; Tanaka, S.; Shinkawa, H.; Ohira, G.; Kinoshita, M.; Amano, R.; Kimura, K.; Nishio, K.; Tauchi, J.; Uchida-Kobayashi, S.; et al. Impact of frailty on long-term outcomes after liver resection for hepatocellular carcinoma in elderly patients: A prospective study. Asian J. Surg. 2024, 47, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Shahrestani, S.; Silverstein, M.; Nasrollahi, T.; Nasrollahi, T.; Maas, M.; Ugarte, C.; Kulkarni, S.; Lenz, H.J.; Genyk, Y. The influence of frailty on perioperative outcomes in patients undergoing surgical resection of liver metastases: A nationwide readmissions database study. Ann. Gastroenterol. 2023, 36, 333–339. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.; Perera, S.; Patel, K.; Rosano, C.; Faulkner, K.; Inzitari, M.; Brach, J.; Chandler, J.; Cawthon, P.; Connor, E.B.; et al. Gait speed and survival in older adults. JAMA 2011, 305, 50–58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Komici, K.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Carpini, G.D.; Picerno, V.; Avella, P.; Brunese, M.C.; Rengo, G.; Guerra, G.; et al. The Prevalence and the Impact of Frailty in Hepato-Biliary Pancreatic Cancers: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Yan, Y.; Li, B.; Ge, C. Frailty as a predictor of adverse outcomes in patients with hepatectomy—The importance of design studies to improve frailty: A systematic review and meta-analysis of 128868 patients. J. Hepatobiliary Pancreat. Sci. 2024, 31, 851–862. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.J.; Xu, G.X.; Lan, J.R. Impact of frailty on postoperative outcomes after hepatectomy: A systematic review and meta-analysis. World J. Gastrointest. Surg. 2024, 16, 2319–2328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lunca, S.; Morarasu, S.; Rouet, K.; Ivanov, A.A.; Morarasu, B.C.; Roata, C.E.; Clancy, C.; Dimofte, G.M. Frailty Increases Morbidity and Mortality in Patients Undergoing Oncological Liver Resections: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2024, 31, 6514–6525. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, F.; Yan, Y.; Li, B.; Ge, C. Significance of frailty in mortality and complication after hepatectomy for patients with liver cancer: A systematic review and meta-analysis. HPB 2025, 27, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Louwers, L.; Schnickel, G.; Rubinfeld, I. Use of a simplified frailty index to predict Clavien 4 complications and mortality after hepatectomy: Analysis of the National Surgical Quality Improvement Project database. Am. J. Surg. 2016, 211, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Stem, M.; Cerullo, M.; Gearhart, S.L.; Safar, B.; Fang, S.H.; Weiss, M.J.; He, J.; Efron, J.E. The Effect of Frailty Index on Early Outcomes after Combined Colorectal and Liver Resections. J. Gastrointest. Surg. 2018, 22, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Gani, F.; Cerullo, M.; Amini, N.; Buettner, S.; Margonis, G.A.; Sasaki, K.; Kim, Y.; Pawlik, T.M. Frailty as a Risk Predictor of Morbidity and Mortality Following Liver Surgery. J. Gastrointest. Surg. 2017, 21, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, F.B.; Ahmad, M.; Aguirre, K.; Elhanafi, S.; Chiba, S.; Philipovskiy, A.; Tyroch, A.H.; Konstantinidis, I.T. The impact of minimally invasive surgery and frailty on post-hepatectomy outcomes. HPB 2022, 24, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Lunca, S.; Morarasu, S.; Ivanov, A.A.; Clancy, C.; O’Brien, L.; Zaharia, R.; Musina, A.M.; Roata, C.E.; Dimofte, G.M. Is Frailty Associated with Worse Outcomes After Major Liver Surgery? An Observational Case-Control Study. Diagnostics 2025, 15, 512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamada, S.; Shimada, M.; Morine, Y.; Imura, S.; Ikemoto, T.; Arakawa, Y.; Saito, Y.; Yoshikawa, M.; Miyazaki, K. Significance of Frailty in Prognosis After Hepatectomy for Elderly Patients with Hepatocellular Carcinoma. Ann. Surg. Oncol. 2021, 28, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Osei-Bordom, D.; Hall, L.; Hodson, J.; Joshi, K.; Austen, L.; Bartlett, D.; Isaac, J.; Mirza, D.F.; Marudanayagam, R.; Roberts, K.; et al. Impact of Frailty on Short-Term Outcomes After Laparoscopic and Open Hepatectomy. World J. Surg. 2022, 46, 2444–2453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McKechnie, T.; Bao, T.; Fabbro, M.; Ruo, L.; Serrano, P.E. Frailty as a Predictor of Postoperative Morbidity and Mortality Following Liver Resection. Am. Surg. 2021, 87, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Zhang, P.; Wu, B.; Wang, S.-Y.; Guo, H.-W.; Zheng, Q.-X.; Chen, T.-H.; Li, J.; Wang, X.-M.; Liang, Y.-J.; et al. Preoperative frailty as a key predictor of short- and long-term outcomes among octogenarians undergoing hepatectomy for hepatocellular carcinoma: A multicenter comprehensive analysis. HPB 2024, 26, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- HoHosoda, K.; Shimizu, A.; Kubota, K.; Notake, T.; Masuo, H.; Yoshizawa, T.; Sakai, H.; Hayashi, H.; Yasukawa, K.; Soejima, Y. Usefulness of frailty to predict short- and long-term outcomes in patients who have undergone major hepatectomy for perihilar cholangiocarcinoma. Ann. Gastroenterol. Surg. 2022, 6, 833–841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakajima, H.; Yokoyama, Y.; Inoue, T.; Nagaya, M.; Mizuno, Y.; Kadono, I.; Nishiwaki, K.; Nishida, Y.; Nagino, M. Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann. Surg. Oncol. 2019, 26, 264–272. [Google Scholar] [CrossRef]
- Yamada, M.; Arai, H. Long-Term Care System in Japan. Ann. Geriatr. Med. Res. 2020, 24, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Schmelzle, M.; Krenzien, F.; Dahlke, P.; Krombholz, A.; Nevermann, N.; Feldbrügge, L.; Winter, A.; Schöning, W.; Benzing, C.; Pratschke, J.; et al. Validation of the Enhanced Recovery after Surgery (ERAS) society recommendations for liver surgery: A prospective, observational study. Hepatobiliary Surg. Nutr. 2023, 12, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Skořepa, P.; Ford, K.L.; Alsuwaylihi, A.; O’Connor, D.; Prado, C.M.; Gomez, D.; Lobo, D.N. The impact of prehabilitation on outcomes in frail and high-risk patients undergoing major abdominal surgery: A systematic review and meta-analysis. Clin. Nutr. 2024, 43, 629–648. [Google Scholar] [CrossRef]
- Paredes, A.Z.; Hyer, J.M.; Tsilimigras, D.I.; Merath, K.; Mehta, R.; Sahara, K.; Farooq, S.A.; Wu, L.; White, S.; Pawlik, T.M. Skilled nursing facility (SNF) utilization and impact of SNF star-quality ratings on outcomes following hepatectomy among Medicare beneficiaries. HPB 2020, 22, 109–115. [Google Scholar] [CrossRef]
- Dalmacy, D.M.; Hyer, J.M.; Diaz, A.; Paro, A.; Tsilimigras, D.I.; Pawlik, T.M. Trends in Discharge Disposition Following Hepatectomy for Hepatocellular Carcinoma Among Medicare Beneficiaries. J. Gastrointest. Surg. 2021, 25, 2842–2850. [Google Scholar] [CrossRef] [PubMed]
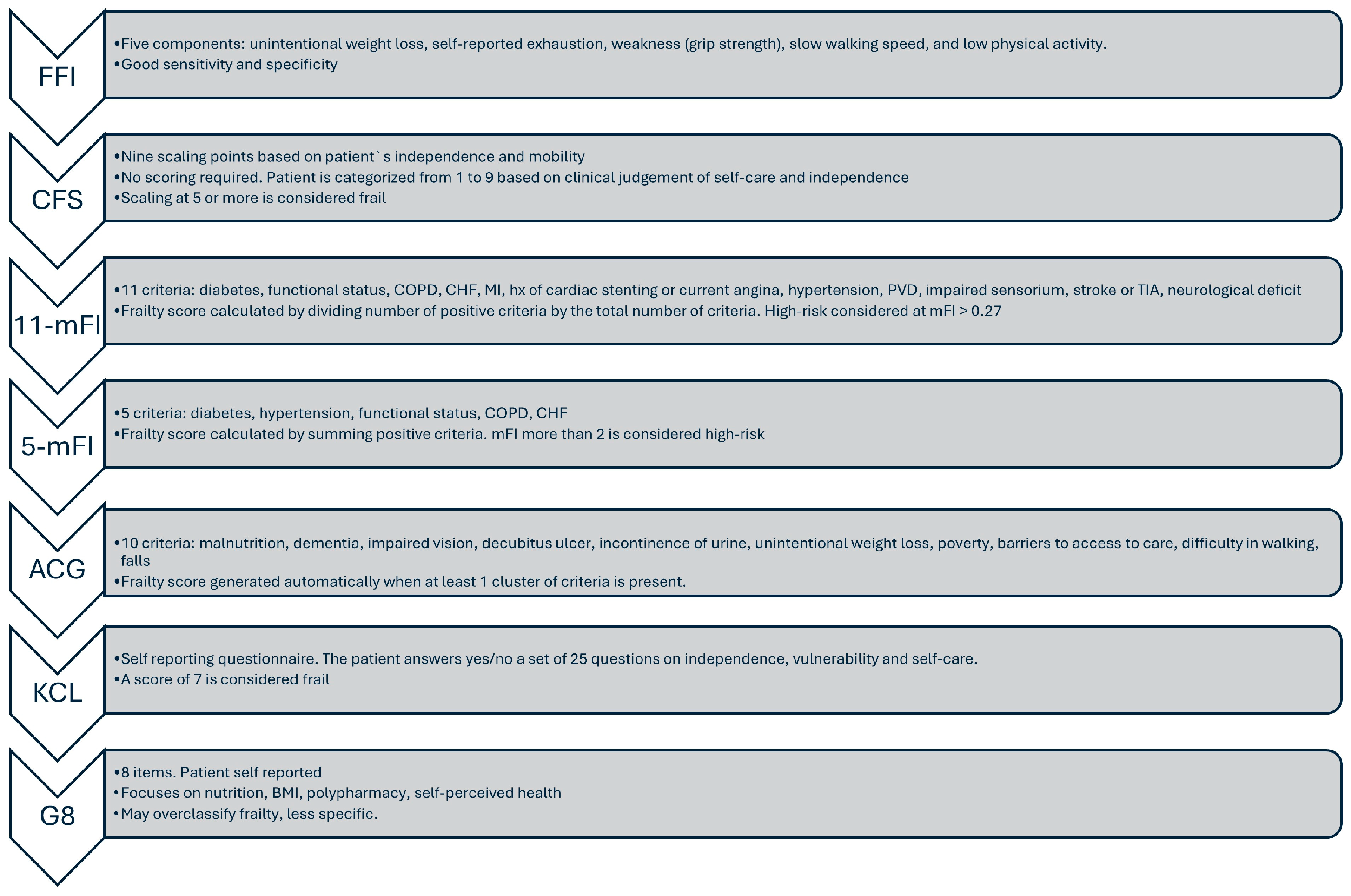
| Tool | Type | Assessment Method | Components | Strengths | Limitations |
|---|---|---|---|---|---|
| Fried frailty scale (FFI) | Phenotypic model of frailty/Focuses primarily on physical frailty | Clinician-administered or research-based tool | Five observable criteria | Well-validated and widely used; Simple and objective; Physical focus | Only assesses physical aspects; self-reported components; less suitable for very ill, cognitively impaired |
| Modified Frailty Index (mFI) | Claims-based/Clinical | Derived from NSQIP variables | 5- or 11-item version based on comorbidities (e.g., diabetes, CHF, COPD) | Easy to calculate from registry data; widely validated | Comorbidity-heavy; limited functional or cognitive assessment |
| Clinical Frailty Scale (CFS) | Clinical judgment | 1–9 scale based on clinical impression | Assesses physical fitness, function, and independence | Quick and intuitive; validated in elderly populations | Subjective; inter-observer variability |
| Kihon Checklist (KCL) | Patient questionnaire | 25 yes/no items | Covers nutrition, social, cognitive, and physical domains | Comprehensive multidimensional assessment | Lengthy; requires patient cooperation |
| Adjusted Clinical Groups (ACG) Frailty Indicator | Claims-based | Based on ICD-10 diagnostic clusters | Flags frailty from diagnoses like malnutrition, falls, dementia, etc. | Works in administrative datasets; scalable for large cohorts | Binary; lacks granularity and functional assessment |
| Geriatric-8 (G8) | Screening tool | 8 items; mostly patient-reported | Focuses on nutrition, BMI, polypharmacy, self-perceived health | Good sensitivity for frailty screening in cancer patients | May overclassify frailty; less specific |
| Study (Year) | Frailty Measure | Patient Cohort | Key Short-Term Outcomes (Complications, Mortality, LOS) |
|---|---|---|---|
| Lv et al., 2024 [28] | Meta-analysis (13 studies) | n = 84,096 (23,964 frail) | Frail patients had significantly increased risk of overall (RR~1.7) and major complications (RR~2.7). Mortality was substantially higher: 30-day mortality was 4.6× higher (RR 4.60), and 90-day mortality 2.5× higher (RR 2.52) in frail patients. LOS was prolonged by an average of 3.7 days. |
| Lunca et al., 2024 [29] | Meta-analysis (10 studies) | n = 71,102 (17,167 frail) | Frailty was linked to significantly higher morbidity, increased rate of major complications, and higher incidence of PHLF (all p < 0.001). Perioperative mortality and readmission rates were also significantly higher among frail patients. |
| Zhang et al., 2025 [30] | Not specified | n = 38,157 (35% frail) | Frail patients had increased odds of major complications (OR 4.01), and higher 30- and 90-day mortality risk (HRs 7.03 and 4.59, respectively), although these findings were not statistically significant. |
| Louwers et al., 2016 [31] | 11-item mFI (≥1 = frail) | n = 10,300 (NSQIP) | Higher frailty scores were associated with increased rates of Clavien IV complications, 30-day mortality, and prolonged LOS. The relationship remained consistent across various types of hepatectomies. |
| Chen et al., 2018 [32] | 5-item mFI (≥2 = frail) | n = 1928 (liver + colorectal resections) | Frail patients experienced significantly more overall and severe complications, longer LOS, and higher 30-day mortality (5.3% vs. 1.2%, p < 0.01). On multivariate analysis, frailty was an independent predictor of morbidity, whereas age was not. |
| Shahrestani et al., 2023 [23] | JHACG Frailty Indicator | n = 1515 (NRD) | Frailty was associated with increased inpatient complications (e.g., infections, DVT, UTI), higher in-hospital mortality, and prolonged LOS. Frail patients were more frequently discharged to nursing/rehabilitation facilities. Including frailty in prediction models improved outcome prediction over age alone. |
| Maegawa et al., 2022 [34] | 5-item mFI (≥1 = frail) | n = 24,150 [NSQIP 2014–19] | Frailty was linked to higher rates of major complications, 30-day mortality, and PHLF. The mFI improved predictive accuracy when added to the ALBI score. Laparoscopic surgery was associated with better outcomes than open surgery in frail patients. |
| Osei-Bordom et al., 2022 [37] | mFI | n = 1826 (34.7% frail) | Frail patients had significantly higher 90-day mortality (6.6% vs. 2.9%) and postoperative complication rates (36.3% vs. 26.1%). LOS was longer for frail patients undergoing open surgery compared to laparoscopic, with similar trends observed in non-frail patients. |
| Tanaka et al., 2018 [21] | KCL (≥8 = frail) | n = 217 (≥70 years, multicenter) | Although overall complication rates were comparable between groups, frail patients had higher 90-day mortality (4.8% vs. 0%). Frailty independently predicted age-related adverse outcomes such as cardiopulmonary complications and functional decline. |
| McKechnie et al., 2021 [38] | mFI (≥0.27 = frail) | n = 409 (Canada, mixed tumors) | Frail patients had significantly more postoperative complications (79% vs. 46%) including major (50% vs. 13%) and minor (69% vs. 42%) events, longer median LOS (9.5 vs. 5 days), and higher 90-day mortality (12% vs. 3.4%). Frailty independently predicted major complications. |
| Study (Year) | Frailty Measure | Patient Cohort & Follow-Up | Long-Term Survival Findings |
|---|---|---|---|
| Lv et al., 2024 [28] | Meta-analysis | Various (meta-analysis of 13 studies), ~5-year outcomes (pooled) | Frail patients demonstrated significantly poorer long-term survival across pooled studies, with a nearly threefold increased hazard of death (HR 2.89, 95% CI: 1.84–4.53). |
| Okada et al., 2024 [22] | KCL (frail = KCL ≥ 8) | n = 81, ≥65 y with HCC (prospective; median 36 mo follow-up) | Five-year overall survival was markedly lower in frail patients (42.7%) compared to non-frail (77.2%) (p = 0.005). Frailty independently predicted worse OS on multivariate analysis. While DFS did not differ significantly, frail patients experienced more extrahepatic recurrences and underwent fewer salvage treatments. |
| Yamada et al., 2021 [36] | CFS (frail = CFS ≥ 4) | n = 92, >75 y with HCC (mean follow-up 2.6 years) | Frail patients had significantly reduced cancer-specific 3-year survival (72.0% vs. 94.3%, p < 0.01) and OS (p < 0.01). DFS was also significantly worse in the frailty group (p = 0.01). Multivariate analysis identified frailty as the only independent prognostic factor. Frail patients had a higher rate of extrahepatic recurrence (50% vs. 4.8%) and were significantly less likely to receive treatment for recurrence (50% vs. 95.2%). |
| Tokuda et al., 2021 [20] | CFS (frail = CFS ≥ 4) | n = 87, median age 78 (CRLM; mean follow-up 46.2 months) | Three-year OS and CSS were significantly lower in frail patients (OS: 63.9% vs. 89.1%; CSS: 69.3% vs. 91.0%; both p < 0.01). Frailty was the only independent predictor of worse survival (p = 0.0477). No significant difference in recurrence rates |
| Hosoda et al., 2022 [40] | CFS (score 1–2 = non-frail, 3–9 = frail) | n = 87, mean age 71 (perihilar cholangiocarcinoma) | Five-year OS was significantly lower in frail patients (10.2%) compared to non-frail (41.8%) (p = 0.01), with a similar trend for disease-specific survival. This survival gap was more pronounced in early-stage disease (stage 0–II: 44.5% vs. 13.0%; p = 0.02), whereas outcomes in advanced-stage patients (stage III/IV) showed no significant difference (OS: 40.0% vs. 0%; p = 0.46). On multivariate analysis, a CFS score of 3–9 remained an independent predictor of OS (HR 2.31, 95% CI: 1.14–4.87; p = 0.02). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunca, S.; Morarasu, S.; Zaharia, R.; Musina, A.M.; Ong, W.L.; Dimofte, G.M.; Roata, C.E. Should We Fear the Frail? A Review on the Impact of Frailty on Liver Surgery. Med. Sci. 2025, 13, 253. https://doi.org/10.3390/medsci13040253
Lunca S, Morarasu S, Zaharia R, Musina AM, Ong WL, Dimofte GM, Roata CE. Should We Fear the Frail? A Review on the Impact of Frailty on Liver Surgery. Medical Sciences. 2025; 13(4):253. https://doi.org/10.3390/medsci13040253
Chicago/Turabian StyleLunca, Sorinel, Stefan Morarasu, Raluca Zaharia, Ana Maria Musina, Wee Liam Ong, Gabriel Mihail Dimofte, and Cristian Ene Roata. 2025. "Should We Fear the Frail? A Review on the Impact of Frailty on Liver Surgery" Medical Sciences 13, no. 4: 253. https://doi.org/10.3390/medsci13040253
APA StyleLunca, S., Morarasu, S., Zaharia, R., Musina, A. M., Ong, W. L., Dimofte, G. M., & Roata, C. E. (2025). Should We Fear the Frail? A Review on the Impact of Frailty on Liver Surgery. Medical Sciences, 13(4), 253. https://doi.org/10.3390/medsci13040253






