Multimodal Imaging of Ductal Carcinoma In Situ: A Single-Center Study of 75 Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patitent Cohort
2.2. Imaging Acquisition and Analysis
2.3. Pathological Correlation
2.4. Statystical Analysis
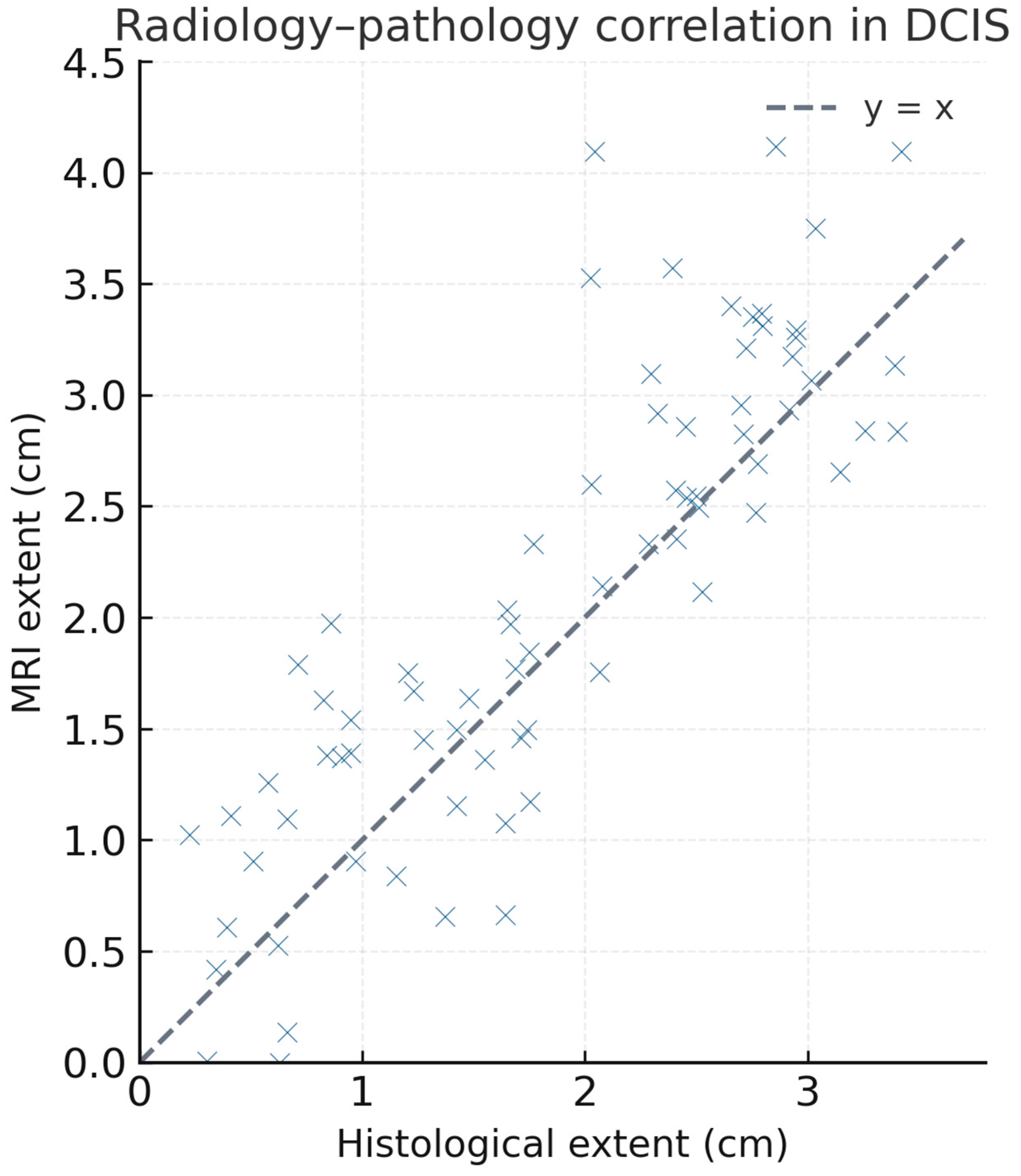
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, G.M.; Dinh, P.; Pathmanathan, N.; Graham, J.D. Ductal Carcinoma In Situ: Molecular Changes Accompanying Disease Progression. J. Mammary Gland Biol. Neoplasia 2022, 27, 101–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Luo, M.; Huang, J.; Zhang, K.; Zheng, S.; Zhang, S.; Zhou, J. Progression from ductal carcinoma in situ to invasive breast cancer: Molecular features and clinical significance. Signal Transduct. Target. Ther. 2024, 9, 83. [Google Scholar] [CrossRef]
- Brown, J.P.; Pinder, S.E. Ductal carcinoma in situ: Current morphological and molecular subtypes. Diagn. Histopathol. 2012, 18, 112–118. [Google Scholar] [CrossRef]
- Bane, A. Ductal carcinoma in situ: What the pathologist needs to know and why. Int. J. Breast Cancer 2013, 2013, 914053. [Google Scholar] [CrossRef]
- Risom, T.; Glass, D.R.; Averbukh, I.; Liu, C.C.; Baranski, A.; Kagel, A.; McCaffrey, E.F.; Greenwald, N.F.; Rivero-Gutiérrez, B.; Strand, S.H.; et al. Transition to invasive breast cancer is associated with progressive changes in the structure and composition of tumor stroma. Cell 2022, 185, 299–310.e218. [Google Scholar] [CrossRef] [PubMed]
- Gibson, S.V.; Roozitalab, R.M.; Allen, M.D.; Jones, J.L.; Carter, E.P.; Grose, R.P. Everybody needs good neighbours: The progressive DCIS microenvironment. Trends Cancer 2023, 9, 326–338. [Google Scholar] [CrossRef]
- Prajzendanc, K. DCIS Progression and the Tumor Microenvironment: Molecular Insights and Prognostic Challenges. Cancers 2025, 17, 1925. [Google Scholar] [CrossRef]
- Neal, C.H.; Joe, A.I.; Patterson, S.K.; Pujara, A.C.; Helvie, M.A. Digital Mammography Has Persistently Increased High-Grade and Overall DCIS Detection Without Altering Upgrade Rate. Am. J. Roentgenol. 2021, 216, 912–918. [Google Scholar] [CrossRef]
- Ernster, V.L.; Barclay, J. Increases in Ductal Carcinoma In Situ (DCIS) of the Breast in Relation to Mammography: A Dilemma. JNCI Monogr. 1997, 1997, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Badruddoja, M. Ductal carcinoma in situ of the breast: A surgical perspective. Int. J. Surg. Oncol. 2012, 2012, 761364. [Google Scholar] [CrossRef]
- Campa, D.; Gentiluomo, M.; Stein, A.; Aoki, M.N.; Oliverius, M.; Vodičková, L.; Jamroziak, K.; Theodoropoulos, G.; Pasquali, C.; Greenhalf, W.; et al. The PANcreatic Disease ReseArch (PANDoRA) consortium: Ten years’ experience of association studies to understand the genetic architecture of pancreatic cancer. Crit. Rev. Oncol./Hematol. 2023, 186, 104020. [Google Scholar] [CrossRef] [PubMed]
- Oyucu Orhan, S.; Orhan, B.; Esenbuğa, Ş.; Sali, S.; Caner, B.; Ocak, B.; Şahin, A.B.; Deligönül, A.; Evrensel, T.; Çubukçu, E. Managing Bone Metastases with Denosumab: Real-World Data and Critical Monitoring Points in Breast, Lung, and Prostate Cancers. Medicina 2025, 61, 1637. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.; Craighead, F.; MacKenzie, J.D.; Aujayeb, A. Day Case Local Anaesthetic Thoracoscopy: Experience from 2 District General Hospitals in the United Kingdom. Med. Sci. 2023, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Liang, H.-L.; Chou, C.-P.; Huang, J.-S.; Yang, T.-L.; Chou, Y.-H.; Pan, H.-B. Easily recognizable sonographic patterns of ductal carcinoma in situ of the breast. J. Chin. Med. Assoc. 2016, 79, 493–499. [Google Scholar] [CrossRef][Green Version]
- Raza, S.; Vallejo, M.; Chikarmane, S.A.; Birdwell, R.L. Pure Ductal Carcinoma in Situ: A Range of MRI Features. Am. J. Roentgenol. 2008, 191, 689–699. [Google Scholar] [CrossRef]
- Evans, A. The diagnosis and management of pre-invasive breast disease: Radiological diagnosis. Breast Cancer Res. 2003, 5, 250–253. [Google Scholar] [CrossRef]
- Liu, X.; Bao, Y.; Sui, L.; Cao, J.; Wang, Y.; Yu, C.; Qiao, G.; Cong, Y. Mammographically detected breast clustered microcalcifications localized by chest thin-section computed tomography. World J. Surg. Oncol. 2024, 22, 72. [Google Scholar] [CrossRef]
- ElSayad, M.M.T.M.; Helal, M.H.M.; Omar, O.H.; Othman, A.G.E.; Ibrahim, M.E.A. Comparative analysis of digital mammography and contrast-enhanced mammography in diagnosing suspicious breast calcifications: Implications for surgical decision-making. Egypt. J. Radiol. Nucl. Med. 2025, 56, 126. [Google Scholar] [CrossRef]
- Zubair, M.; Hussain, M.; Albashrawi, M.A.; Bendechache, M.; Owais, M. A comprehensive review of techniques, algorithms, advancements, challenges, and clinical applications of multi-modal medical image fusion for improved diagnosis. Comput. Methods Programs Biomed. 2025, 272, 109014. [Google Scholar] [CrossRef]
- Hou, R.; Peng, Y.; Grimm, L.J.; Ren, Y.; Mazurowski, M.A.; Marks, J.R.; King, L.M.; Maley, C.C.; Hwang, E.S.; Lo, J.Y. Anomaly Detection of Calcifications in Mammography Based on 11,000 Negative Cases. IEEE Trans. Biomed. Eng. 2022, 69, 1639–1650. [Google Scholar] [CrossRef]
- Guo, W.; Wang, T.; Li, F.; Jia, C.; Zheng, S.; Zhang, X.; Bai, M. Non-mass Breast Lesions: Could Multimodal Ultrasound Imaging Be Helpful for Their Diagnosis? Diagnostics 2022, 12, 2923. [Google Scholar] [CrossRef]
- Uematsu, T. Non-mass lesions on breast ultrasound: Why does not the ACR BI-RADS breast ultrasound lexicon add the terminology? J. Med. Ultrason. (2001) 2023, 50, 341–346. [Google Scholar] [CrossRef]
- Korpraphong, P.; Tritanon, O.; Tangcharoensathien, W.; Angsusinha, T.; Chuthapisith, S. Ultrasonographic characteristics of mammographically occult small breast cancer. J. Breast Cancer 2012, 15, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Jurj, E.-D.; Colibășanu, D.; Vasii, S.-O.; Suciu, L.; Dehelean, C.A.; Udrescu, L. Redefining Breast Cancer Care by Harnessing Computational Drug Repositioning. Medicina 2025, 61, 1640. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Giacobbe, G.; Del Canto, M.T.; Alessandrella, M.; Balestrucci, G.; Urraro, F.; Russo, G.M.; Gallo, L.; Danti, G.; Frittoli, B.; et al. Peritoneal Carcinosis: What the Radiologist Needs to Know. Diagnostics 2023, 13, 1974. [Google Scholar] [CrossRef]
- Tucunduva, T.C.d.M.; Zanetta, V.C.; Chala, L.F.; Torres, U.S.; Viana, M.P.; Lee, M.V.; Silva, M.M.; Shimizu, C.; Aguillar, V.L.N.; de Mello, G.G.N. Advancements in Detection and Management of Ductal Carcinoma in Situ. RadioGraphics 2025, 45, e240174. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, A.P.; Ortiz-Perez, T.; Ebuoma, L.O.; Sepulveda, K.A.; Severs, F.J.; Roark, A.; Wang, T.; Sedgwick, E.L. Is breast magnetic resonance imaging (MRI) useful for diagnosis of additional sites of disease in patients recently diagnosed with pure ductal carcinoma in situ (DCIS)? Eur. J. Radiol. 2017, 96, 74–79. [Google Scholar] [CrossRef]
- Esserman, L.J.; Kumar, A.S.; Herrera, A.F.; Leung, J.; Au, A.; Chen, Y.Y.; Moore, D.H.; Chen, D.F.; Hellawell, J.; Wolverton, D.; et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J. Clin. Oncol. 2006, 24, 4603–4610. [Google Scholar] [CrossRef]
- Viehweg, P.; Lampe, D.; Buchmann, J.; Heywang-Köbrunner, S.H. In situ and minimally invasive breast cancer: Morphologic and kinetic features on contrast-enhanced MR imaging. Magn. Reson. Mater. Phys. Biol. Med. 2000, 11, 129–137. [Google Scholar] [CrossRef]
- Jansen, S.A.; Shimauchi, A.; Zak, L.; Fan, X.; Wood, A.M.; Karczmar, G.S.; Newstead, G.M. Kinetic Curves of Malignant Lesions Are Not Consistent Across MRI Systems: Need for Improved Standardization of Breast Dynamic Contrast-Enhanced MRI Acquisition. Am. J. Roentgenol. 2009, 193, 832–839. [Google Scholar] [CrossRef][Green Version]
- Nadrljanski, M.M.; Marković, B.B.; Milošević, Z. Breast ductal carcinoma in situ: Morphologic and kinetic MRI findings. Iran. J. Radiol. 2013, 10, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Chen, J.H.; Agrawal, G.; Lin, M.; Mehta, R.S.; Carpenter, P.M.; Nalcioglu, O.; Su, M.Y. Characterization of Pure Ductal Carcinoma In Situ on Dynamic Contrast-Enhanced MR Imaging: Do Nonhigh Grade and High Grade Show Different Imaging Features? J. Oncol. 2010, 2010, 431341. [Google Scholar] [CrossRef]
- Agrawal, G.; Su, M.Y.; Nalcioglu, O.; Feig, S.A.; Chen, J.H. Significance of breast lesion descriptors in the ACR BI-RADS MRI lexicon. Cancer 2009, 115, 1363–1380. [Google Scholar] [CrossRef]
- Nardone, V.; Ruggiero, D.; Chini, M.G.; Bruno, I.; Lauro, G.; Terracciano, S.; Nebbioso, A.; Bifulco, G.; Cappabianca, S.; Reginelli, A. From Bench to Bedside: Translational Approaches to Cardiotoxicity in Breast Cancer, Lung Cancer, and Lymphoma Therapies. Cancers 2025, 17, 1059. [Google Scholar] [CrossRef]
- Indolfi, C.; Cappabianca, S.; Rossi, F.; Perrotta, S.; Procaccini, E.; Pota, E.; Martino, M.D.; Pinto, D.D.; Casale, F.; Indolfi, P. Abbreviated breast magnetic resonance imaging (FAST-MRI): A novel approach to breast cancer screening in patients with previous Hodgkin lymphoma. Pediatr. Blood Cancer 2019, 66, e27666. [Google Scholar] [CrossRef]
- Hurmuz, I.; Barna, R.; Natarâș, B.; Trăilă, I.-A.; Anderco, D.; Dema, S.; Jurescu, A.; Lăzureanu, D.-C.; Tăban, S.; Dema, A. Endometrial Carcinoma and Associated Secondary Neoplasia: The Role of Clinical Features, Pathology, and Comorbidities in a University-Affiliated Clinical Center from Western Romania. Medicina 2025, 61, 1748. [Google Scholar] [CrossRef]
- Abdelrahman, E.M.; Mohsen, S.M.; Mohamed, A.G.; Abdeen, M.S.; Elsayed, M.A.; Ibrahim, Z.M.; Abdelraouf, O.R.; Hegazy, H.; Abdelhalim, M.G. Outcome of Matrix Rotation Versus Single Incision Lateral Sulcus Mammoplasty in Upper Quadrant Breast Carcinomas. Medicina 2025, 61, 1609. [Google Scholar] [CrossRef]
- Fitrianti, A.E.; Wardani, N.O.; Astuti, A.; Anggadiredja, K.; Amalia, L.; Putri, R.A.; Zazuli, Z. Cardiotoxicity in Breast Cancer Therapy: Risks, Mechanisms, and Prevention Strategies. Med. Sci. 2025, 13, 130. [Google Scholar] [CrossRef]
- Albanghali, M.A.; Alnemari, R.K.; Al Ghamdi, R.B.; Gomaa, F.A.M.; Alzahrani, T.A.; Al Ghamdi, A.S.; Albanghali, B.M.; Kofiah, Y.M.; Alhassan, E.M.; Othman, B.A. Assessing Breast Cancer Awareness Among Women in Al Baha, Saudi Arabia: A Cross-Sectional Study Using the Breast Cancer Awareness Measure (BCAM). Med. Sci. 2025, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, F.; Avendano, D.; Cicero, G.; Garza-Montemayor, M.; Sofia, C.; Venanzi Rullo, E.; Ascenti, G.; Pinker-Domenig, K.; Marino, M.A. High-risk lesions of the breast: Concurrent diagnostic tools and management recommendations. Insights Imaging 2021, 12, 63. [Google Scholar] [CrossRef]
- Rubio, I.T.; Wyld, L.; Marotti, L.; Athanasiou, A.; Regitnig, P.; Catanuto, G.; Schoones, J.W.; Zambon, M.; Camps, J.; Santini, D.; et al. European guidelines for the diagnosis, treatment and follow-up of breast lesions with uncertain malignant potential (B3 lesions) developed jointly by EUSOMA, EUSOBI, ESP (BWG) and ESSO. Eur. J. Surg. Oncol. 2024, 50, 107292. [Google Scholar] [CrossRef]
- El Sharouni, M.A.; Postma, E.L.; Menezes, G.L.; van den Bosch, M.A.; Pijnappel, R.M.; Witkamp, A.J.; van der Pol, C.C.; Verkooijen, H.M.; van Diest, P.J. High Prevalence of MRI-Detected Contralateral and Ipsilateral Malignant Findings in Patients With Invasive Ductolobular Breast Cancer: Impact on Surgical Management. Clin. Breast Cancer 2016, 16, 269–275. [Google Scholar] [CrossRef]
- Pettit, K.; Swatske, M.E.; Gao, F.; Salavaggione, L.; Gillanders, W.E.; Aft, R.L.; Monsees, B.S.; Eberlein, T.J.; Margenthaler, J.A. The impact of breast MRI on surgical decision-making: Are patients at risk for mastectomy? J. Surg. Oncol. 2009, 100, 553–558. [Google Scholar] [CrossRef]
- Palmér, M.; Åkesson, Å.; Ljungberg, M.; Kuzcera, S.; Gryska, E.; de Coursey, E.; Heckemann, R.A.; Dahm Kähler, P.; Maier, S.E.; Leonhardt, H. Preoperative risk assessment of endometrial cancer using histogram analysis of weighted and quantitative MRI images. Abdom. Radiol. 2025, 1–11. [Google Scholar] [CrossRef]
- Gatta, G.; Di Grezia, G.; Cuccurullo, V.; Sardu, C.; Iovino, F.; Comune, R.; Ruggiero, A.; Chirico, M.; La Forgia, D.; Fanizzi, A.; et al. MRI in Pregnancy and Precision Medicine: A Review from Literature. J. Pers. Med. 2021, 12, 9. [Google Scholar] [CrossRef]
- Reginelli, A.; Nardone, V.; Giacobbe, G.; Belfiore, M.P.; Grassi, R.; Schettino, F.; Del Canto, M.; Grassi, R.; Cappabianca, S. Radiomics as a New Frontier of Imaging for Cancer Prognosis: A Narrative Review. Diagnostics 2021, 11, 1796. [Google Scholar] [CrossRef]
- Renzulli, M.; Zanotti, S.; Clemente, A.; Mineo, G.; Tovoli, F.; Reginelli, A.; Barile, A.; Cappabianca, S.; Taffurelli, M.; Golfieri, R. Hereditary breast cancer: Screening and risk reducing surgery. Gland Surg. 2019, 8, S142–S149. [Google Scholar] [CrossRef] [PubMed]
- Kanadys, W.; Barańska, A.; Malm, M.; Błaszczuk, A.; Polz-Dacewicz, M.; Janiszewska, M.; Jędrych, M. Use of Oral Contraceptives as a Potential Risk Factor for Breast Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies Up to 2010. Int. J. Environ. Res. Public Health 2021, 18, 4638. [Google Scholar] [CrossRef] [PubMed]
- Phillips, L.S.; Millikan, R.C.; Schroeder, J.C.; Barnholtz-Sloan, J.S.; Levine, B.J. Reproductive and hormonal risk factors for ductal carcinoma in situ of the breast. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1507–1514. [Google Scholar] [CrossRef]
- Claus, E.B.; Stowe, M.; Carter, D. Oral contraceptives and the risk of ductal breast carcinoma in situ. Breast Cancer Res. Treat. 2003, 81, 129–136. [Google Scholar] [CrossRef]
- Nardone, V.; Barbarino, M.; Angrisani, A.; Correale, P.; Pastina, P.; Cappabianca, S.; Reginelli, A.; Mutti, L.; Miracco, C.; Giannicola, R.; et al. CDK4, CDK6/cyclin-D1 Complex Inhibition and Radiotherapy for Cancer Control: A Role for Autophagy. Int. J. Mol. Sci. 2021, 22, 8391. [Google Scholar] [CrossRef]
- Sardu, C.; Gatta, G.; Pieretti, G.; Viola, L.; Sacra, C.; Di Grezia, G.; Musto, L.; Minelli, S.; La Forgia, D.; Capodieci, M.; et al. Pre-Menopausal Breast Fat Density Might Predict MACE During 10 Years of Follow-Up: The BRECARD Study. JACC Cardiovasc. Imaging 2021, 14, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Net, J.; Hamedi-Sangsari, A.; Schwartz, T.; Barrios, M.; Brofman, N.; Pluguez-Turull, C.; Spoont, J.; Stamler, S.; Yepes, M. Change in Indications and Outcomes for Stereotactic Biopsy Following Transition from Full Field Digital Mammography + Digital Breast Tomosynthesis to Full Field Synthetic Mammography + Digital Breast Tomosynthesis. Med. Sci. 2025, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Reginelli, A.; Urraro, F.; Sangiovanni, A.; Russo, G.M.; Russo, C.; Grassi, R.; Agostini, A.; Belfiore, M.P.; Cellina, M.; Floridi, C.; et al. Extranodal Lymphomas: A pictorial review for CT and MRI classification. Acta Bio Medica Atenei Parm. 2020, 91, 34–42. [Google Scholar] [CrossRef]
- Lewin, A.A.; Heller, S.L.; Jaglan, S.; Elias, K.; Newburg, A.; Melsaether, A.; Moy, L. Radiologic-Pathologic Discordance and Outcome After MRI-Guided Vacuum-Assisted Biopsy. AJR Am. J. Roentgenol. 2017, 208, W17–W22. [Google Scholar] [CrossRef]
- Gemici, A.A.; Inci, E. Agreement between dynamic contrast-enhanced magnetic resonance imaging and pathologic tumour size of breast cancer and analysis of the correlation with BI-RADS descriptors. Pol. J. Radiol. 2019, 84, 616–624. [Google Scholar] [CrossRef]

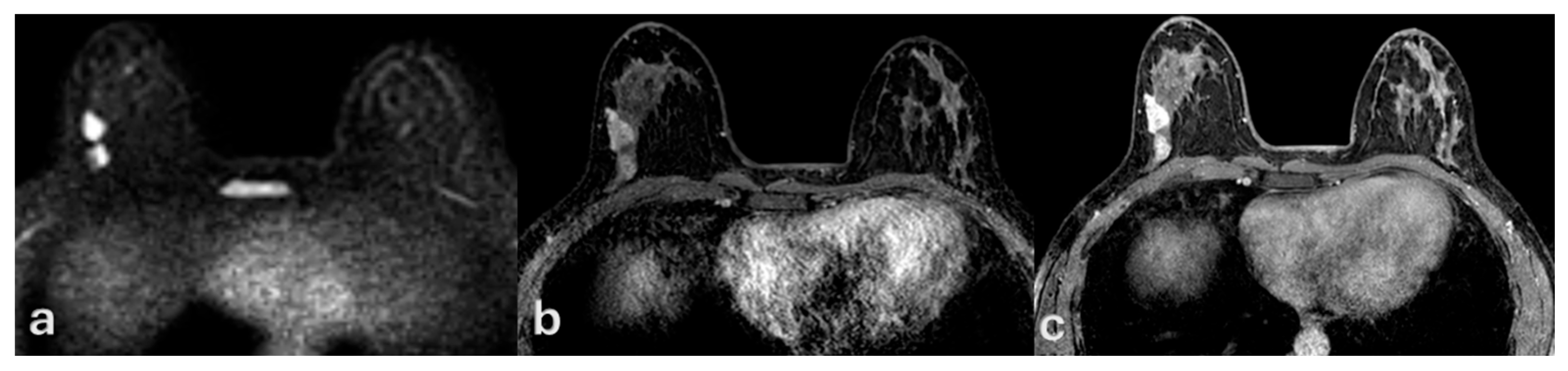
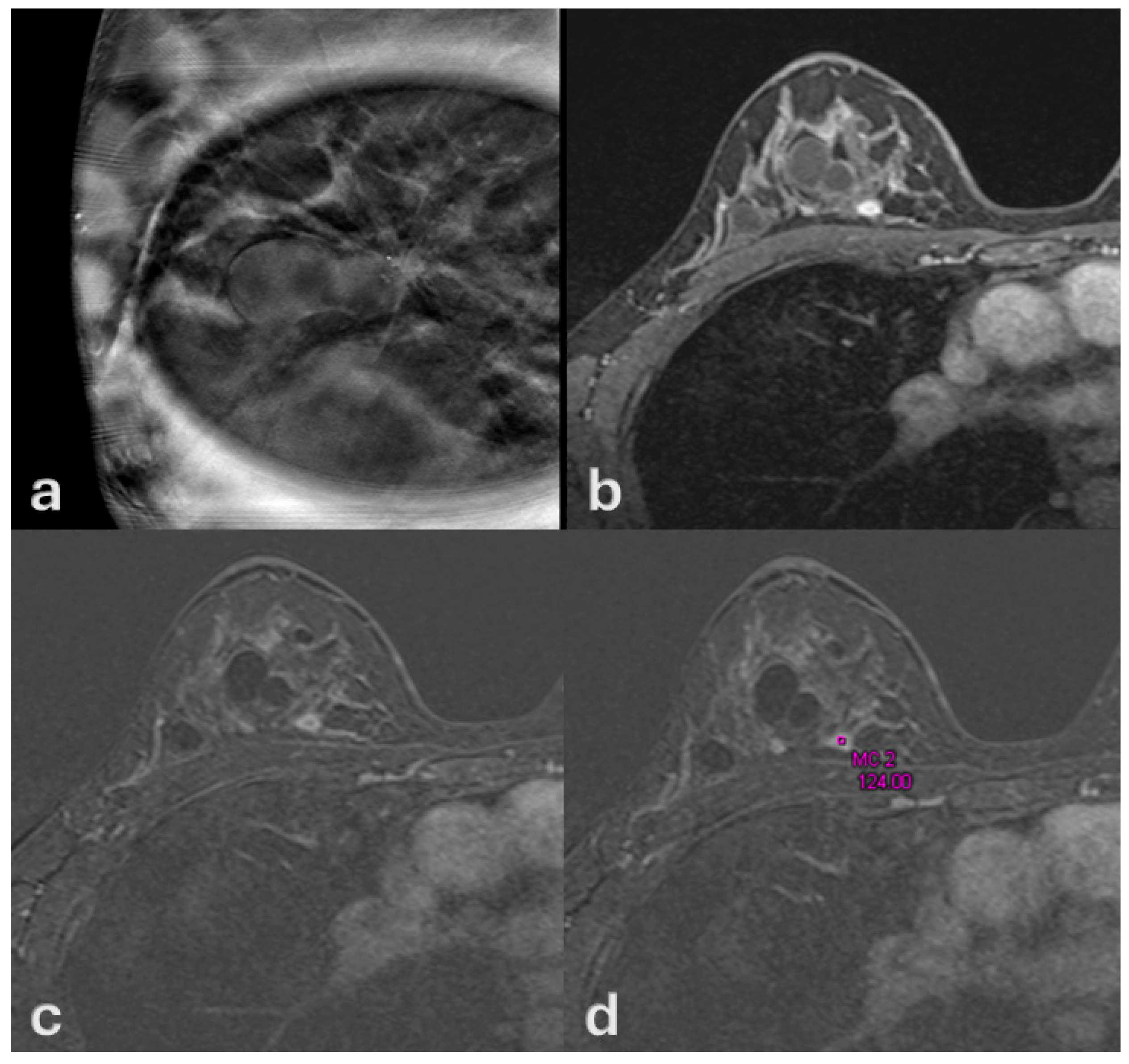
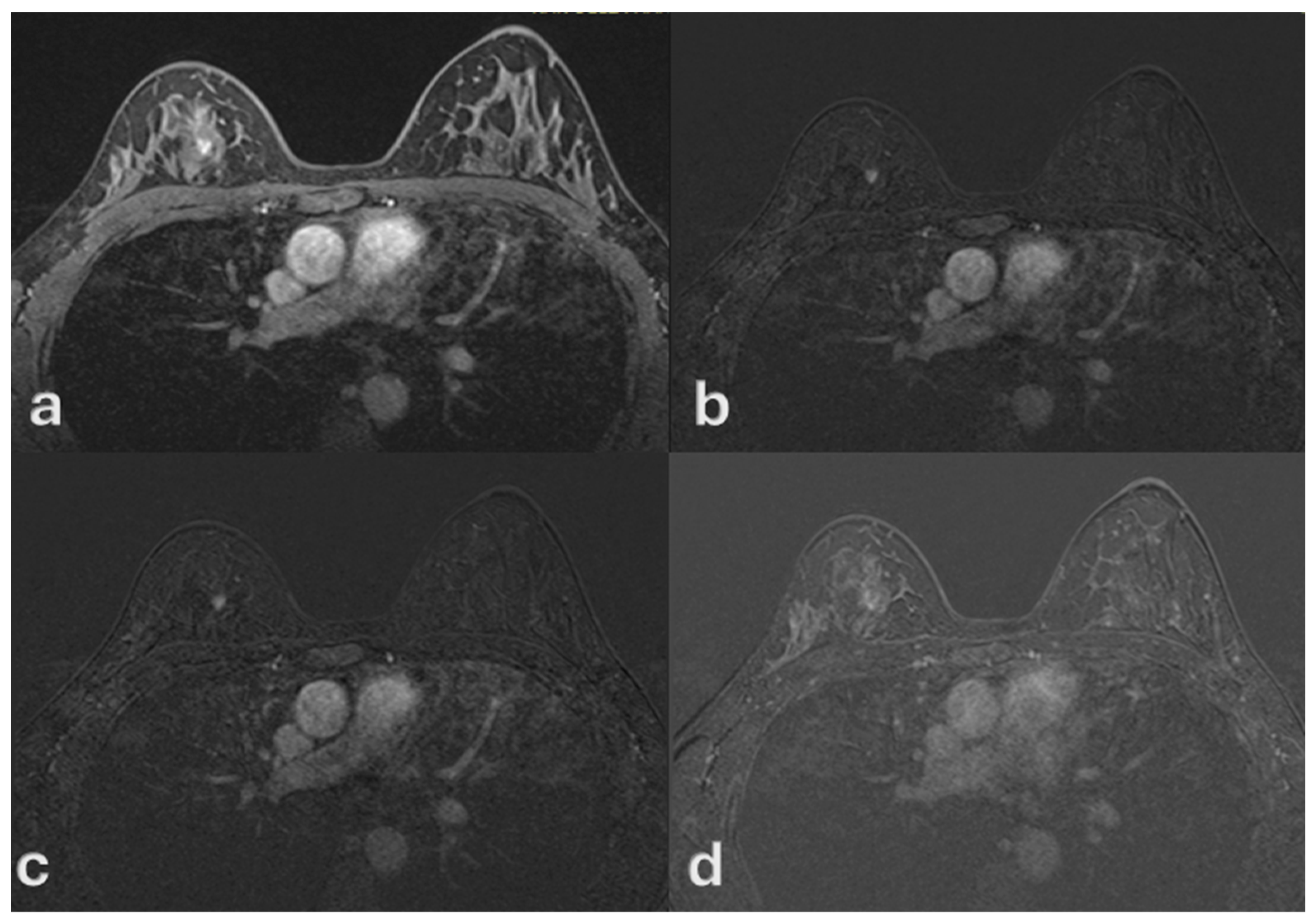
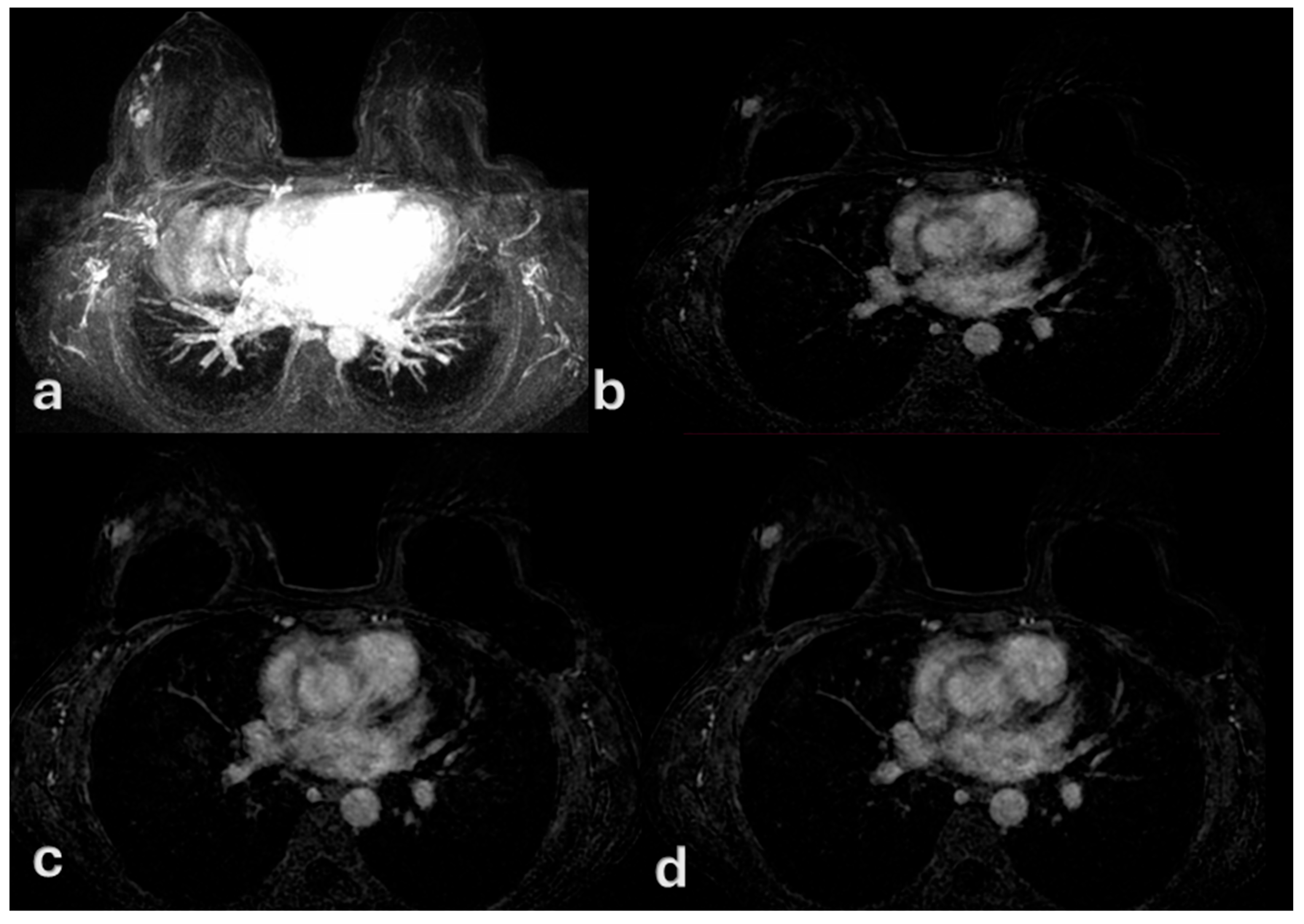


| Variable | Mean ± SD or n (%) | Description |
|---|---|---|
| Age (years) | 44.8 ± 4.5 (range 36–52) | - |
| Microcalcifications on mammography | 40 (53.8%) | Present |
| No microcalcification on mammography | 35 (46.2%) | Absent |
| MRI kinetics: persistent | 52 (69.2%) | - |
| MRI kinetics: washout | 23 (30.8%) | - |
| Radiology–pathology discordance | 17 (23.1%) | Overestimation on MRI |
| Oral contraceptive use | 46 (61.5%) | - |
| Low grade DCIS among OC users | 23 (30.7%) | - |
| Variable | Number (n—Out of Cohort of 75) | % of Cohort |
|---|---|---|
| Mammography: microcalcifications present | 40 | 53.8% |
| Mammography: no microcalcifications | 35 | 46.2% |
| MRI kinetics: persistent | 52 | 69.2% |
| MRI kinetics: rapid washout | 23 | 30.8% |
| Radiology–pathology mismatch | 17 | 23.1% |
| Any OC exposusre | 46 | 61.5% |
| Low-grade DCIS among OC users | 23 | 30.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urraro, F.; Giordano, N.; Patanè, V.; Brunese, M.C.; Varelli, C.; Russo, C.; Brunese, L.; Cappabianca, S. Multimodal Imaging of Ductal Carcinoma In Situ: A Single-Center Study of 75 Cases. Med. Sci. 2025, 13, 245. https://doi.org/10.3390/medsci13040245
Urraro F, Giordano N, Patanè V, Brunese MC, Varelli C, Russo C, Brunese L, Cappabianca S. Multimodal Imaging of Ductal Carcinoma In Situ: A Single-Center Study of 75 Cases. Medical Sciences. 2025; 13(4):245. https://doi.org/10.3390/medsci13040245
Chicago/Turabian StyleUrraro, Fabrizio, Nicoletta Giordano, Vittorio Patanè, Maria Chiara Brunese, Carlo Varelli, Carolina Russo, Luca Brunese, and Salvatore Cappabianca. 2025. "Multimodal Imaging of Ductal Carcinoma In Situ: A Single-Center Study of 75 Cases" Medical Sciences 13, no. 4: 245. https://doi.org/10.3390/medsci13040245
APA StyleUrraro, F., Giordano, N., Patanè, V., Brunese, M. C., Varelli, C., Russo, C., Brunese, L., & Cappabianca, S. (2025). Multimodal Imaging of Ductal Carcinoma In Situ: A Single-Center Study of 75 Cases. Medical Sciences, 13(4), 245. https://doi.org/10.3390/medsci13040245






