Non-Invasive Myocardial Work Detects Extensive Coronary Disease in Orthotopic Heart Transplant Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Demographic and Clinical Data
2.3. Two-Dimensional TTE
2.4. CCTA Protocol and Study Analysis
2.5. Statistical Analysis
3. Results
Advanced Echocardiography Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A wave | late diastolic mitral inflow velocity |
| ANT | Anterior |
| ANT_SEPT | Anteroseptal |
| AUC | area under the curve |
| BMI | body mass index |
| CAV | cardiac allograft vasculopathy |
| CCTA | coronary computed tomography angiography |
| CI | confidence interval |
| CO | cardiac output |
| DBP | diastolic blood pressure |
| eGFR | estimated glomerular filtration rate |
| GCW | global constructed work |
| GWE | global work efficiency |
| GWW | global wasted work |
| ICA | invasive coronary angiography |
| INF | Inferior |
| ISHLT | International Society for Heart and Lung Transplantation |
| IVUS | intravascular ultrasound |
| LAT | Lateral |
| LV | left ventricle, left ventricular |
| LVEF | left ventricular ejection fraction |
| GLS | left ventricular global longitudinal strain |
| mmHg | millimeters of mercury |
| MW | myocardial work |
| MWI | myocardial work index |
| NPV | negative predictive value |
| OCL | obstructive coronary lesions |
| OHT | orthotopic heart transplantation |
| OHT< 4 | orthotopic heart transplantation patients with coronary artery disease of fewer than 4 segments or no disease on CCTA |
| OHT ≥ 4 | orthotopic heart transplantation patients with disease of 4 or more coronary artery segments on CCTA |
| OR | odds ratio |
| POST | Posterior |
| PRA | panel reactive antibodies |
| ROC | receiver operating characteristic |
| SBP | systolic blood pressure |
| SEPT | Septal |
| TTE | transthoracic echocardiography |
References
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Harhay, M.O.; Hayes, D.; Hsich, E.; Meiser, B.; Potena, L.; Robinson, A.; Rossano, J.W.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2018, 37, 1155–1168. [Google Scholar] [CrossRef]
- Rahmani, M.; Cruz, R.P.; Granville, D.J.; McManus, B.M. Allograft vasculopathy versus atherosclerosis. Circ. Res. 2006, 99, 801–815. [Google Scholar] [CrossRef]
- Pober, J.S.; Chih, S.; Kobashigawa, J.; Madsen, J.C.; Tellides, G. Cardiac allograft vasculopathy: Current review and future research directions. Cardiovasc. Res. 2021, 117, 2624–2638. [Google Scholar] [CrossRef]
- Chih, S.; Chong, A.Y.; Mielniczuk, L.M.; Bhatt, D.L.; Beanlands, R.S.B. Allograft Vasculopathy: The Achilles’ Heel of Heart Transplantation. J. Am. Coll. Cardiol. 2016, 68, 80–91. [Google Scholar] [CrossRef]
- Lee, F.; Nair, V.; Chih, S. Cardiac allograft vasculopathy: Insights on pathogenesis and therapy. Clin. Transplant. 2020, 34, e13794. [Google Scholar] [CrossRef]
- Velleca, A.; Shullo, M.A.; Dhital, K.; Azeka, E.; Colvin, M.; DePasquale, E.; Farrero, M.; García-Guereta, L.; Jamero, G.; Khush, K.; et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2023, 42, e1–e141. [Google Scholar] [CrossRef]
- Johnson, D.E.; Alderman, E.L.; Schroeder, J.S.; Gao, S.-Z.; Hunt, S.; DeCampli, W.M.; Stinson, E.; Billingham, M. Transplant coronary artery disease: Histopathologic correlations with angiographic morphology. J. Am. Coll. Cardiol. 1991, 17, 449–457. [Google Scholar] [CrossRef]
- Spes, C.H.; Klauss, V.; Mudra, H.; Schnaack, S.D.; Tammen, A.R.; Rieber, J.; Siebert, U.; Henneke, K.-H.; Uberfuhr, P.; Reichart, B.; et al. Diagnostic and prognostic value of serial dobutamine stress echocardiography for noninvasive assessment of cardiac allograft vasculopathy: A comparison with coronary angiography and intravascular ultrasound. Circulation 1999, 100, 509–515. [Google Scholar] [CrossRef]
- Tuzcu, E.M.; Hobbs, R.E.; Rincon, G.; Bott-Silverman, C.; De Franco, A.C.; Robinson, K.; McCarthy, P.M.; Stewart, R.W.; Guyer, S.; Nissen, S.E. Occult and frequent transmission of atherosclerotic coronary disease with cardiac transplantation. Insights from intravascular ultrasound. Circulation 1995, 91, 1706–1713. [Google Scholar] [CrossRef]
- Sharples, L.D.; Jackson, C.H.; Parameshwar, J.; Wallwork, J.; Large, S.R. Diagnostic accuracy of coronary angiography and risk factors for post-heart-transplant cardiac allograft vasculopathy. Transplantation 2003, 76, 679–682. [Google Scholar] [CrossRef]
- Romero, J.; Wever-Pinzon, O.; Golive, A.; Kelesidis, I.; Manrique, C.; Drakos, S.; Pina, I.; Kfoury, A.; Stehlik, J.; Garcia, M. Coronary computed tomography angiography for the detection of cardiac allograft vasculopathy: A meta-analysis of prospective trials. J. Am. Coll. Cardiol. 2014, 63, 1992–2004. [Google Scholar] [CrossRef]
- Cerqueira, M.D.; Weissman, N.J.; Dilsizian, V.; Jacobs, A.K.; Kaul, S.; Laskey, W.K.; Pennell, D.J.; Rumberger, J.A.; Ryan, T.; Verani, M.S. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart. Circulation 2002, 105, 539–542. [Google Scholar] [CrossRef]
- García-Baizán, A.; Caballeros, M.; Ezponda, A.; Manrique, R.; Gavira, J.J.; Rábago, G.; Bastarrika, G. Long-Term Prognostic Value of Coronary CTA in Orthotopic Heart Transplant Recipients. AJR Am. J. Roentgenol. 2021, 216, 1216–1221. [Google Scholar] [CrossRef]
- Badano, L.P.; Miglioranza, M.H.; Edvardsen, T.; Colafranceschi, A.S.; Muraru, D.; Bacal, F.; Nieman, K.; Zoppellaro, G.; Braga, F.G.M.; Binder, T.; et al. European Association of Cardiovascular Imaging/Cardiovascular Imaging Department of the Brazilian Society of Cardiology recommendations for the use of cardiac imaging to assess and follow patients after heart transplantation. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 919–948. [Google Scholar] [CrossRef]
- Clemmensen, T.S.; Løgstrup, B.B.; Eiskjær, H.; Poulsen, S.H. Evaluation of longitudinal myocardial deformation by 2-dimensional speckle-tracking echocardiography in heart transplant recipients: Relation to coronary allograft vasculopathy. J. Heart Lung Transplant. Off. Publ. Int. Soc. Heart Transplant. 2015, 34, 195–203. [Google Scholar] [CrossRef]
- Sciaccaluga, C.; Ghionzoli, N.; Mandoli, G.; Sisti, N.; D’aScenzi, F.; Focardi, M.; Bernazzali, S.; Vergaro, G.; Emdin, M.; Valente, S.; et al. The role of non-invasive imaging modalities in cardiac allograft vasculopathy: An updated focus on current evidences. Heart Fail. Rev. 2022, 27, 1235–1246. [Google Scholar] [CrossRef]
- Moya, A.; Buytaert, D.; Penicka, M.; Bartunek, J.; Vanderheyden, M. State-of-the-Art: Noninvasive Assessment of Left Ventricular Function Through Myocardial Work. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2023, 36, 1027–1042. [Google Scholar] [CrossRef]
- Borrie, A.; Goggin, C.; Ershad, S.; Robinson, W.; Sasse, A. Noninvasive Myocardial Work Index: Characterizing the Normal and Ischemic Response to Exercise. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2020, 33, 1191–1200. [Google Scholar] [CrossRef]
- Ran, H.; Yao, Y.; Wan, L.; Ren, J.; Sheng, Z.; Zhang, P.; Schneider, M. Characterizing stenosis severity of coronary heart disease by myocardial work measurement in patients with preserved ejection fraction. Quant. Imaging Med. Surg. 2023, 13, 5022–5033. [Google Scholar] [CrossRef]
- Lin, J.; Gao, L.; He, J.; Liu, M.; Cai, Y.; Niu, L.; Zhao, Y.; Li, X.; Wang, J.; Wu, W.; et al. Comparison of Myocardial Layer-Specific Strain and Global Myocardial Work Efficiency During Treadmill Exercise Stress in Detecting Significant Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 786943. [Google Scholar] [CrossRef]
- Cacioli, G.; Ciabatti, M.; Cristiano, E.; Notari, C.; Papisca, I.; Distefano, G.; Menafra, G.; Della Monica, P.L.; Feccia, M.A.; Pergolini, A.; et al. Myocardial Work by Speckle-Tracking Echocardiography in Heart Transplant Recipients: Association Between Global Work Efficiency and Coronary Allograft Vasculopathy. Am. J. Cardiol. 2024, 228, 1–9. [Google Scholar] [CrossRef]
- Antón, R.M.; Izco, M.P.; Bastarrika, G.; Dorronsoro, A.D.; Ezponda, A.; Carazo, F.d.l.T.; Salteráin, N.; Martín, L.J.-S.; Martín-Calvo, N.; Iribarren, M.J.; et al. Non-Invasive Myocardial Work Identifies Patients with Obstructive Coronary Lesions After Orthotopic Heart Transplantation. Diagnostics 2025, 15, 1352. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef]
- Samset, E.; Healthcare, G. Evaluation of Segmental Myocardial Work in the Left Ventricle; GE Healthcare Website. 2017. Available online: https://www.gehealthcare.com/-/media/8cab29682ace4ed7841505f813001e33.pdf@line-2@?srsltid=AfmBOopPgNu39xr-DeqnqU54O318wdIr29IkGPzYBCD7LPJDA5nnS4R7 (accessed on 30 April 2025).
- Austen, W.; Edwards, J.; Frye, R.; Gensini, G.; Gott, V.; Griffith, L.; McGoon, D.; Murphy, M.; Roe, B. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 1975, 51 (Suppl. 4), 5–40. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Crespo-Leiro, M.G.; Dipchand, A.; Ensminger, S.M.; Hiemann, N.E.; Kobashigawa, J.A.; Madsen, J.; Parameshwar, J.; Starling, R.C.; Uber, P.A. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy: 2010. J. Heart Lung Transplant. 2010, 29, 717–727, Erratum in J. Heart Lung Transplant. 2011, 30, 360. [Google Scholar] [CrossRef]
- Daud, A.; Xu, D.; Revelo, M.P.; Shah, Z.; Drakos, S.G.; Dranow, E.; Stoddard, G.; Kfoury, A.G.; Hammond, M.E.H.; Nativi-Nicolau, J.; et al. Microvascular Loss and Diastolic Dysfunction in Severe Symptomatic Cardiac Allograft Vasculopathy. Circ. Heart Fail. 2018, 11, e004759. [Google Scholar] [CrossRef] [PubMed]
- Van Keer, J.M.; Van Aelst, L.N.L.; Rega, F.; Droogne, W.; Voros, G.; Meyns, B.; Vanhaecke, J.; Emonds, M.P.; Janssens, S.; Naesens, M.; et al. Long-term outcome of cardiac allograft vasculopathy: Importance of the International Society for Heart and Lung Transplantation angiographic grading scale. J. Heart Lung Transplant. 2019, 38, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Mullikin, A.; Zang, H.; Ollberding, N.J.; Stark, S.; Hill, G.D.; Chin, C.; Tretter, J.T. Decreased Global Myocardial Work Efficiency Correlates with Coronary Vasculopathy in Pediatric Heart Transplant Patients. Pediatr. Cardiol. 2022, 43, 515–524. [Google Scholar] [CrossRef]
- Bittencourt, M.S.; Hulten, E.; Ghoshhajra, B.; O’Leary, D.; Christman, M.P.; Montana, P.; Truong, Q.A.; Steigner, M.; Murthy, V.L.; Rybicki, F.J.; et al. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ. Cardiovasc. Imaging 2014, 7, 282–291. [Google Scholar] [CrossRef]
- Ayoub, C.; Erthal, F.; Abdelsalam, M.A.; Murad, M.H.; Wang, Z.; Erwin, P.J.; Hillis, G.S.; Kritharides, L.; Chow, B.J. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: A systematic review and meta-analysis. J. Cardiovasc. Comput. Tomogr. 2017, 11, 258–267. [Google Scholar] [CrossRef]
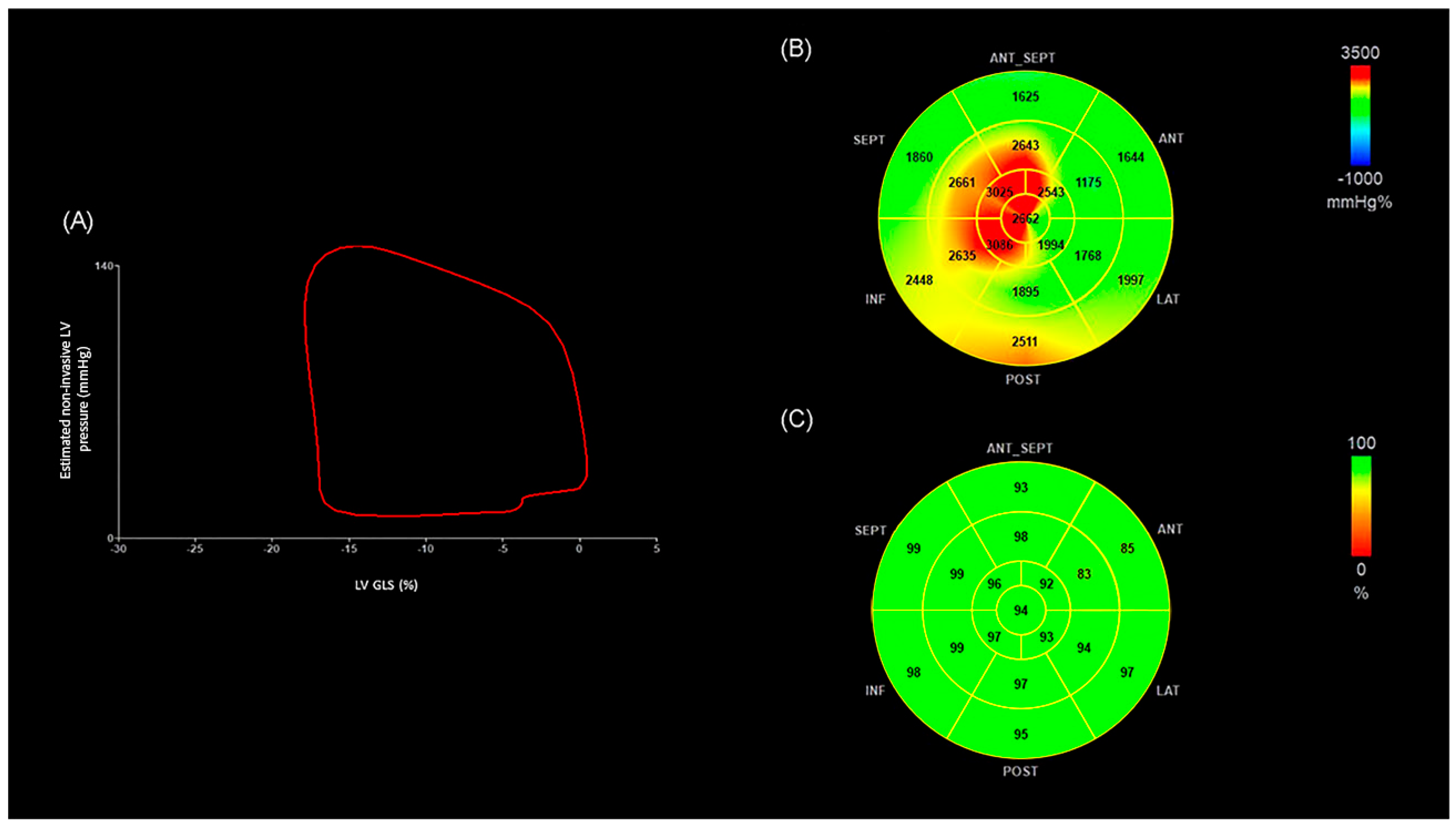
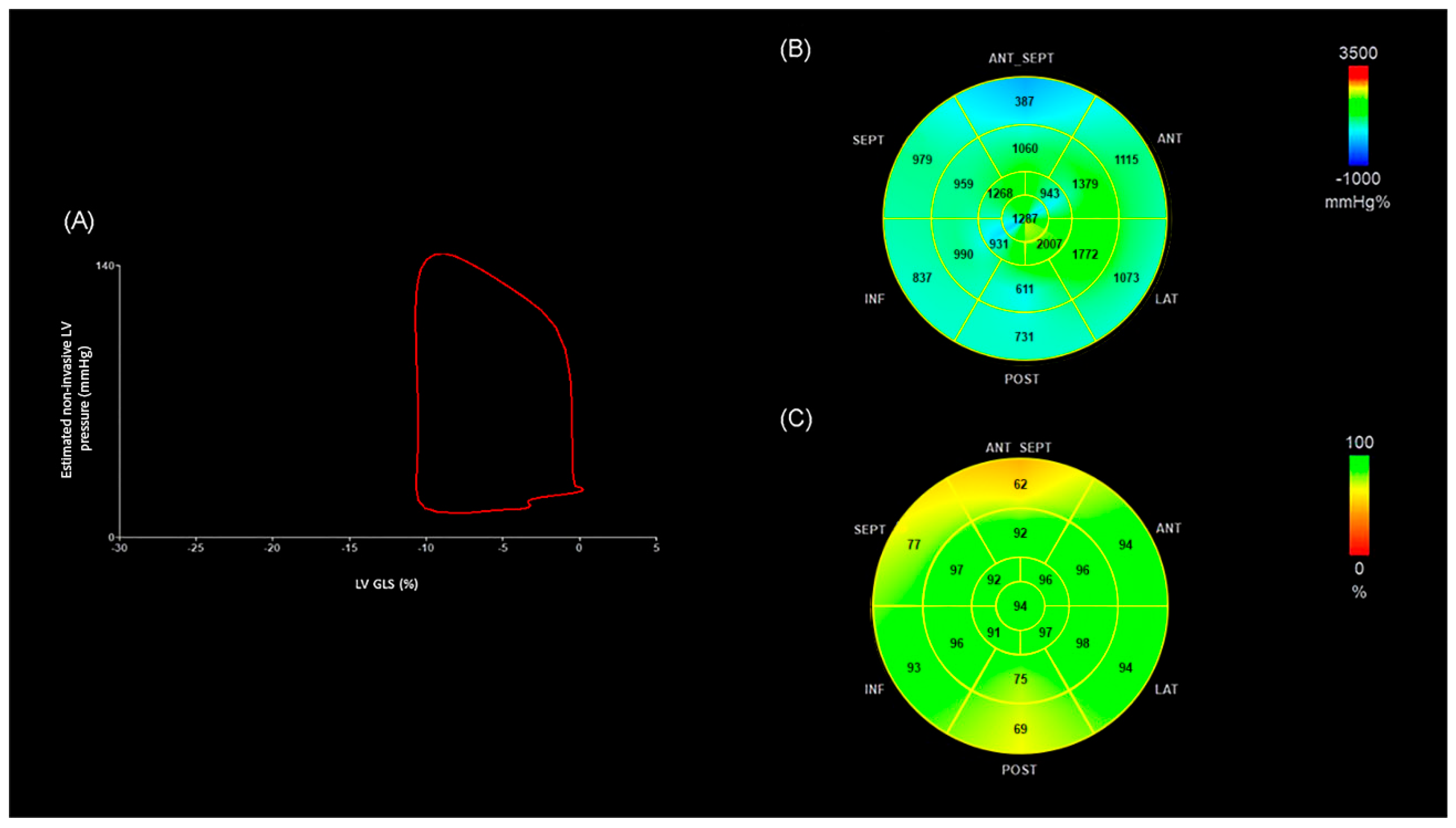
| OHT < 4 (n = 17) | OHT ≥ 4 (n = 38) | p | |
|---|---|---|---|
| Age (years) | 60.43 (13.94) | 64.68 (11.51) | 0.27 |
| Sex (male) | 8 (47.06) | 34 (89.47) | <0.01 |
| BMI (kg/m2) | 27.44 (6.26) | 25.45 (3.42) | 0.39 |
| SBP (mmHg) | 122.94 (17.18) | 127.58 (20.08) | 0.48 |
| DBP (mmHg) | 82.24 (13.86) | 79.63 (12.50) | 0.55 |
| Hypertension | 11 (64.71) | 24 (63.16) | 1 |
| Dyslipidemia | 11 (64.71) | 28 (73.68) | 0.53 |
| Diabetes mellitus | 2 (11.76) | 11 (28.95) | 0.30 |
| Tobacco use Never Current Former | 23 (48.94) 2 (4.26) 22(46.81) | 1 (14.29) 0 (0.00) 6 (85.71) | 0.89 |
| Age at OHT (years) | 53.24 (13.97) | 53.32 (12.08) | 0.73 |
| Retransplant | 1 (5.88) | 0 (0.00) | 0.30 |
| eGFR (mL/min/1.73 m2) | 55.12 (9.88) | 56.26 (7.50) | 0.77 |
| Hemoglobin (g/dL) | 14.38 (2.85) | 13.60 (2.01) | 0.57 |
| Cytomegalovirus infection | 8 (47.06) | 16 (42.11) | 0.77 |
| Cardiac device | 0 (0.00) | 2 (5.26) | 1 |
| Peripheral artery disease | 3 (17.65) | 3 (7.89) | 0.35 |
| Etiology of the cardiopathy: Dilated Ischemic Valvular Congenital CAV Autoimmune | 3 (17.65) 8 (47.06) 1 (5.88) 3 (17.65) 1 (5.88) 1 (5.88) | 20 (52.63) 13 (34.21) 4 (10.53) 1 (2.63) 0 (0.00) 0 (0.00) | <0.05 0.38 1 0.08 0.30 0.30 |
| Antiplatelet: Acetylsalicylic acid Clopidogrel Ticlopidine | 15 (88.24) 1 (5.88) 1 (5.88) | 28 (73.68) 7 (18.42) 4 (10.53) | 0.30 0.41 1 |
| Immunosuppressive agents: Tacrolimus Mycophenolate mofetil Everolimus Steroids Cyclosporine Sirolimus | 17 (100.00) 17 (100.00) 0 (0.00) 3 (17.65) 0 (0.00) 0 (0.00) | 35 (92.11) 25 (65.79) 13 (34.21) 5 (13.16) 2 (5.26) 1 (2.63) | 0.54 <0.01 <0.01 0.69 1 1 |
| Lipid-lowering drugs: Statin Ezetimib | 14 (82.35) 3 (17.65) | 34 (89.47) 7 (18.42) | 0.664 1 |
| Time from OHT to CCTA (months) | 62.93 (37.43–129.70) | 94.70 (68.46–198.27) | 0.028 |
| OHT < 4 (n = 17) | OHT ≥ 4 (n = 38) | p | ||
|---|---|---|---|---|
| GLS (%) | Mean | −15.47 (2.28) | −2.28 (5.83) | 0.27 |
| p50 | −16 | −15 | ||
| p25 | −17 | −16 | ||
| p75 | −14 | −11 | ||
| MWI (mmHg%) | Mean | 1419.77 (373.91) | 1391.55 (380.38) | 0.87 |
| p50 | 1406 | 1374 | ||
| p25 | 1213 | 1145 | ||
| p75 | 1538 | 1663 | ||
| GCW (mmHg%) | Mean | 1637.12 (377.06) | 1645 (446.05) | 0.66 |
| p50 | 1573 | 1713 | ||
| p25 | 1387 | 1360 | ||
| p75 | 1767 | 1934 | ||
| GWW (mmHg%) | Mean | 96.59 (82.91) | 111.42 (66.27) | 0.08 |
| p50 | 78 | 91 | ||
| p25 | 52 | 73 | ||
| p75 | 95 | 132 | ||
| GWE (%) | Mean | 93.18 (5.45) | 91.42 (4.64) | 0.05 |
| p50 | 95 | 92 | ||
| p25 | 93 | 88 | ||
| p75 | 96 | 95 |
| Cutoff | |
|---|---|
| GLS | >−12.6 |
| MWI | <1131 |
| GCW | <1767 |
| GWW | >88 |
| GWE | <94 |
| Pathological Values (%) | OHT < 4 (n = 17) | OHT ≥ 4 (n = 38) | p |
|---|---|---|---|
| GLS | 5.88 | 31.58 | 0.04 |
| MWI | 11.76 | 23.68 | 0.31 |
| GCW | 70.59 | 55.26 | 0.28 |
| GWW | 29.41 | 65.79 | 0.01 |
| GWE | 29.41 | 60.53 | 0.03 |
| Pathological Values | OR (CI 95%) | p |
|---|---|---|
| GLS | 1.22 (1.02–1.47) | 0.033 |
| MWI | 1.14 (0.95–1.40) | 0.152 |
| GCW | 0.92 (0.79–1.06) | 0.236 |
| GWW | 1.20 (1.03–1.40) | 0.018 |
| GWE | 1.10 (0.96–1.27) | 0.175 |
| Pathological Values | OR (CI 95%) | p |
|---|---|---|
| GLS | 7.38 (0.87–62.32) | 0.07 |
| MWI | 2.32 (0.44–12.17) | 0.32 |
| GCW | 0.51 (0.15–1.75) | 0.24 |
| GWW | 4.61 (1.33–15.95) | 0.02 |
| GWE | 3.68 (1.07–12.58) | 0.04 |
| Cutoff Value | AUC (CI 95%) | Sensitivity | Specificity | |
|---|---|---|---|---|
| GLS | −12.6 | 0.59 (0.43–0.74) | 31.58 | 94.12 |
| MWI | 1131 | 0.48 (0.32–0.65) | 88.24 | 23.68 |
| GCW | 1767 | 0.46 (0.29–0.63) | 29.41 | 55.26 |
| GWW | 88 | 0.64 (0.47–81.3) | 65.79 | 70.59 |
| GWE | 94 | 0.61 (0.45–0.77) | 70.59 | 60.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manrique Antón, R.; Pascual Izco, M.; Díaz Dorronsoro, A.; Ezponda, A.; de la Torre Carazo, F.; Salteráin, N.; Jimeno-San Martín, L.; Martín-Calvo, N.; Manrique Antón, Á.; Iribarren, M.J.; et al. Non-Invasive Myocardial Work Detects Extensive Coronary Disease in Orthotopic Heart Transplant Patients. Med. Sci. 2025, 13, 212. https://doi.org/10.3390/medsci13040212
Manrique Antón R, Pascual Izco M, Díaz Dorronsoro A, Ezponda A, de la Torre Carazo F, Salteráin N, Jimeno-San Martín L, Martín-Calvo N, Manrique Antón Á, Iribarren MJ, et al. Non-Invasive Myocardial Work Detects Extensive Coronary Disease in Orthotopic Heart Transplant Patients. Medical Sciences. 2025; 13(4):212. https://doi.org/10.3390/medsci13040212
Chicago/Turabian StyleManrique Antón, Rebeca, Marina Pascual Izco, Agnés Díaz Dorronsoro, Ana Ezponda, Fátima de la Torre Carazo, Nahikari Salteráin, Leticia Jimeno-San Martín, Nerea Martín-Calvo, Áurea Manrique Antón, María Josefa Iribarren, and et al. 2025. "Non-Invasive Myocardial Work Detects Extensive Coronary Disease in Orthotopic Heart Transplant Patients" Medical Sciences 13, no. 4: 212. https://doi.org/10.3390/medsci13040212
APA StyleManrique Antón, R., Pascual Izco, M., Díaz Dorronsoro, A., Ezponda, A., de la Torre Carazo, F., Salteráin, N., Jimeno-San Martín, L., Martín-Calvo, N., Manrique Antón, Á., Iribarren, M. J., Bastarrika, G., & Rábago, G. (2025). Non-Invasive Myocardial Work Detects Extensive Coronary Disease in Orthotopic Heart Transplant Patients. Medical Sciences, 13(4), 212. https://doi.org/10.3390/medsci13040212






