Prevalence and Epidemiological Patterns of Enterobius vermicularis Infection in Thailand: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Registration and Protocol
2.2. Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis
3. Results
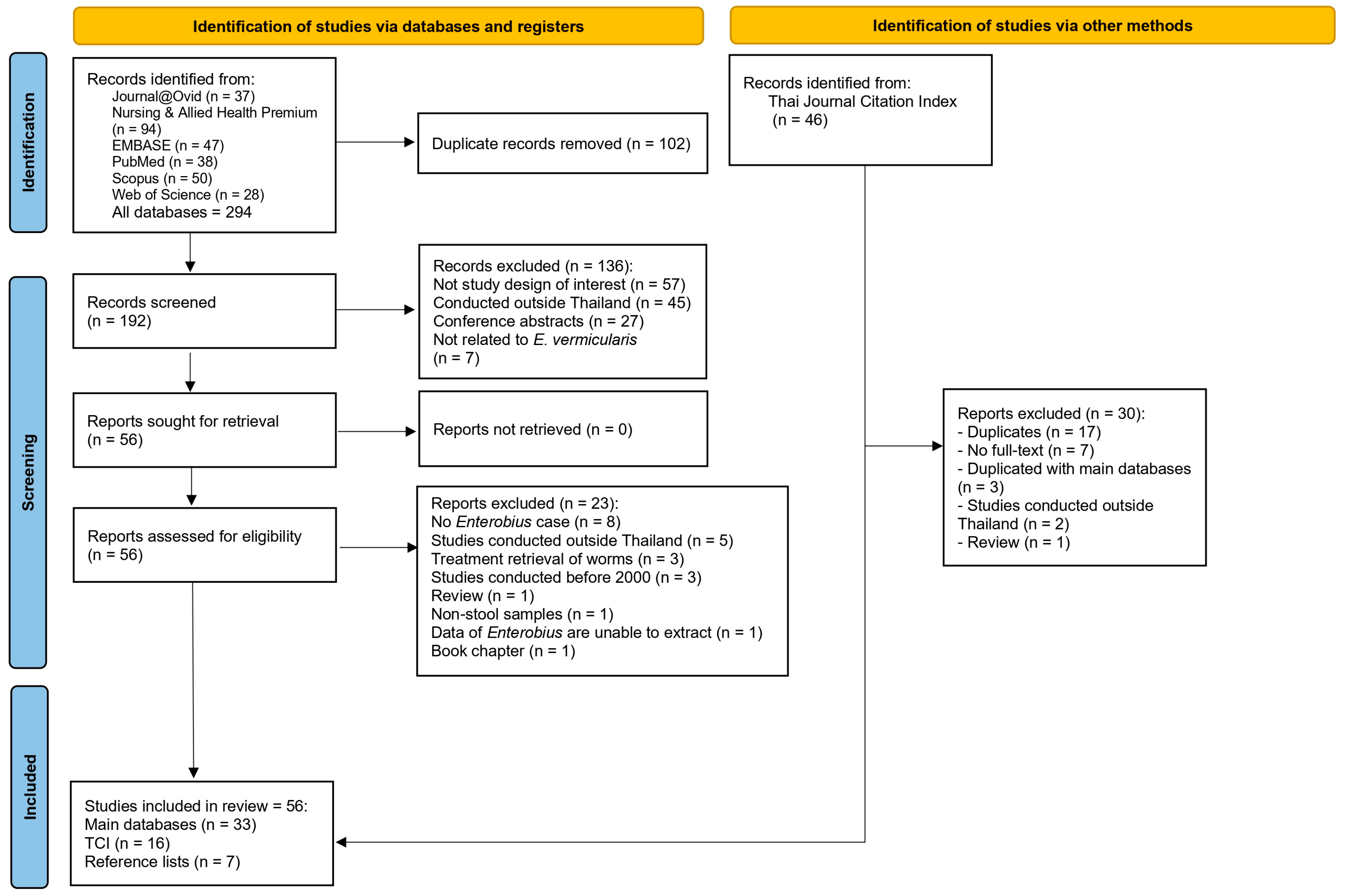
3.1. Search Results
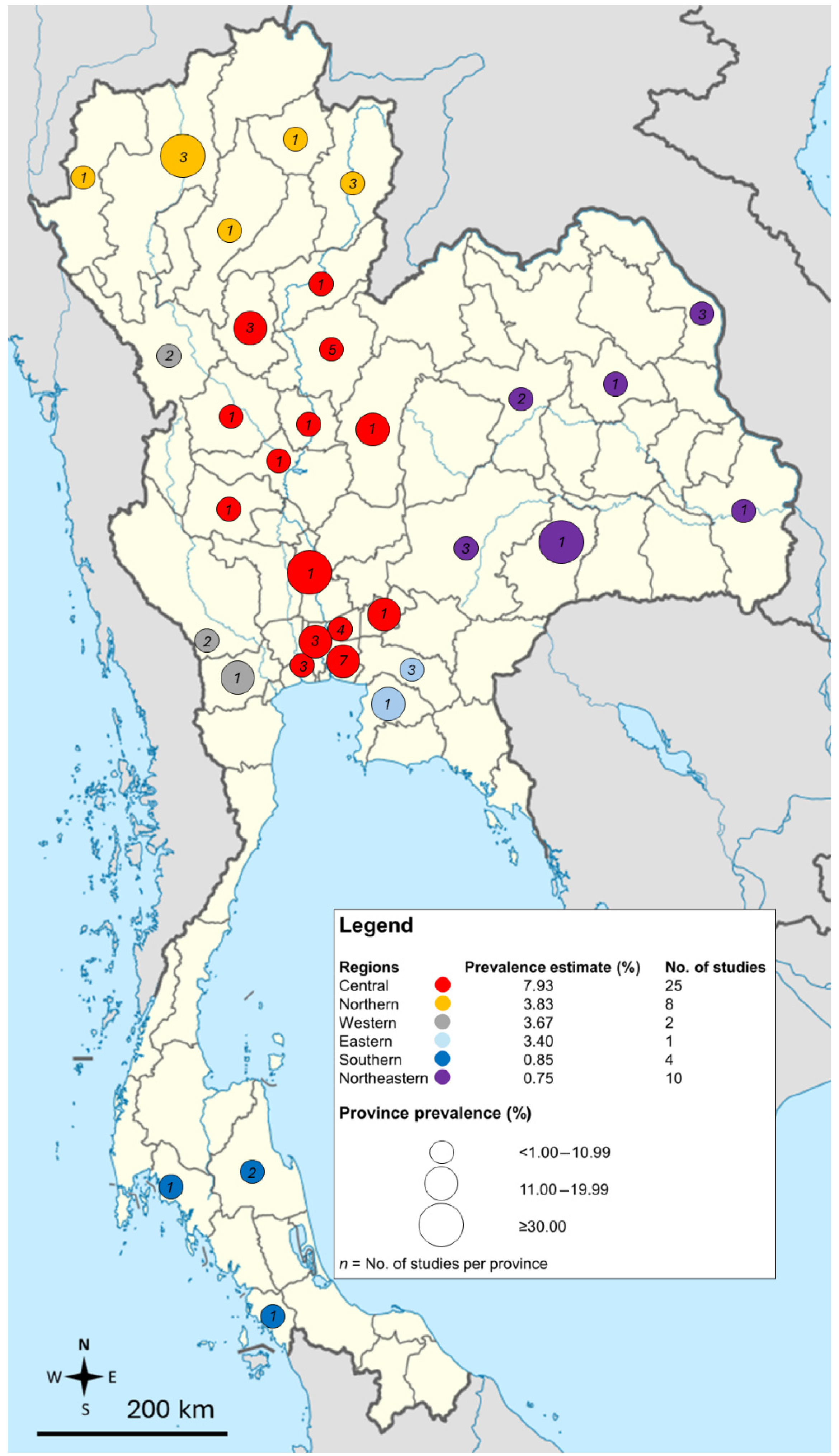
3.2. General Characteristics of Included Studies
3.3. Risk of Bias Assessment Results
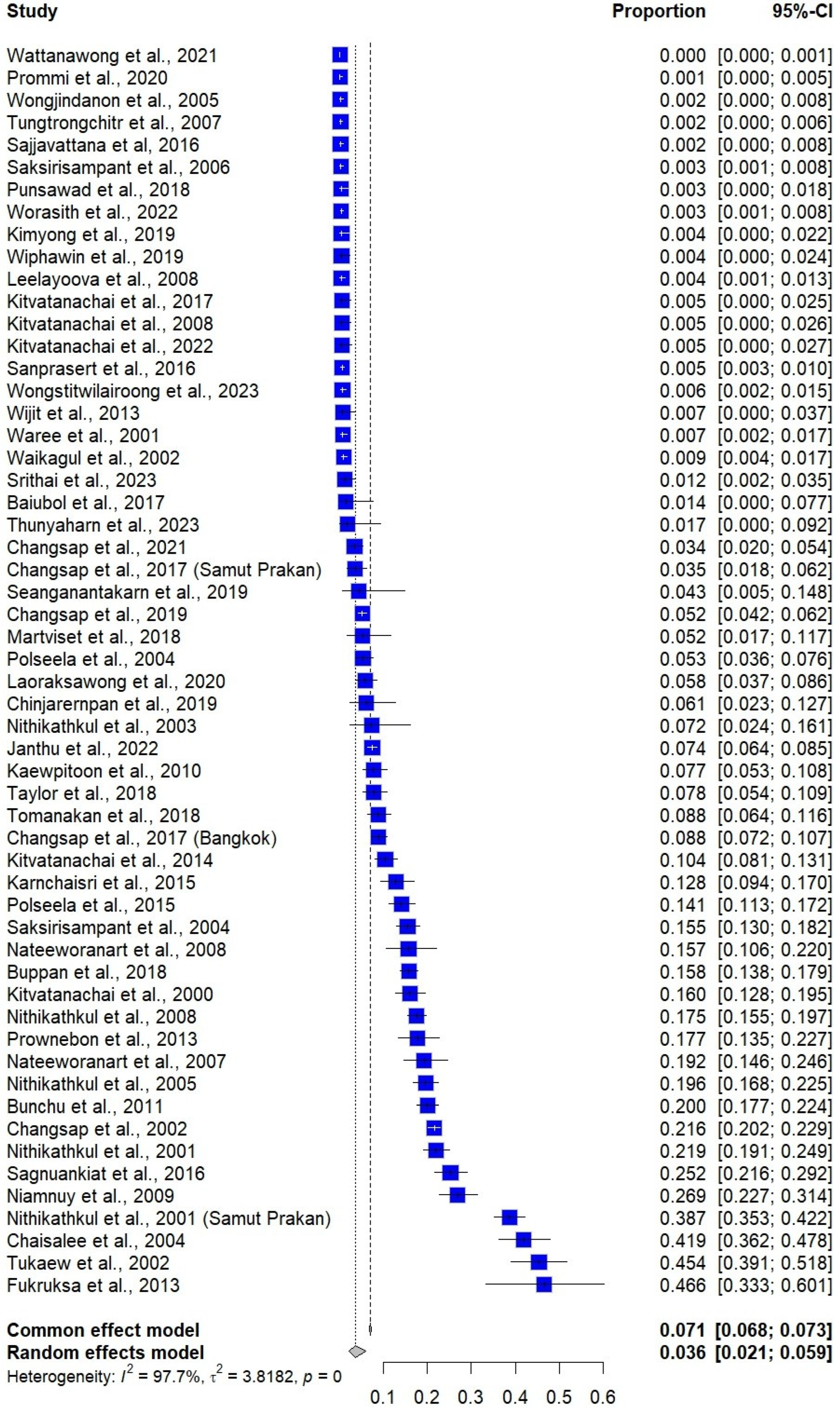
3.4. Prevalence Estimate of E. vermicularis Infections
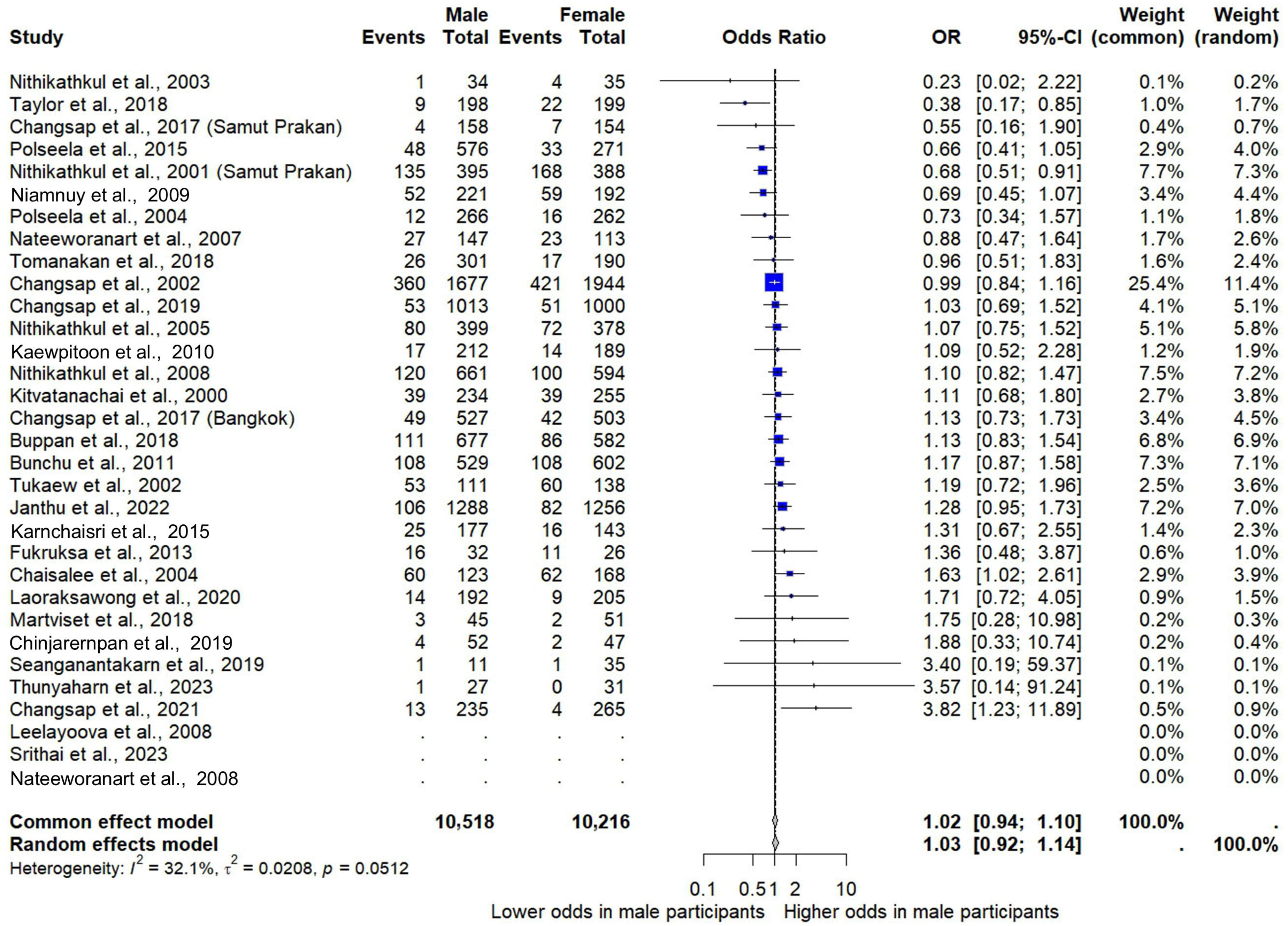
3.5. Gender and Risk of E. vermicularis Infections
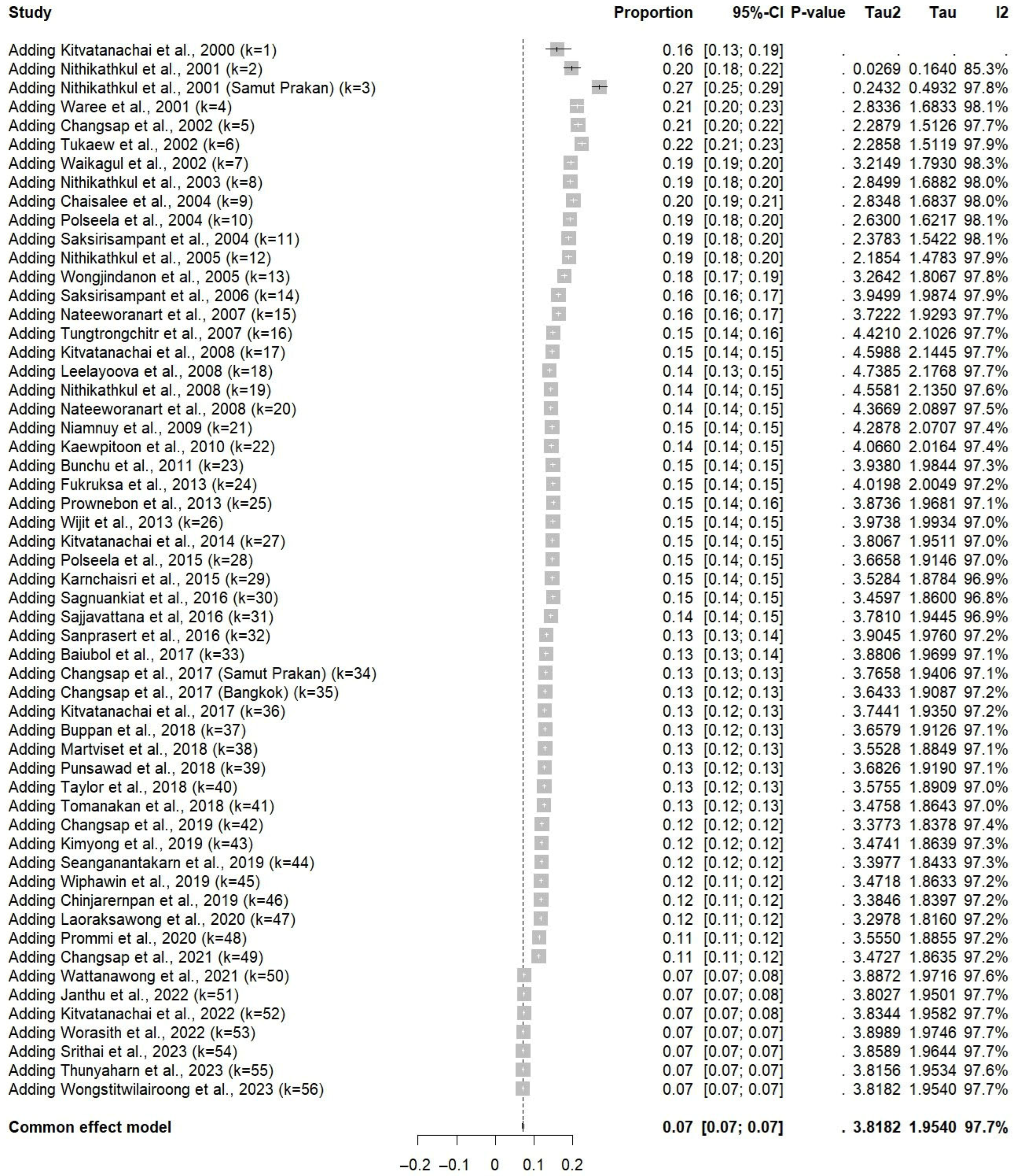
3.6. Sensitivity Analysis
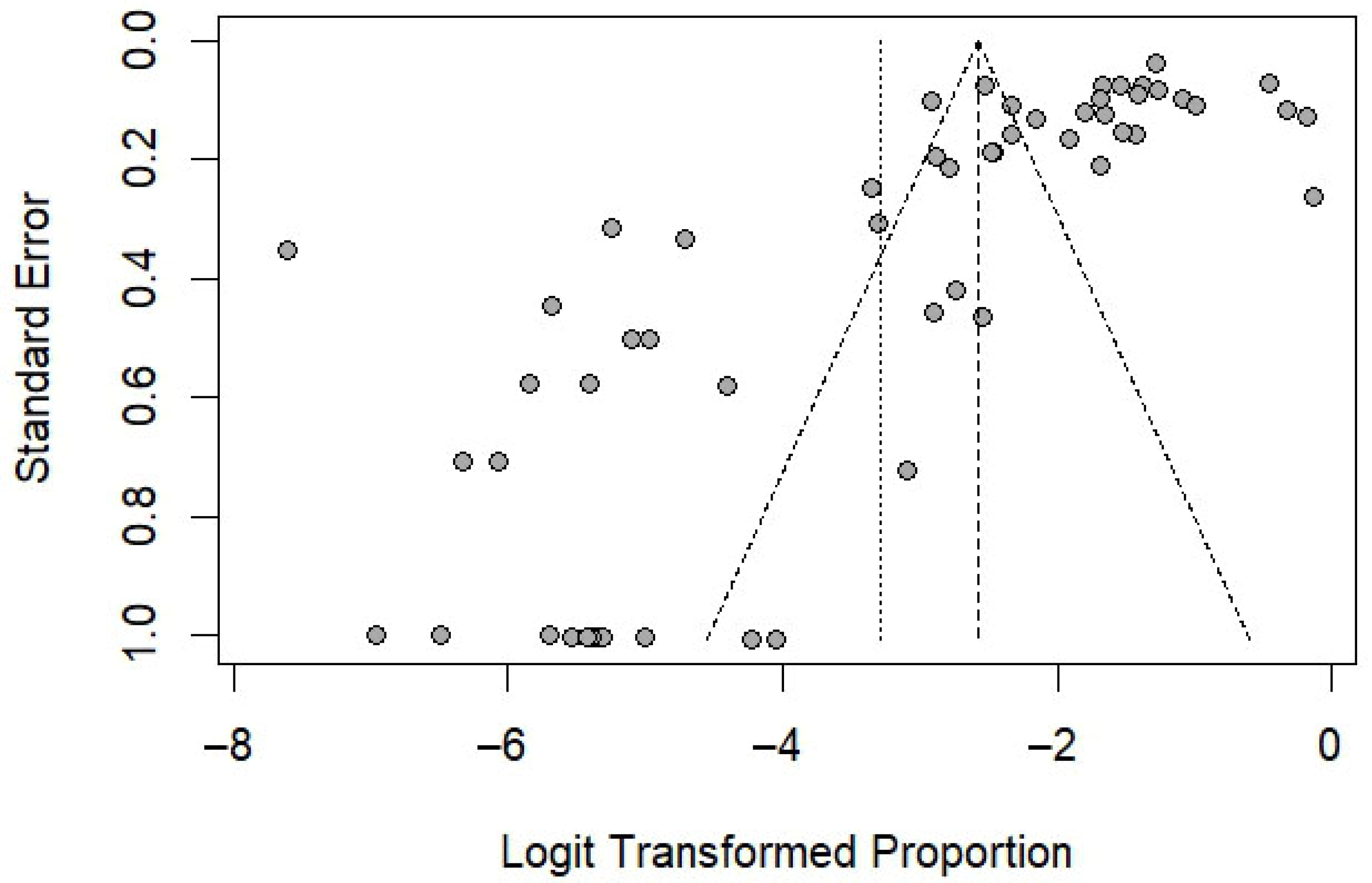
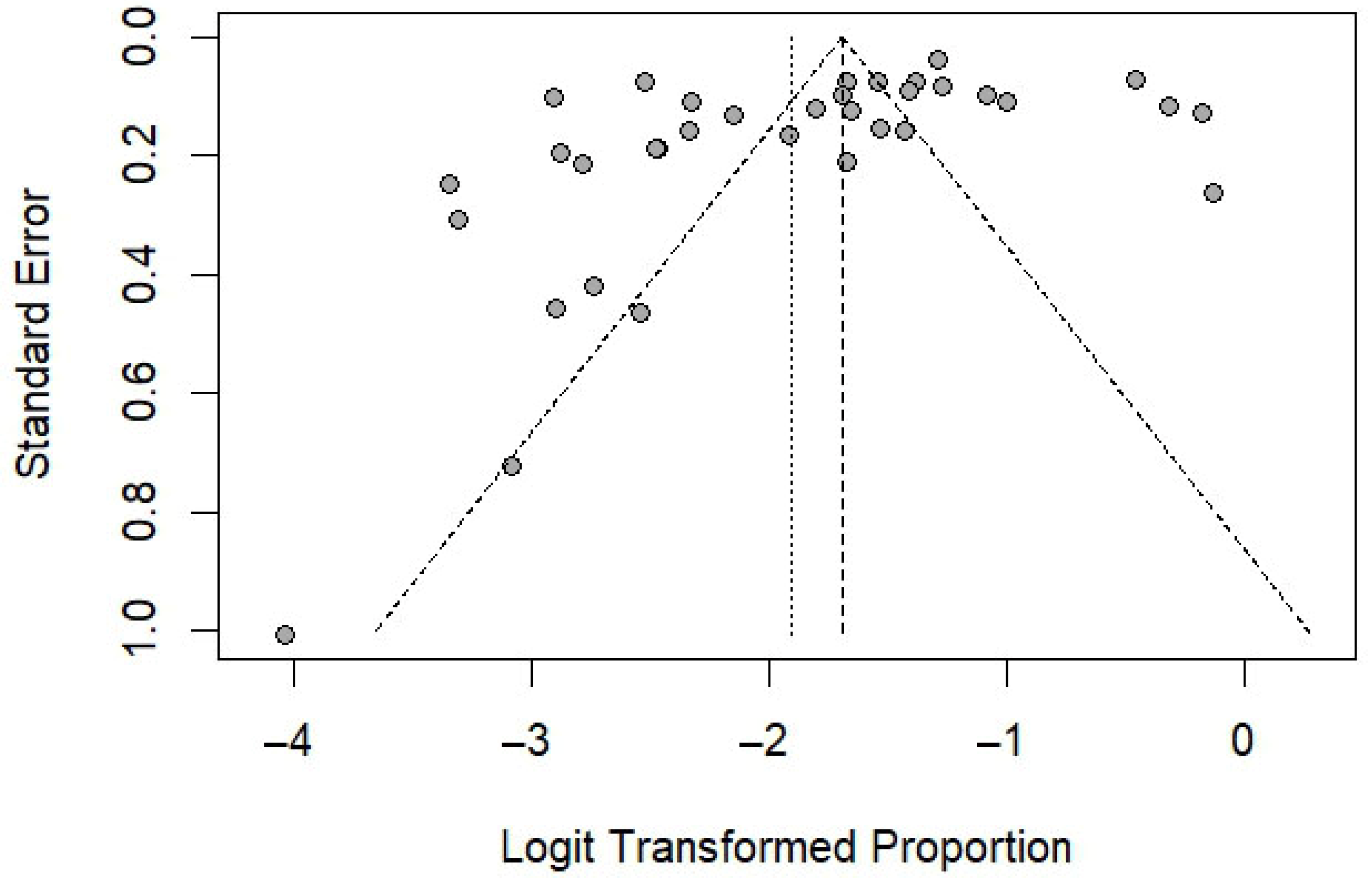
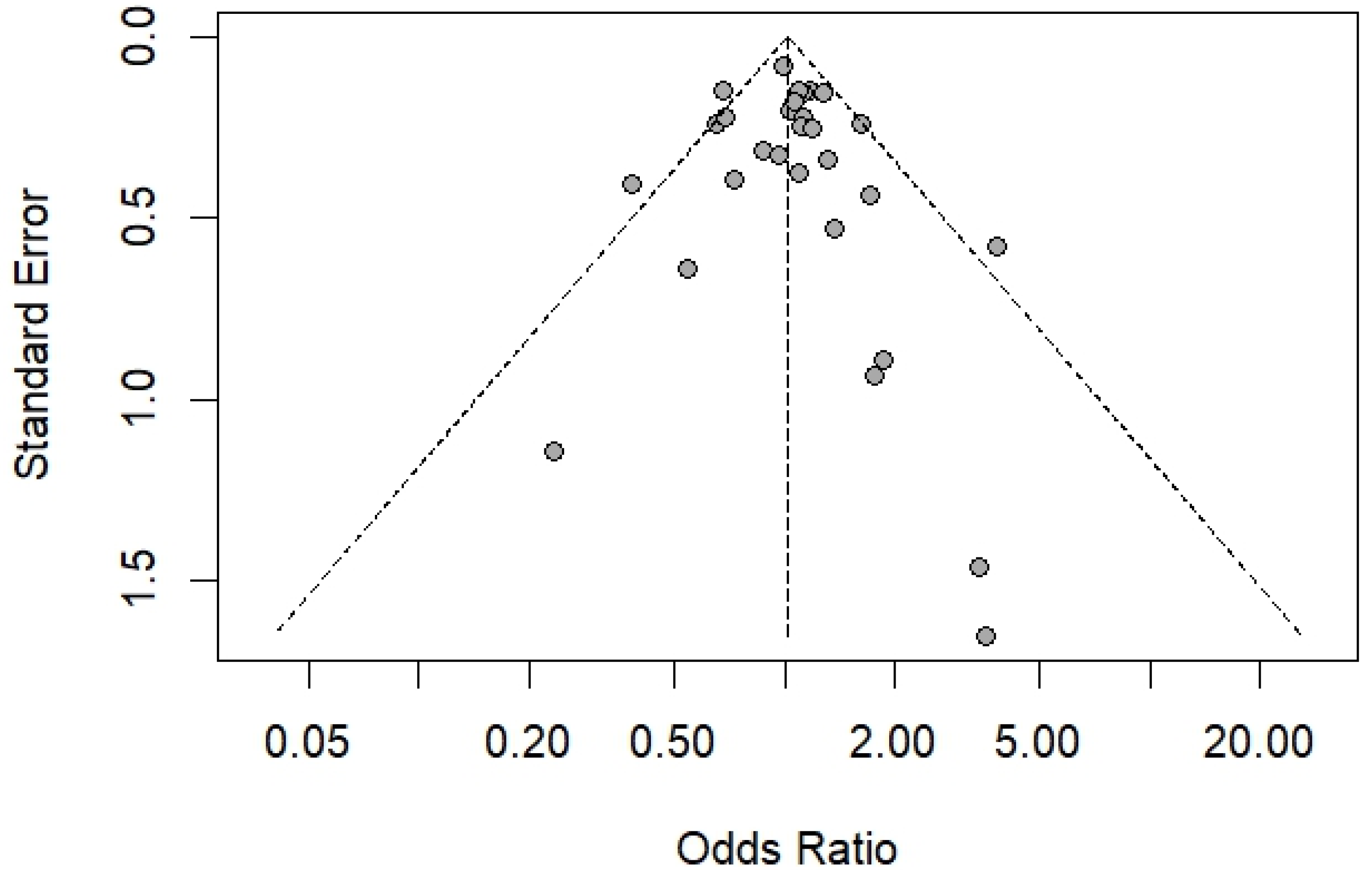
3.7. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cook, G.C. Enterobius vermicularis infection. Gut 1994, 35, 1159–1162. [Google Scholar] [CrossRef]
- Wendt, S.; Trawinski, H.; Schubert, S.; Rodloff, A.C.; Mossner, J.; Lubbert, C. The diagnosis and treatment of pinworm infection. Dtsch. Arztebl. Int. 2019, 116, 213–219. [Google Scholar] [CrossRef] [PubMed]
- CDC. Parasites—Enterobiasis (Also Known as Pinworm Infection). 2020. Available online: https://www.cdc.gov/pinworm/about/?CDC_AAref_Val=https://www.cdc.gov/parasites/pinworm (accessed on 4 August 2025).
- Rawla, P.; Sharma, S. Enterobius vermicularis; [Updated 1 August 2023]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536974/ (accessed on 8 August 2025).
- Chen, K.Y.; Yen, C.M.; Hwang, K.P.; Wang, L.C. Enterobius vermicularis infection and its risk factors among pre-school children in Taipei, Taiwan. J. Microbiol. Immunol. Infect. 2018, 51, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, S.; Al Mamari, A.H.; Al Awfi, M.M.; Al Khaldi, N.H.N.; Abed, N.M.; Pandak, N.; Khamis, F.; Balushi, Z.A.; Alalawi, R.M.K.; Al Lawati, S.; et al. Enterobius vermicularis related acute appendicitis: A case report and review of the literature. Infect. Dis. Rep. 2023, 15, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Adorisio, O.; De Peppo, F.; Rivosecchi, M.; Silveri, M. Enterobius vermicularis as a cause of intestinal occlusion: How to avoid unnecessary surgery. Pediatr. Emerg. Care 2016, 32, 235–236. [Google Scholar] [CrossRef]
- Laoraksawong, P.; Pansuwan, P.; Krongchon, S.; Pongpanitanont, P.; Janwan, P. Prevalence of Enterobius vermicularis infections and associated risk factors among schoolchildren in Nakhon Si Thammarat, Thailand. Trop. Med. Health 2020, 48, 83. [Google Scholar] [CrossRef]
- WHO. Deworming for Health and Development: Report of the Third Global Meeting of the Partners for Parasite Control. 2005. Available online: https://www.who.int/publications/i/item/WHO-CDS-CPE-PVC-2005.14 (accessed on 14 August 2025).
- Waheed, A.M.A. Enterobius vermicularis infection: Prevalence and risk factors among primary school children in Al-mudhafar Directorate, Taiz, Republic of Yemen. Enhanc. Knowl. Sci. Technol. 2022, 2, 441–449. [Google Scholar]
- Lashaki, E.K.; Mizani, A.; Hosseini, S.A.; Habibi, B.; Taherkhani, K.; Javadi, A.; Taremiha, A.; Dodangeh, S. Global prevalence of enterobiasis in young children over the past 20 years: A systematic review and meta-analysis. Osong Public. Health Res. Perspect. 2023, 14, 441–450. [Google Scholar] [CrossRef]
- Tepmongkol, M.; Suntadwoot, C.L. Enterobius infection in young school children in slum Klongtoei. Siriraj Hosp. Gaz. 1980, 32, 597–600. [Google Scholar]
- Saksirisampant, W.; Prownebon, J.; Kanmarnee, P.; Thaisom, S.; Yenthakam, S.; Nuchprayoon, S. Prevalence of parasitism among students of the Karen hill-tribe in Mae Chame district, Chiang Mai province, Thailand. J. Med. Assoc. Thai 2004, 87 (Suppl. 2), S278–S283. [Google Scholar]
- Changsap, B.; Wannapinyosheep, S.; Tantravanich, S.; Plaikaew, K.; Saguansit, P.; Siridet, R. Prevalence of pinworm (Enterobius vermicularis ) infection among preschool and lower primary school children in Bangbo District, Samut Prakarn Province, Thailand. Thai J. Public Health 2019, 49, 221–233. [Google Scholar]
- Sagnuankiat, S.; Wanichsuwan, M.; Bhunnachet, E.; Jungarat, N.; Panraksa, K.; Komalamisra, C.; Maipanich, W.; Yoonuan, T.; Pubampen, S.; Adisakwattana, P.; et al. Health status of immigrant children and environmental survey of child daycare centers in Samut Sakhon Province, Thailand. J. Immigr. Minor. Health 2016, 18, 21–27. [Google Scholar] [CrossRef]
- Taylor, A.; Saichua, P.; Rhongbutsri, P.; Tiengtip, R.; Kitvatanachai, S.; Taylor, W.R.J. A preliminary epidemiological study of pinworm infection in Thaklong Municipal Early Childhood Development Center and Rangsit Babies’ Home, Pathum Thani, Thailand. BMC Res. Notes 2018, 11, 603. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufanaru, C.; Aromataris, E.; Sears, K.; Sfetcu, R.; Currie, M.; Qureshi, R.; Mattis, P.; Lisy, K.; et al. Chapter 7: Systematic reviews of etiology and risk. In JBI Manual for Evidence Synthesis; JBI: Adelaide, Australia, 2020; Available online: https://synthesismanual.jbi.global (accessed on 1 August 2025).
- Sukati, S.; Rattanatham, R.; Masangkay, F.R.; Tseng, C.P.; Kotepui, M. Alterations in von Willebrand factor levels in patients with malaria: A systematic review and meta-analysis of disease severity. Medicina 2025, 61, 767. [Google Scholar] [CrossRef] [PubMed]
- Melo, G.; Dutra, K.L.; Rodrigues Filho, R.; Ortega, A.O.L.; Porporatti, A.L.; Dick, B.; Flores-Mir, C.; De Luca Canto, G. Association between psychotropic medications and presence of sleep bruxism: A systematic review. J. Oral. Rehabil. 2018, 45, 545–554. [Google Scholar] [CrossRef]
- Team R. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 3 August 2025).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4; (Updated August 2023); Cochrane: Los Angeles, CA, USA, 2023; Available online: www.training.cochrane.org/handbook (accessed on 1 August 2025).
- Chaisalee, T.; Tukaew, A.; Wiwanitkit, V.; Suyaphun, A.; Thiamtip, S.; Suwansaksri, J. Very high prevalence of enterobiasis among the hilltribal children in rural district “Mae Suk,” Thailand. Medscape Gen. Med. 2004, 6, 5. [Google Scholar]
- Changsap, B.; Nithikathkul, C.; Boontan, P.; Wannapinyosheep, S.; Vongvanich, N.; Poister, C. Enterobiasis in primary schools in Bang Khun Thian District, Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33 (Suppl. 3), 72–75. [Google Scholar] [PubMed]
- Janthu, P.; Dumidae, A.; Subkrasae, C.; Ardpairin, J.; Nateeworanart, S.; Thanwisai, A.; Vitta, A. Prevalence and genetic analysis of Enterobius vermicularis in schoolchildren in lower northern Thailand. Parasitol. Res. 2022, 121, 2955–2965. [Google Scholar] [CrossRef]
- Kitvatanachai, S.; Boonsilp, S.; Watanasatitarpa, S. Intestinal parasitic infections in Srimum suburban area of Nakhon Ratchasima Province, Thailand. Trop. Biomed. 2008, 25, 237–242. [Google Scholar]
- Kitvatanachai, S.; Kritsiriwutthinan, K.; Taylor, A.; Rhongbutsri, P. Modified nonnutrient agar plate culture for the diagnosis of Strongyloides stercoralis and hookworm infections in La-Ngu District, Satun Province, Southern Thailand. J. Parasitol. Res. 2022, 2022, 1117400. [Google Scholar] [CrossRef]
- Kitvatanachai, S.; Taylor, A.; Rhongbutsri, P.; Pongstaporn, W. Determine the prevalence of intestinal and soil-transmitted helminths using different copromicroscopic techniques in Krabi Province, Thailand. Asian Pac. J. Trop. Dis. 2017, 7, 719–723. [Google Scholar] [CrossRef]
- Leelayoova, S.; Siripattanapipong, S.; Thathaisong, U.; Naaglor, T.; Taamasri, P.; Piyaraj, P.; Mungthin, M. Drinking water: A possible source of blastocystis spp. subtype 1 infection in schoolchildren of a rural community in central Thailand. Am. J. Trop. Med. Hyg. 2008, 79, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Nithikadikul, C.; Sukthana, Y.; Wongsawad, C.; Nithikathkul, A.; Nithikethkul, B.; Wichmann, O.; Gonzalez, J.P.; Hugot, J.P.; Herbreteau, V. Enterobiasis infections among Thai school children: Spatial analysis using a geographic information system. Asian Biomed. 2008, 2, 283–288. [Google Scholar]
- Nithikathkul, C.; Akarachantachote, N.; Wannapinyosheep, S.; Pumdonming, W.; Brodsky, M.; Sukthana, Y. Impact of health educational programmes on the prevalence of enterobiasis in schoolchildren in Thailand. J. Helminthol. 2005, 79, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Nithikathkul, C.; Changsap, B.; Wannapinyosheep, S.; Poister, C.; Boontan, P. The prevalence of enterobiasis in children attending mobile health clinic of Huachiew Chalermprakiet University. Southeast Asian J. Trop. Med. Public Health 2001, 32 (Suppl. 2), 138–142. [Google Scholar] [PubMed]
- Nithikathkul, C.; Changsap, B.; Wannapinyosheep, S.; Poister, C.; Boontan, P. The prevalence of Enterobius vermicularis among primary school students in Samut Prakan Province, Thailand. Southeast Asian J. Trop. Med. Public Health 2001, 32 (Suppl. S2), 133–137. [Google Scholar]
- Nithikathkul, C.; Polseela, P.; Poodendan, W.; Brodsky, M.; Rakprapapant, D.; Chadchatreechan, S.; Phethleart, A.; Sukthana, Y.; Leemingsawat, S. Malaria and enterobiasis among Karen Long-neck tribe in Mae Hong Son Province. Southeast Asian J. Trop. Med. Public Health 2003, 34 (Suppl. S2), 25–28. [Google Scholar]
- Polseela, P.; Poodendan, W.; Tangchaisuriya, U.; Nithikathkul, C.; Arnat, N.; Pannarunotha, S.; Radomyos, P. Parasitic infection among primary school students in Meuang District, Phitsanulok Province, Thailand. Southeast Asian J. Trop. Med. Public Health 2004, 35, 120–122. [Google Scholar]
- Polseela, R.; Vitta, A. Prevalence of intestinal parasitic infections among schoolchildren in Phitsanulok Province, Northern Thailand. Asian Pac. J. Trop. Dis. 2015, 5, 539–542. [Google Scholar] [CrossRef]
- Prommi, A.; Prombutara, P.; Watthanakulpanich, D.; Adisakwattana, P.; Kusolsuk, T.; Yoonuan, T.; Poodeepiyasawat, A.; Homsuwan, N.; Prummongkol, S.; Tanita, M.; et al. Intestinal parasites in rural communities in Nan Province, Thailand: Changes in bacterial gut microbiota associated with minute intestinal fluke infection. Parasitology 2020, 147, 972–984. [Google Scholar] [CrossRef]
- Prownebon, J.; Charupoonphol, P.; Saksirisampant, P.; Limvorapitak, T.; Seepongpun, U.; Saksirisampant, W. Intestinal parasitic infections: High prevalence of Giardia intestinalis in children living in an orphanage compared with hill-tribe children as detected by microscopy and ELISA. Asian Biomed. 2013, 7, 855–863. [Google Scholar]
- Punsawad, C.; Phasuk, N.; Bunratsami, S.; Thongtup, K.; Viriyavejakul, P.; Palipoch, S.; Koomhin, P.; Nongnaul, S. Prevalence of intestinal parasitic infections and associated risk factors for hookworm infections among primary schoolchildren in rural areas of Nakhon Si Thammarat, southern Thailand. BMC Public Health 2018, 18, 1118. [Google Scholar] [CrossRef]
- Saksirisampant, W.; Prownebon, J.; Kulkumthorn, M.; Yenthakam, S.; Janpla, S.; Nuchprayoon, S. Prevalence of intestinal parasitic infections among school children in the central region of Thailand. J. Med. Assoc. Thai. 2006, 89, 1928–1933. [Google Scholar]
- Sanprasert, V.; Srichaipon, N.; Bunkasem, U.; Srirungruang, S.; Nuchprayoon, S. Prevalence of intestinal protozoan infections among children in Thailand: A large-scale screening and comparative study of three standard detection methods. Southeast Asian J. Trop. Med. Public Health 2016, 47, 1123–1133. [Google Scholar]
- Tomanakan, K.; Sanpool, O.; Chamavit, P.; Lulitanond, V.; Intapan, P.M.; Maleewong, W. Genetic variation of Enterobius vermicularis among schoolchildren in Thailand. J. Helminthol. 2018, 94, e7. [Google Scholar] [CrossRef]
- Tukaew, A.; Chaisalee, T.; Nithiuthai, S.; Thiamtip, S.; Suyaphun, A.; Wiwanitkit, V.; Suwansaksri, J. Enterobius vermicularis infection among pre-school children in Karen hilltribe villages in Chiang Mai, Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33 (Suppl. 3), 70–71. [Google Scholar] [PubMed]
- Tungtrongchitr, A.; Chiworaporn, C.; Praewanich, R.; Radomyos, P.; Boitano, J.J. The potential usefulness of the modified Kato thick smear technique in the detection of intestinal sarcocystosis during field surveys. Southeast Asian J. Trop. Med. Public Health 2007, 38, 232–238. [Google Scholar] [PubMed]
- Waikagul, J.; Krudsood, S.; Radomyos, P.; Radomyos, B.; Chalemrut, K.; Jonsuksuntigul, P.; Kojima, S.; Looareesuwan, S.; Thaineau, W. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan Province, Northern Thailand. Southeast Asian J. Trop. Med. Public Health 2002, 33, 218–223. [Google Scholar]
- Waree, P.; Polseela, P.; Pannarunothai, S.; Pipitgool, V. The present situation of paragonimiasis in endemic area in Phitsanulok Province. Southeast Asian J. Trop. Med. Public Health 2001, 32 (Suppl. S2), 51–54. [Google Scholar] [PubMed]
- Wattanawong, O.; Iamsirithaworn, S.; Kophachon, T.; Nak-ai, W.; Wisetmora, A.; Wongsaroj, T.; Dekumyoy, P.; Nithikathkul, C.; Suwannatrai, A.T.; Sripa, B. Current status of helminthiases in Thailand: A cross-sectional, nationwide survey, 2019. Acta Trop. 2021, 223, 106082. [Google Scholar] [CrossRef] [PubMed]
- Wijit, A.; Morakote, N.; Klinchid, J. High prevalence of haplorchiasis in Nan and Lampang provinces, Thailand, proven by adult worm recovery from suspected opisthorchiasis cases. Korean J. Parasitol. 2013, 51, 767–769. [Google Scholar] [CrossRef] [PubMed]
- Wongjindanon, N.; Suksrichavalit, T.; Subsutti, W.; Sarachart, T.; Worapisuttiwong, U.; Norramatha, P. Current infection rate of Giardia lamblia in two provinces of Thailand. Southeast Asian J. Trop. Med. Public Health 2005, 36 (Suppl. S4), 21–25. [Google Scholar]
- Wongstitwilairoong, B.; Anothaisintawee, T.; Ruamsap, N.; Lertsethtakarn, P.; Kietsiri, P.; Oransathid, W.; Oransathid, W.; Gonwong, S.; Silapong, S.; Suksawad, U.; et al. Prevalence of intestinal parasitic infections, genotypes, and drug susceptibility of giardia lamblia among preschool and school-aged children: A cross-sectional study in Thailand. Trop. Med. Infect. Dis. 2023, 8, 394. [Google Scholar] [CrossRef]
- Worasith, C.; Wongphutorn, P.; Homwong, C.; Kopolrat, K.Y.; Techasen, A.; Thanan, R.; Eamudomkarn, C.; Wangboon, C.; Khuntikeo, N.; Loilome, W.; et al. Effects of day-to-day variation of Opisthorchis viverrini antigen in urine on the accuracy of diagnosing opisthorchiasis in Northeast Thailand. PLoS ONE 2022, 17, e0271553. [Google Scholar] [CrossRef]
- Baiubol, P.; Wiriyawattana, C.; Tayanram, S.; Suradej, B.; Srisuwan, W. The prevalent of intestinal parasite infestation among primary school students in a district. Health Sci. Sci. Technol. Rev. 2017, 10, 12–14. [Google Scholar]
- Bunchu, N.; Vitta, A.; Thongwat, D.; Lamlertthon, S.; Pimolsri, U.; Waree, P.; Wongwigkarn, J.; Khamsri, B.; Cheewapat, R.; Wichai, S.; et al. Enterobius vermicularis infection among children in lower northern Thailand. J. Trop. Med. Parasitol. 2011, 34, 36–40. [Google Scholar]
- Buppan, P.; Kosuwin, R.; Srimee, P. Infection rate of Enterobius vermicularis in Elementary school Students 1–3, Ongkharak District, Nakhonnayok Province. Thammasat Med. J. 2018, 18, 186–192. [Google Scholar]
- Changsap, B.; Mongkolcharatchai, K.; Choosakul, K.; Teansuwang, M.; Komyan, S.; Nithiketkul, C. The infection rate of Enterobius vermicularis among children in Bang Saothong district, Samutprakan province. Huachiew Chalermprakiet Sci. Technol. J. 2017, 3, 34–42. [Google Scholar]
- Changsap, B.; Wannapinyosheep, S.; Nithikathkul, C.; Tangpong, J. A survey on enterobiasis along with potential risk factors among children in Khlong Toei Community, Bangkok and comparative study of the past. J. Health Sci. Thail. 2017, 26, 829–837. [Google Scholar]
- Kimyong, T.; Chuangchaiya, S.; Laoprom, N.; Raiyawa, N.; Patthaisong, W.; Phromnophas, T.; Roobkom, P. Prevalence and risk factors for Opisthorchis viverrini infection in the That Phanom district, Nakhon Phanom Province, Thailand. Res. Dev. Health Syst. J. 2019, 12, 271–279. [Google Scholar]
- Kitvatanachai, S.; Rhongbutsri, P. Pinworm infections in suburban government schools in Lak Hok Subdistrict, Muang Patumthani, Thailand. J. Curr. Sci. Technol. 2014, 4, 117–122. [Google Scholar]
- Martviset, P.; Kitvatanachai, S.; Watanasatitarpa, S.; Trakulsomboon, S.; Bunchaleamchai, A. Intestinal parasitic infection among school age students in Lakhok subdistrict, Pathumthani province, Thailand. Thammasat Med. J. 2018, 18, 179–185. [Google Scholar]
- Sajjavattana, T.; Sungkhabut, W. Spatial distribution and risk behaviors of parasitic helminth infection among students in the schools under the Border Patrol Police Sub-division 21. J. Health Syst. Res. 2016, 10, 394–401. [Google Scholar]
- Srithai, C.; Chuangchaiya, S.; Ponrachom, C.; Khabuankeaw, N.; Turyghai, S.; Sodamuk, P.; Wongkhammang, A.; Oopkaew, S. Prevalence and risk factors for opisthorchiasis in Nakhamin Sub-District, Phonsawan District, Nakhon Phanom Province, Thailand. J. Bamrasnaradura Infect. Dis. Inst. 2023, 17, 171–181. [Google Scholar]
- Thunyaharn, S.; Yingsiwaphat, V.; Saichanma, S.; Silasaeng, N.; Yusoh, N.; Ngoenprong, S.; Ayohsae, F.; Sarutipaiboon, I.; Paenganan, P.; Sungsirin, N. Prevalence and related factors of pinworm infection in preschool children of Ban Mai Municipal Child Development Center, Nakhon Ratchasima Province, Thailand. Prog. Appl. Sci. Tech. 2023, 13, 1–8. [Google Scholar]
- Vipawin, C.; Laopromo, N.; Chuangchaiya, S.; Banchonhattakit, P.; Kraiklang, R.; Phromnophas, T.; Ongarj, P. The prevalence of Opisthorchis viverrini and knowledges behaviors attitudes about opisthorchiasis among people living near Songkhram River and prevalence of metacercariae in cyprinoid fish in Songkhram River, Nakhon Phanom Province. Res. Dev. Health Syst. J. 2019, 12, 546–560. [Google Scholar]
- Karnchaisri, K.; Sareebot, T. The infection rate and risk factors of Enterobius vermicularis in school children in Donyaihom Sub-district, Mueang Nakhon Pathom District, Nakhon Pathom Province. Christ. Univ. Thail. J. 2015, 21, 282–293. [Google Scholar]
- Kaewpitoon, N.; Kaewpitoon, S.J. Enterobius vermiclaris infection among pre-school children in Warinchamrap District, Ubonratchathani Province. J. Sci. Technol. Ubon Ratchathani Univ. 2010, 12, 47–53. [Google Scholar]
- Nateeworanart, S.; Limmongkon, A.; Sanpool, O.; Homcharoen, K.; Kornpanichsakun, M.; Khamla, K.; Athi, T. Prevalence of Enterobius vermicularis infection among Hmong schoolchildren at Ban Nam Chuang School, Chat Trakan District, Phitsanulok Province (a preliminary study) [Translated Title]. Bull. Chiang Mai Assoc. Med. Sci. 2008, 41, 46. [Google Scholar]
- Niamnuy, N.; Phothikham, J.; Niwo, T.; Towae, S. Prevalence rates of Enterobius vermicularis in school children in Amphur Bang-Plee, Samutprakarn, and Burirum province. Adv. Sci. J. 2009, 9, 162–168. [Google Scholar]
- Changsap, B.; Piapinthong, A.; Puttanantadet, B.; Kaythong, J.; Kanjanavas, P.; Choombuathong, A.; Bangsumruaj, J. Survey on prevalence of Enterobius vermicularis among children in Bang Nam Priao district, Chachoengsao province, Thailand. Dis. Control. J. 2021, 47, 839–847. [Google Scholar] [CrossRef]
- Fukruksa, C.; Limmongkon, A.; Watcharasupat, T.; Tummeepuk, R.; Yotpanya, W.; Yimthin, T.; Eamsaard, W.; Vitta, A. Prevalence of intestinal parasites in people of Ban Pang Sa, Satchanalai District, Sukhothai Province. J. Sci. Technol. MSU 2013, 32, 794–800. [Google Scholar]
- Kitvatanachai, S.; Marujiwat, K.; Petabut, N.; Thawornpol, K. Enterobius vermicularis infection among children living in orphanages in Bangkok and Pathum Thani Province, Thailand. J. Trop. Med. Parasitol. 2000, 23, 28–31. [Google Scholar]
- Nateeworanart, S.; Pimolsri, U.; Vitta, A.; Soypetcasem, S.; Thongthung, A.; Meepayoong, T. Prevalence of Enterobius vermicularis infection in students of rural areas of Tak province. Thammasat Med. J. 2007, 7, 140–143. [Google Scholar]
- Seanganantakarn, P.; Nak-ung, S.; Phokham, S.; Nateeworanart, S. Pinworm infection rate among schoolchildren of Ban Sung Men School, Mae Sin Subdistrict, Si Satchanalai District, Sukhothai Province. Forensic Med. J. 2019, 11, 18–24. [Google Scholar]
- Chinjarernpan, P.; Yingsiwaphat, V.; Thongsuk, P.; Saowana, S.; Matrakool, B.; Panyasai, K.; Kraibamrung, S.; Saichanma, S. Enterobius vermicularis infections in students in Bann Klongbong School, Amphoe Wang Nam Khiao, Nakhon Ratchasima Province. In Proceedings of the 6th National Conference Journal of Nakhonratchasima College, Nakhon Ratchasima, Thailand, 30 March 2019. [Google Scholar]
- Jin, H.; Ryu, K.; Lee, D.; Vonghachack, Y.; Choi, M.H.; Hong, S.T.; Song, H.B. Prevalence and risk factors of intestinal helminthiasis in remote mountainous villages of northern Lao PDR: A cross-sectional study. Korean J. Parasitol. 2021, 59, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Nanthavong, N.; Black, A.P.; Khattignavong, P.; Lorphachan, L.; Vilivong, K.; Goossens, S.; Buisson, Y.; Quet, F.; Muller, C.P.; Nakamura, S. High prevalence of intestinal worms in children up to 5 years of age in Huaphan province, Lao People’s Democratic Republic (PDR). Parasite Epidemiol. Control 2017, 2, 114–117. [Google Scholar] [CrossRef]
- Yong, T.S.; Chai, J.Y.; Sohn, W.M.; Eom, K.S.; Jeoung, H.G.; Hoang, E.H.; Yoon, C.H.; Jung, B.K.; Lee, S.H.; Sinuon, M.; et al. Prevalence of intestinal helminths among inhabitants of Cambodia (2006–2011). Korean J. Parasitol. 2014, 52, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.Y.; Yang, S.K.; Kim, J.W.; Choi, S.L.; Song, G.Y.; Jung, B.K.; Kim, M.J.; Cho, J.; Kim, D.G.; Sohn, W.M.; et al. High Prevalence of Enterobius vermicularis infection among schoolchildren in three townships around Yangon, Myanmar. Korean J. Parasitol. 2015, 53, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Bamrungkhul, S.; Tanaka, T. Until the wilting day: An analysis of urban population changes in provincial cities in Thailand from 2010 to 2019. J. Asian Archit. Build. Eng. 2023, 22, 1244–1267. [Google Scholar] [CrossRef]
- Losiri, C.; Nagai, M. Identification of urban expansion patterns in Bangkok metropolitan region through time series of landsat images and landscape metrics. In Geoinformatics for Sustainable Development in Asian Cities; Springer: Cham, Switzerland, 2020; pp. 32–45. [Google Scholar]
- Leung, A.K.C.; Lam, J.M.; Barankin, B.; Wong, A.H.C.; Leong, K.F.; Hon, K.L. Pinworm (Enterobius vermicularis ) infestation: An updated review. Curr. Pediatr. Rev. 2024, 21, 333–347. [Google Scholar] [CrossRef]
- Özdil, K.; Karataş, N.; Zincir, H. Low socioeconomic level and Enterobius vermicularis: A interventional study to children and their mothers in home. Zoonoses Public Health 2020, 67, 882–891. [Google Scholar] [CrossRef]
- Kim, D.H.; Yu, H.S. Effect of a one-off educational session about enterobiasis on knowledge, preventative practices, and infection rates among schoolchildren in South Korea. PLoS ONE 2014, 9, e112149. [Google Scholar] [CrossRef][Green Version]
| Publication Year | n | % |
|---|---|---|
| 23 | 41.1 |
| 25 | 44.6 |
| 8 | 14.3 |
| Parts of Thailand | ||
| 25 | 44.6 |
| 10 | 17.9 |
| 8 | 14.3 |
| 4 | 7.14 |
| 2 | 3.57 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| Participants | ||
| 34 | 60.7 |
| 12 | 21.4 |
| 2 | 3.57 |
| 2 | 3.57 |
| 2 | 3.57 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| 1 | 1.79 |
| Age groups | ||
| 42 | 75.0 |
| 5 | 8.93 |
| 9 | 16.1 |
| Detection method | ||
| 35 | 62.5 |
| 21 | 37.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jongthawin, J.; Mahittikorn, A.; Suwannatrai, A.T.; Rattanawan, C.; Wangdi, K.; Masangkay, F.R.; Kotepui, M. Prevalence and Epidemiological Patterns of Enterobius vermicularis Infection in Thailand: A Systematic Review and Meta-Analysis. Med. Sci. 2025, 13, 207. https://doi.org/10.3390/medsci13040207
Jongthawin J, Mahittikorn A, Suwannatrai AT, Rattanawan C, Wangdi K, Masangkay FR, Kotepui M. Prevalence and Epidemiological Patterns of Enterobius vermicularis Infection in Thailand: A Systematic Review and Meta-Analysis. Medical Sciences. 2025; 13(4):207. https://doi.org/10.3390/medsci13040207
Chicago/Turabian StyleJongthawin, Jurairat, Aongart Mahittikorn, Apiporn Thinkhamrop Suwannatrai, Chutima Rattanawan, Kinley Wangdi, Frederick Ramirez Masangkay, and Manas Kotepui. 2025. "Prevalence and Epidemiological Patterns of Enterobius vermicularis Infection in Thailand: A Systematic Review and Meta-Analysis" Medical Sciences 13, no. 4: 207. https://doi.org/10.3390/medsci13040207
APA StyleJongthawin, J., Mahittikorn, A., Suwannatrai, A. T., Rattanawan, C., Wangdi, K., Masangkay, F. R., & Kotepui, M. (2025). Prevalence and Epidemiological Patterns of Enterobius vermicularis Infection in Thailand: A Systematic Review and Meta-Analysis. Medical Sciences, 13(4), 207. https://doi.org/10.3390/medsci13040207






