Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23
Abstract
1. Introduction
2. Materials and Methods
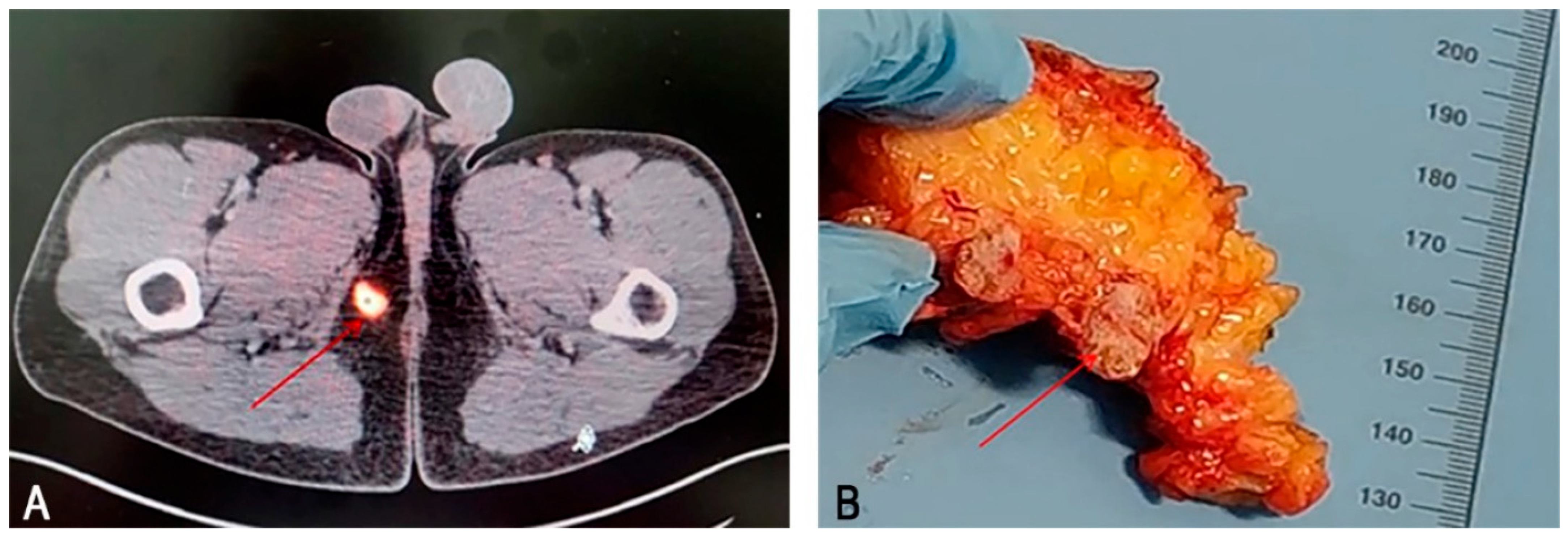
2.1. Case Report
2.2. Sample Preparation
2.3. Immunohistochemistry (IHC) and Histochemistry
2.4. Controls
2.5. Image Acquisition
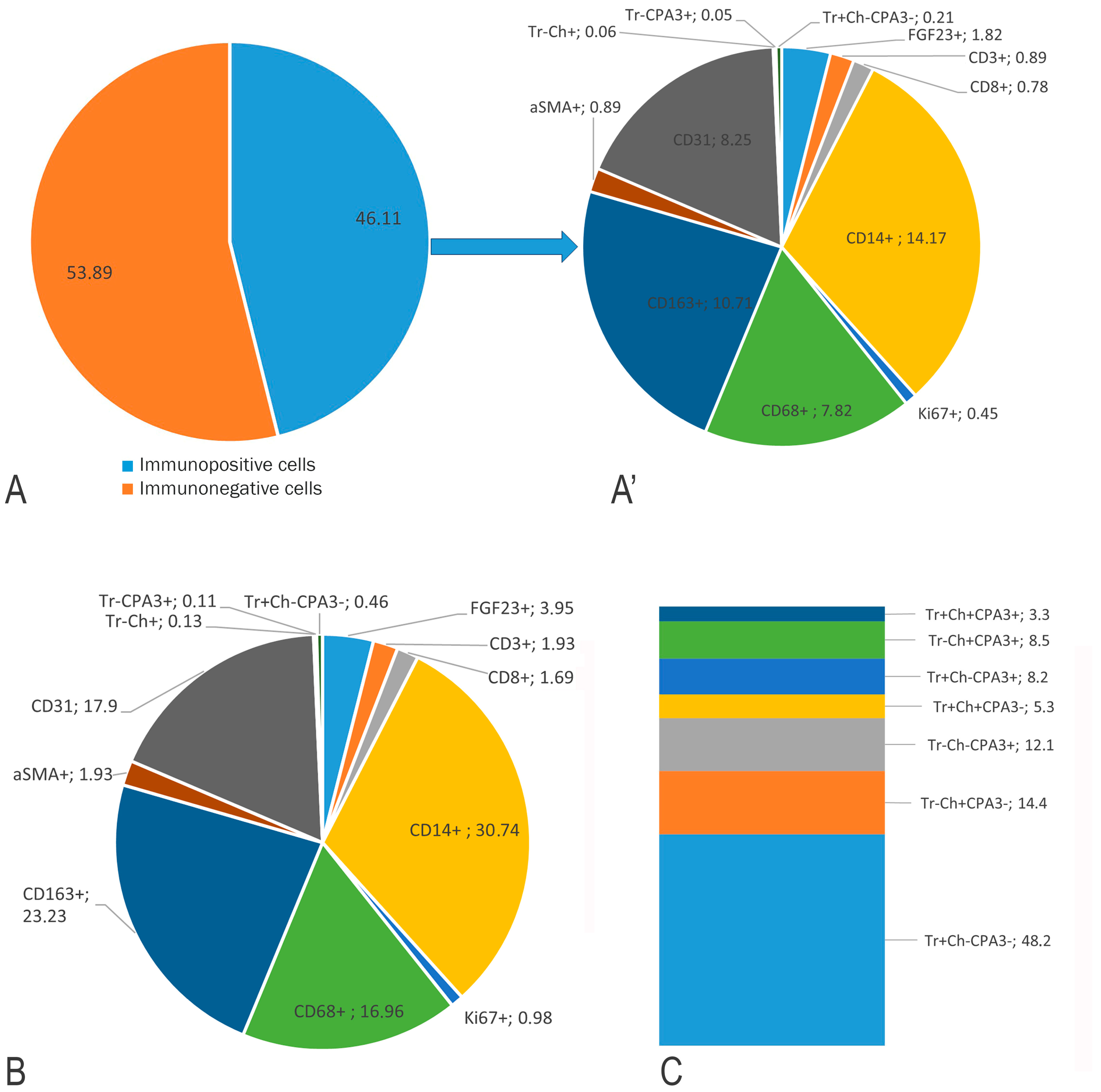
2.6. Quantitative Analysis
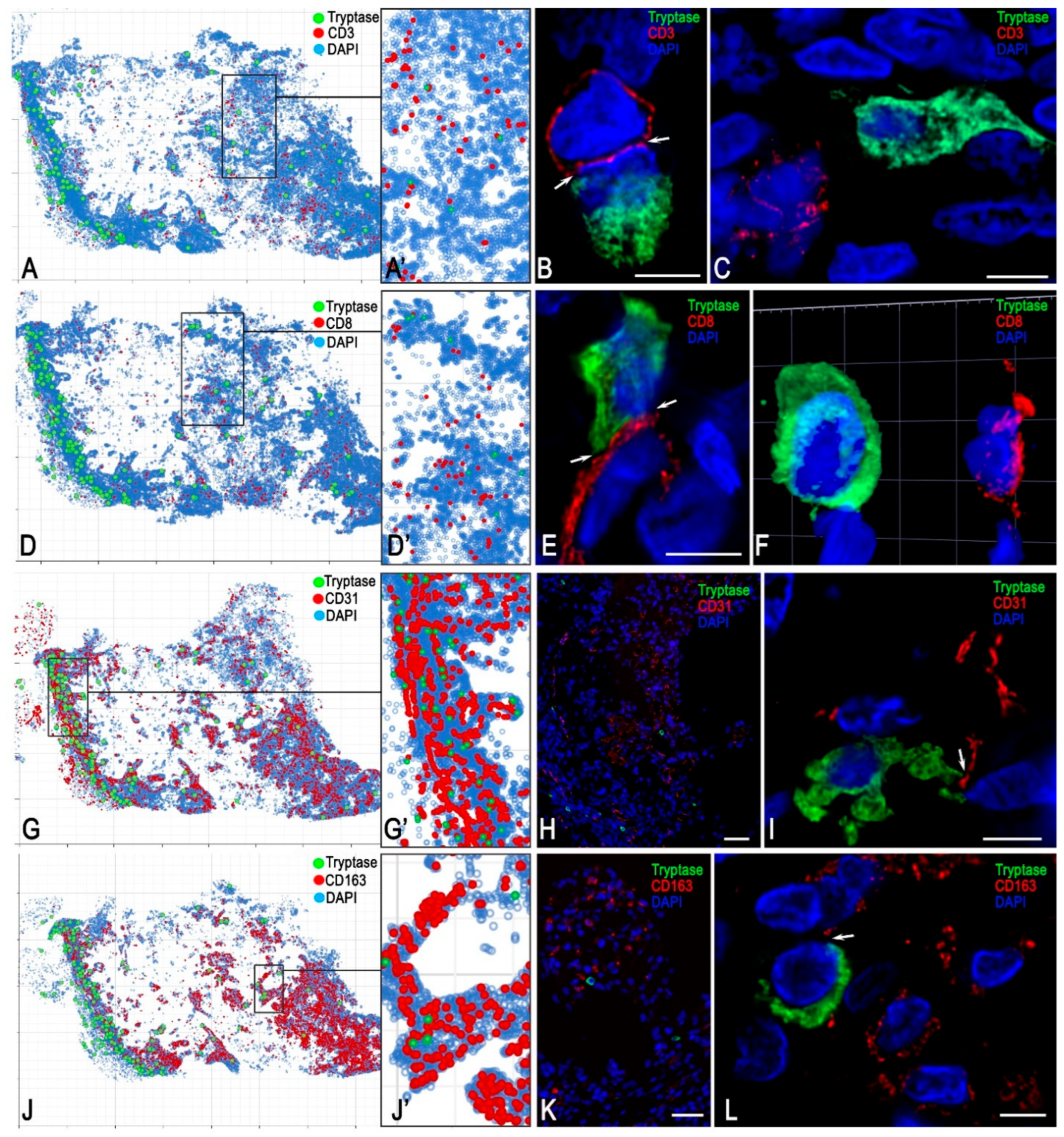
3. Results
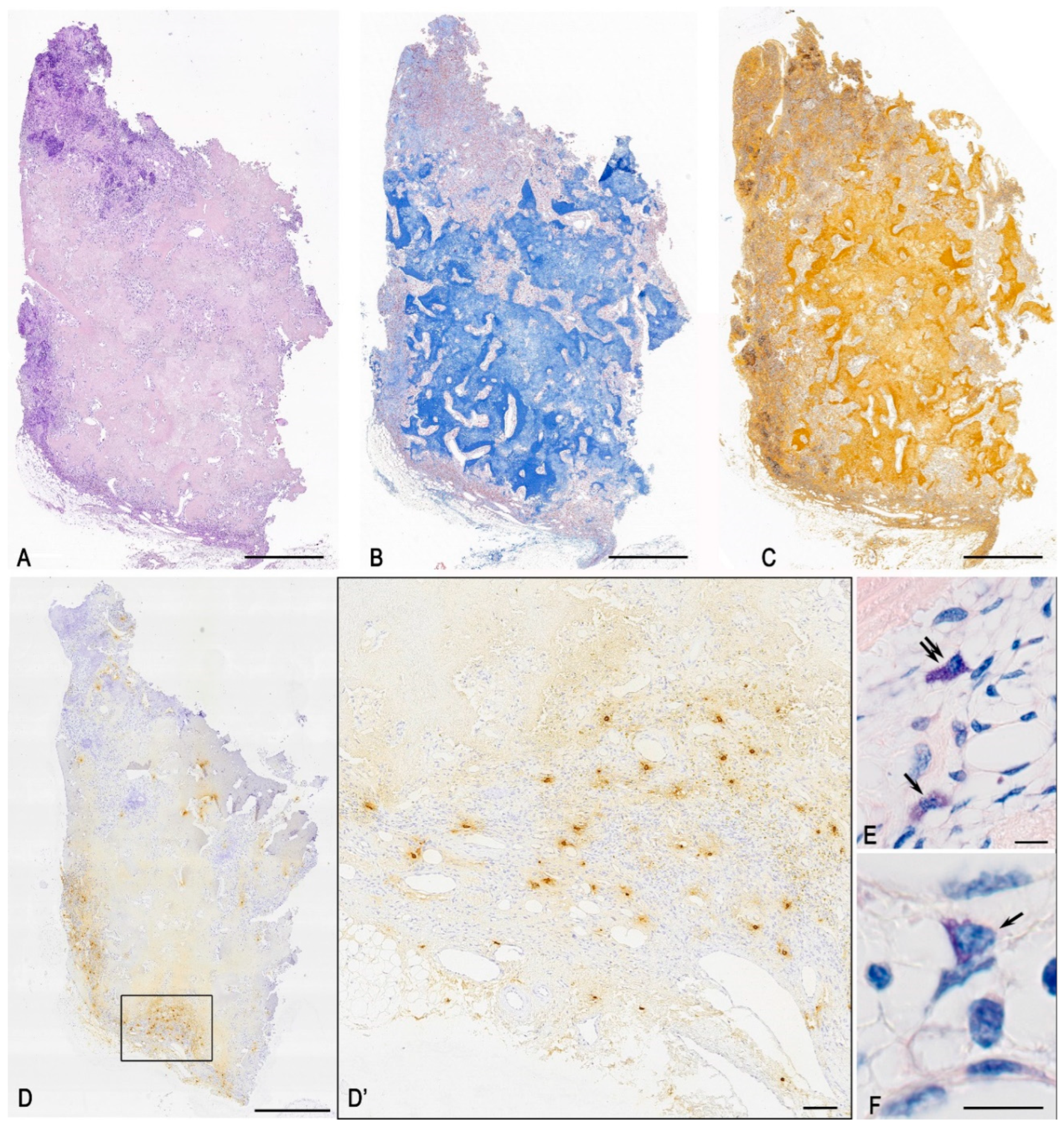
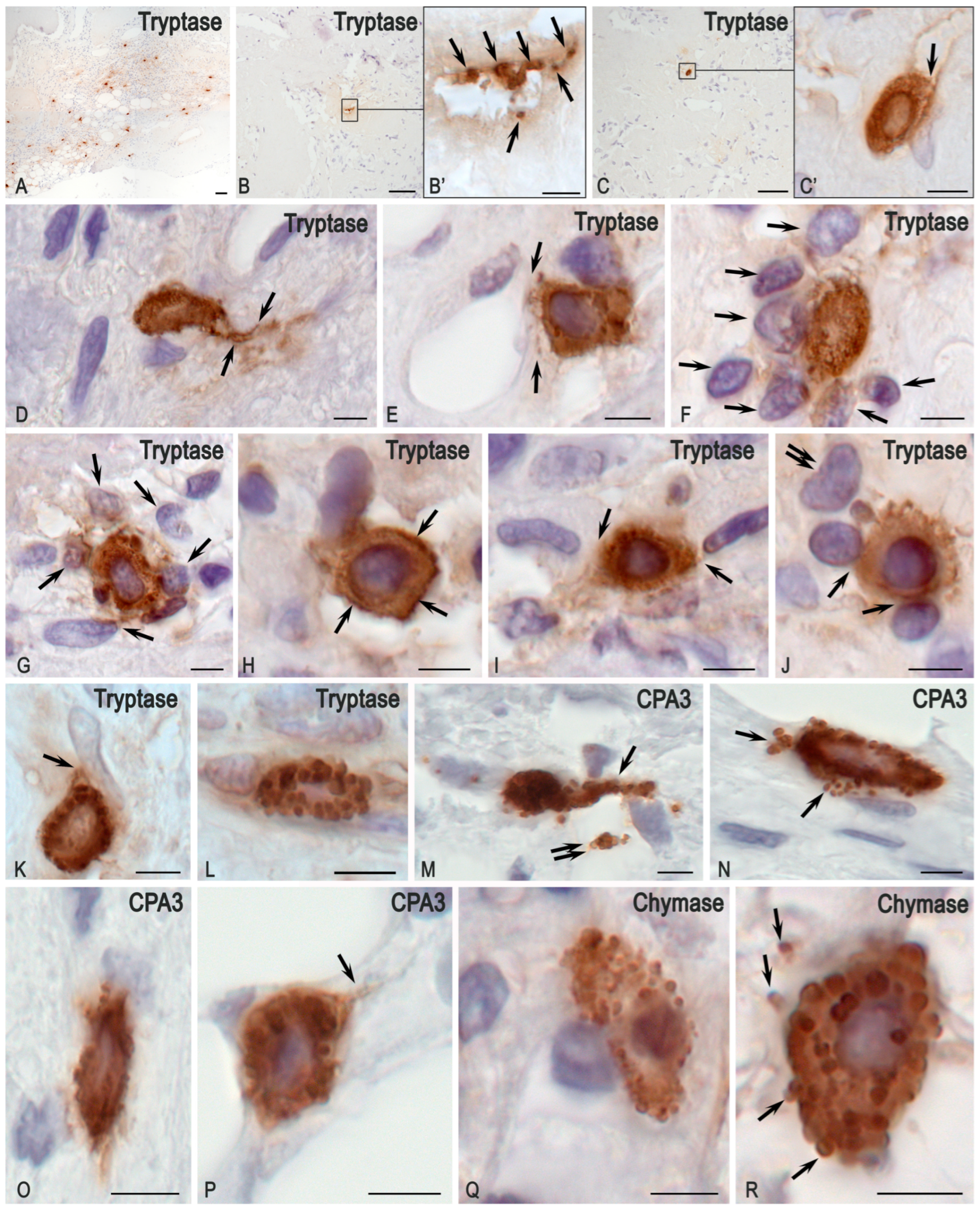
3.1. Histochemical Analysis
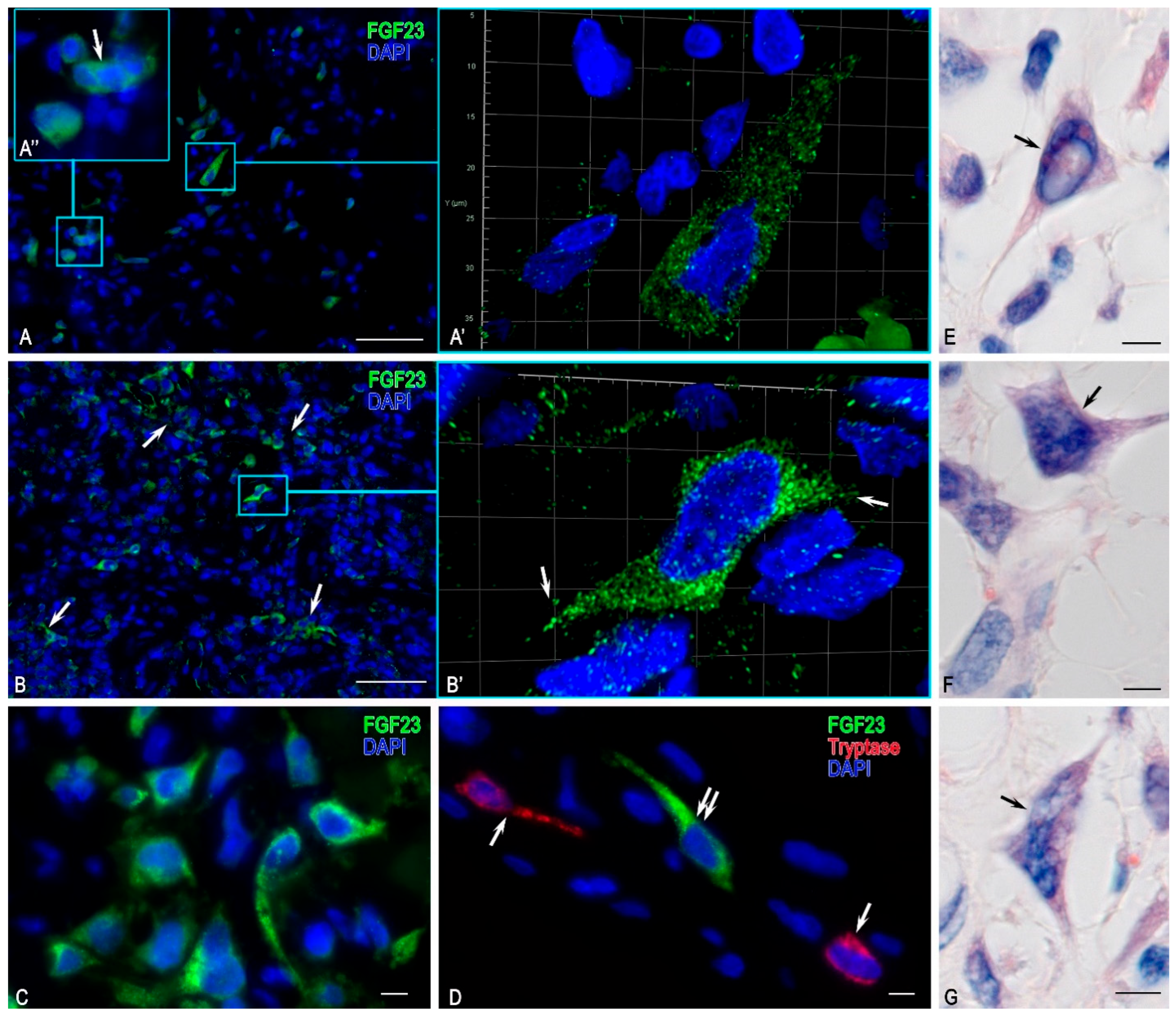
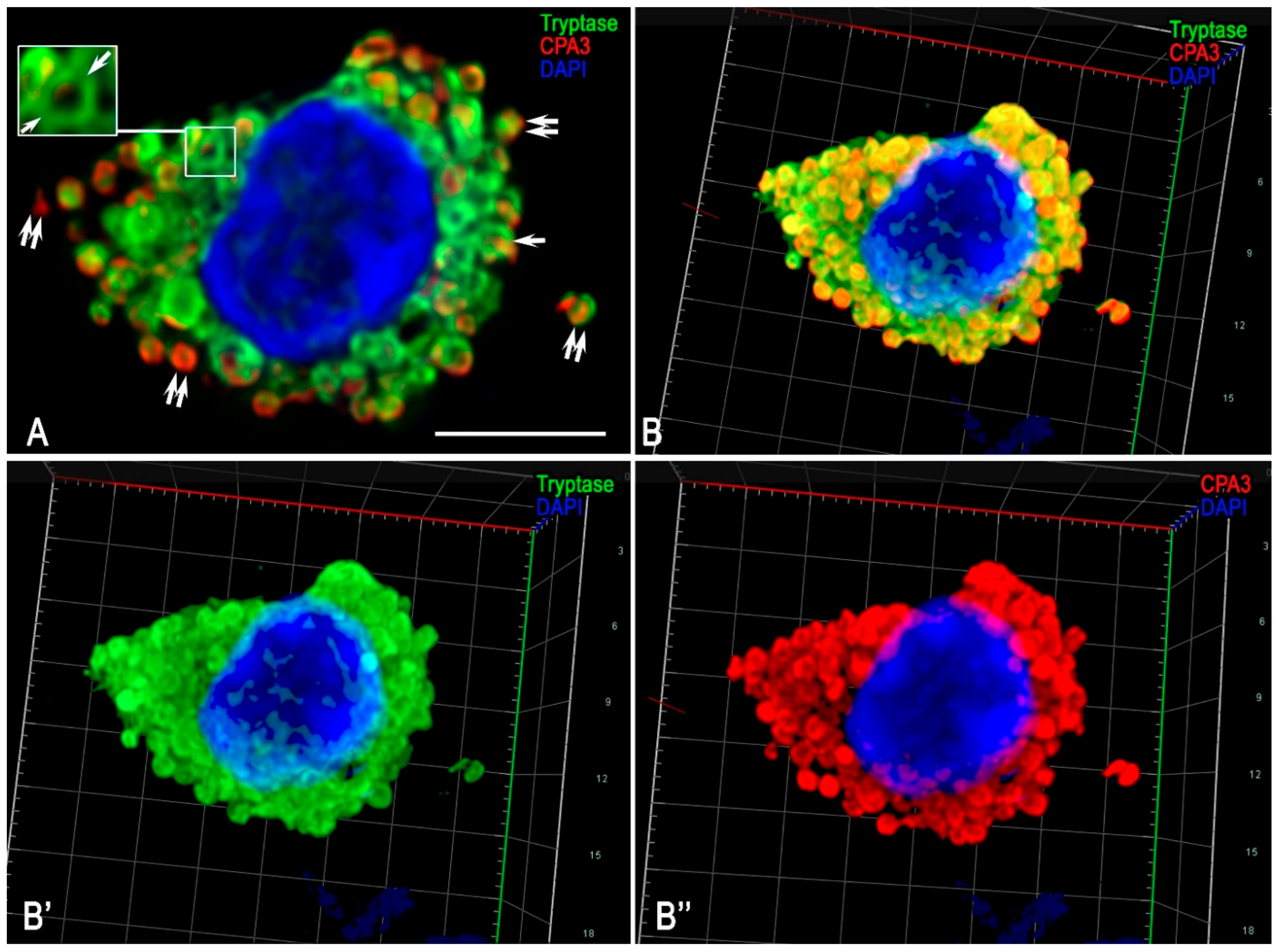
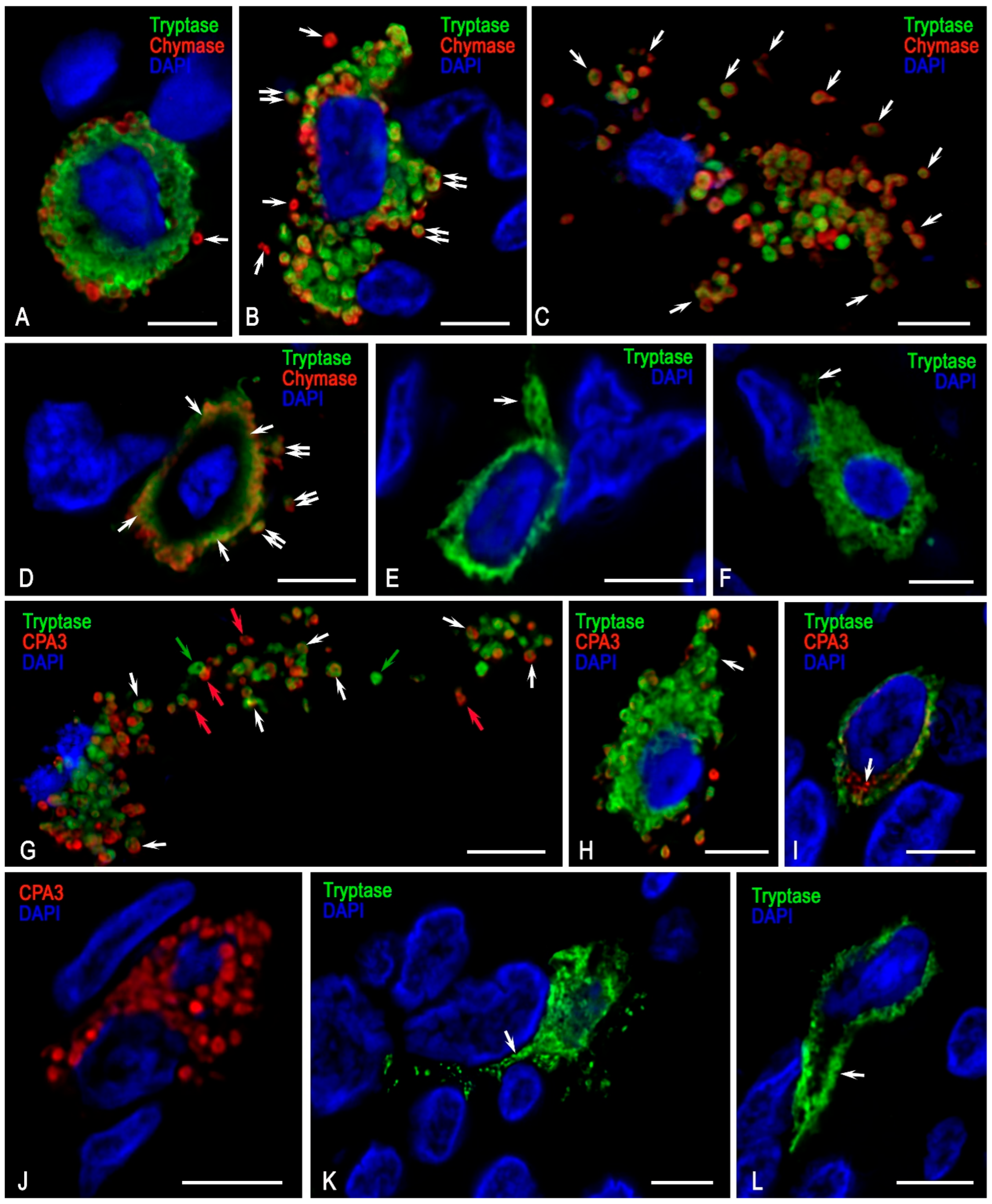
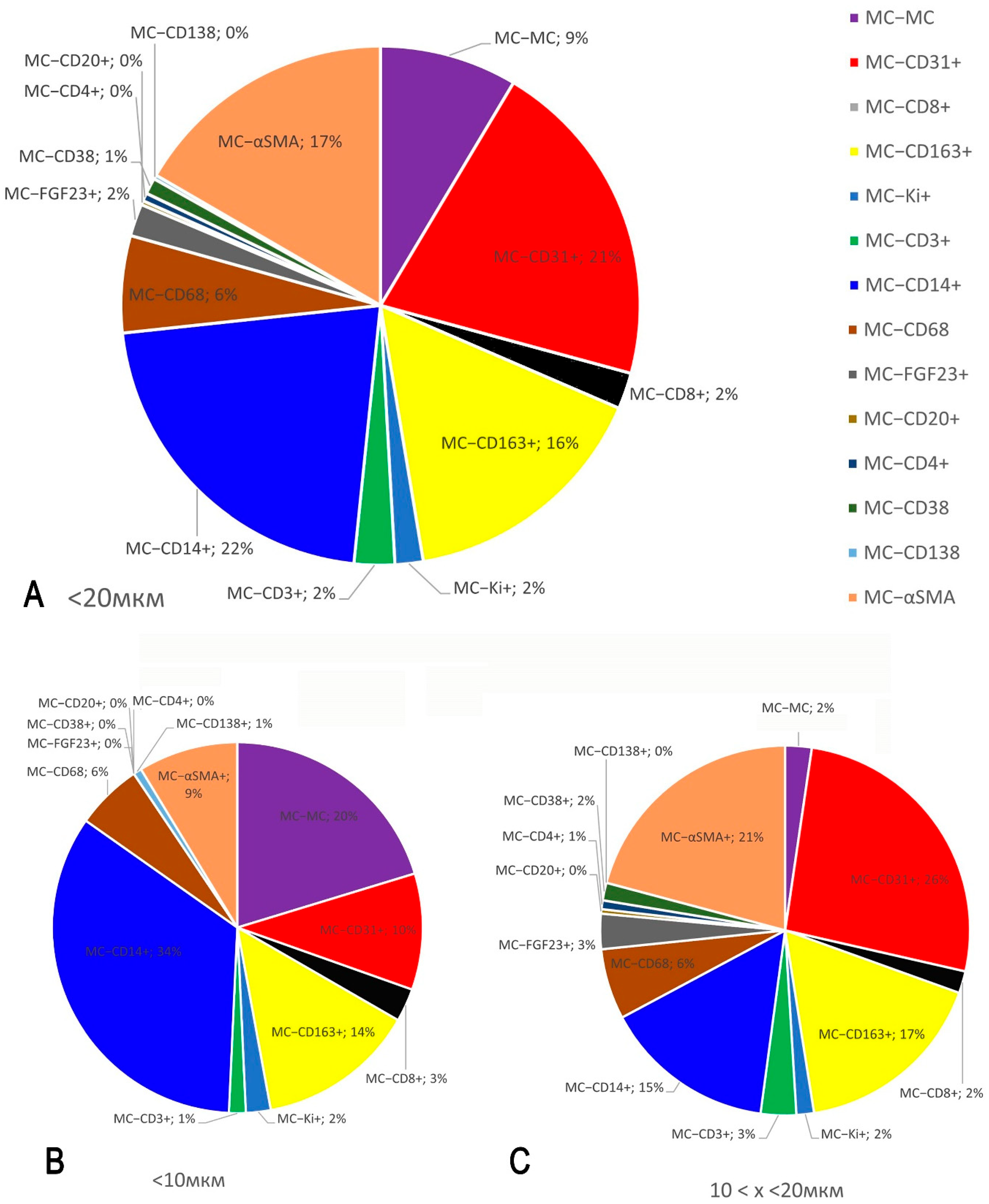
3.2. Monoplex and Multiplex Immunohistochemical Analysis
FGF23-Secreting Cells
3.3. Description of FGF23-Secreting Tumour-Associated MCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Folpe, A.L.; Fanburg-Smith, J.C.; Billings, S.D.; Bisceglia, M.; Bertoni, F.; Cho, J.Y.; Econs, M.J.; Inwards, C.Y.; Jan de Beur, S.M.; Mentzel, T.; et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: An analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 2004, 28, 1–30. [Google Scholar] [CrossRef]
- Ungari, C.; Rocchi, G.; Rinna, C.; Agrillo, A.; Lattanzi, A.; Pagnoni, M. Hypophosphaturic mesenchymal tumor of the ethmoid associated with oncogenic osteomalacia. J. Craniofac. Surg. 2004, 15, 523–527. [Google Scholar] [CrossRef]
- Gronskaia, S.A.; Belaya, Z.E.; Melnichenko, G.A. FGF23 tumor induced osteomalacia. Probl. Endokrinol. 2022, 68, 56–66. [Google Scholar] [CrossRef]
- Villepelet, A.; Casiraghi, O.; Temam, S.; Moya-Plana, A. Ethmoid tumor and oncogenic osteomalacia: Case report and review of the literature. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 365–369. [Google Scholar] [CrossRef]
- Kocelak, P.; Olszanecka-Glinianowicz, M.; Chudek, J. Fibroblast growth factor 23--structure, function and role in kidney diseases. Adv. Clin. Exp. Med. 2012, 21, 391–401. [Google Scholar] [PubMed]
- Burckhardt, M.A.; Schifferli, A.; Krieg, A.H.; Baumhoer, D.; Szinnai, G.; Rudin, C. Tumor-associated FGF-23-induced hypophosphatemic rickets in children: A case report and review of the literature. Pediatr. Nephrol. 2015, 30, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Gronskaia, S.A.; Belaya, Z.E.; Rozhinskaya, L.Y.; Melnichenko, G.A.; Dubovitskaya, T.A.; Mamedova, E.O.; Rodionova, S.S.; Buklemishev, Y.V.; Pigarova, E.A.; Degtyarev, M.V.; et al. Clinical features, diagnostics and treatment of FGF23 secreting tumors: Series of 40 clinical cases. Probl. Endokrinol. 2023, 69, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, R.L. The Roles of Fibroblast Growth Factor (FGF)-23, alpha-Klotho and Furin Protease in Calcium and Phosphate Homeostasis: A Mini-Review. Indian J. Clin. Biochem. 2014, 29, 8–12. [Google Scholar] [CrossRef]
- Clinkenbeard, E.L.; White, K.E. Heritable and acquired disorders of phosphate metabolism: Etiologies involving FGF23 and current therapeutics. Bone 2017, 102, 31–39. [Google Scholar] [CrossRef]
- Beech, T.J.; Rokade, A.; Gittoes, N.; Johnson, A.P. A haemangiopericytoma of the ethmoid sinus causing oncogenic osteomalacia: A case report and review of the literature. Int. J. Oral. Maxillofac. Surg. 2007, 36, 956–958. [Google Scholar] [CrossRef]
- Gonzalez, M.R.; Patel, N.; Connolly, J.J.; Hung, Y.P.; Chang, C.Y.; Lozano-Calderon, S.A. Phosphaturic mesenchymal tumor: Management and outcomes of ten patients treated at a single institution. Skeletal. Radiol. 2024, 53, 1495–1506. [Google Scholar] [CrossRef]
- Ashouri, D.; Kastoon, T. Phosphaturic Tumor-Induced Osteomalacia. Cureus 2024, 16, e54712. [Google Scholar] [CrossRef] [PubMed]
- Qari, H.; Hamao-Sakamoto, A.; Fuselier, C.; Cheng, Y.S.; Kessler, H.; Wright, J. Phosphaturic Mesenchymal Tumor: 2 New Oral Cases and Review of 53 Cases in the Head and Neck. Head Neck Pathol. 2016, 10, 192–200. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Bjermer, L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin. Rev. Allergy Immunol. 2019, 56, 234–247. [Google Scholar] [CrossRef]
- Molin, D.; Edstrom, A.; Glimelius, I.; Glimelius, B.; Nilsson, G.; Sundstrom, C.; Enblad, G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br. J. Haematol. 2002, 119, 122–124. [Google Scholar] [CrossRef]
- Dantas, R.C.M.; de Souza, R.O.; Valverde, L.F.; Vidal, M.T.A.; Sales, C.B.S.; Sousa, L.P.; Dos Santos, J.N.; Ramos, E.A.G.; Gurgel Rocha, C.A. Evaluation of Mast Cell Density in the Tumor Microenvironment in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Appl. Immunohistochem. Mol. Morphol. 2017, 25, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Goffredo, V.; Gadaleta, C.D.; Laterza, A.; Vacca, A.; Ranieri, G. Tryptase serum levels in patients suffering from hepatocellular carcinoma undergoing intra-arterial chemoembolization: Possible predictive role of response to treatment. Mol. Clin. Oncol. 2013, 1, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sperr, W.R.; El-Samahi, A.; Kundi, M.; Girschikofsky, M.; Winkler, S.; Lutz, D.; Endler, G.; Rumpold, H.; Agis, H.; Sillaber, C.; et al. Elevated tryptase levels selectively cluster in myeloid neoplasms: A novel diagnostic approach and screen marker in clinical haematology. Eur. J. Clin. Investig. 2009, 39, 914–923. [Google Scholar] [CrossRef]
- Ranieri, G.; Ammendola, M.; Marech, I.; Laterza, A.; Abbate, I.; Oakley, C.; Vacca, A.; Sacco, R.; Gadaleta, C.D. Vascular endothelial growth factor and tryptase changes after chemoembolization in hepatocarcinoma patients. World J. Gastroenterol. 2015, 21, 6018–6025. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast cells and complement system: Ancient interactions between components of innate immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef]
- Komi, D.E.A.; Redegeld, F.A. Role of Mast Cells in Shaping the Tumor Microenvironment. Clin. Rev. Allergy Immunol. 2020, 58, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Ye, L.; Yu, X.; Jin, K.; Wu, W. Focus on mast cells in the tumor microenvironment: Current knowledge and future directions. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188845. [Google Scholar] [CrossRef]
- Heidarzadeh-Asl, S.; Maurer, M.; Kiani, A.; Atiakshin, D.; Stahl Skov, P.; Elieh-Ali-Komi, D. Novel insights on the biology and immunologic effects of histamine: A road map for allergists and mast cell biologists. J. Allergy Clin. Immunol. 2024, 155, 1095–1114. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Metz, M.; Kolkhir, P.; Kocaturk, E.; Scheffel, J.; Frischbutter, S.; Terhorst-Molawi, D.; Fox, L.; Maurer, M. Chronic urticaria and the pathogenic role of mast cells. Allergol. Int. 2023, 72, 359–368. [Google Scholar] [CrossRef]
- Kolkhir, P.; Elieh-Ali-Komi, D.; Metz, M.; Siebenhaar, F.; Maurer, M. Understanding human mast cells: Lesson from therapies for allergic and non-allergic diseases. Nat. Rev. Immunol. 2022, 22, 294–308. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Wohrl, S.; Bielory, L. Mast Cell Biology at Molecular Level: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2020, 58, 342–365. [Google Scholar] [CrossRef]
- Aponte-Lopez, A.; Munoz-Cruz, S. Mast Cells in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Ligan, C.; Ma, X.H.; Zhao, S.L.; Zhao, W. The regulatory role and mechanism of mast cells in tumor microenvironment. Am. J. Cancer Res. 2024, 14, 1–15. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Shafaghat, F.; Alipoor, S.D.; Kazemi, T.; Atiakshin, D.; Pyatilova, P.; Maurer, M. Immunomodulatory Significance of Mast Cell Exosomes (MC-EXOs) in Immune Response Coordination. Clin. Rev. Allergy Immunol. 2025, 68, 20. [Google Scholar] [CrossRef]
- Cheng, T.; Chen, P.; Chen, J.; Deng, Y.; Huang, C. Landscape Analysis of Matrix Metalloproteinases Unveils Key Prognostic Markers for Patients With Breast Cancer. Front. Genet. 2021, 12, 809600. [Google Scholar] [CrossRef] [PubMed]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Chen, X.; Deng, M.; Wang, Z.; Huang, C. MMP3C: An in-silico framework to depict cancer metabolic plasticity using gene expression profiles. Brief. Bioinform. 2023, 25, bbad471. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Moscat, J.; Diaz-Meco, M. Metabolism shapes the tumor microenvironment. Curr. Opin. Cell Biol. 2017, 48, 47–53. [Google Scholar] [CrossRef]
- Cheng, T.; Wu, Y.; Liu, Z.; Yu, Y.; Sun, S.; Guo, M.; Sun, B.; Huang, C. CDKN2A-mediated molecular subtypes characterize the hallmarks of tumor microenvironment and guide precision medicine in triple-negative breast cancer. Front. Immunol. 2022, 13, 970950. [Google Scholar] [CrossRef]
- Grujic, M.; Hellman, L.; Gustafson, A.M.; Akula, S.; Melo, F.R.; Pejler, G. Protective role of mouse mast cell tryptase Mcpt6 in melanoma. Pigment Cell Melanoma Res. 2020, 33, 579–590. [Google Scholar] [CrossRef]
- Chekmaryova, I.; Kalinin, D.; Kostin, A.; Buchwalow, I.; Tiemann, M.; Elieh-Ali-Komi, D.; Atiakshin, D. Ultrastructural features of tumor-associated mast cells in parasympathetic paragangliomas (chemodectomas) of the neck. Microsc. Res. Tech. 2024, 87, 1373–1383. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Sun, M.; Yang, P.; Meng, X.; Liu, R. Role of mast cells activation in the tumor immune microenvironment and immunotherapy of cancers. Eur. J. Pharmacol. 2023, 960, 176103. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Mast cell proteases and metastasis. Pathol. Res. Pract. 2025, 266, 155801. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Yu, J.; Liu, Y.; Wang, F.; Gou, G.; Wen, M.; Luo, C.; Lu, X.; Hu, Y.; Du, Q.; et al. Mast cells: Key players in digestive system tumors and their interactions with immune cells. Cell Death Discov. 2025, 11, 8. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Böcker, W. Immunohistochemistry: Basics and Methods; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Buchwalow, I.; Samoilova, V.; Boecker, W.; Tiemann, M. Non-specific binding of antibodies in immunohistochemistry: Fallacies and facts. Sci. Rep. 2011, 1, 28. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Schmidt, U.; Weigert, M.; Broaddus, C.; Myers, G. Cell Detection with Star-Convex Polygons. In Proceedings of the Medical Image Computing and Computer Assisted Intervention—MICCAI 2018, Granada, Spain, 16–20 September 2018; pp. 265–273. [Google Scholar]
- The R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Reyes-Mugica, M.; Arnsmeier, S.L.; Backeljauw, P.F.; Persing, J.; Ellis, B.; Carpenter, T.O. Phosphaturic mesenchymal tumor-induced rickets. Pediatr. Dev. Pathol. 2000, 3, 61–69. [Google Scholar] [CrossRef]
- Mathis, D.A.; Stehel, E.J., Jr.; Beshay, J.E.; Mickey, B.E.; Folpe, A.L.; Raisanen, J. Intracranial phosphaturic mesenchymal tumors: Report of 2 cases. J. Neurosurg. 2013, 118, 903–907. [Google Scholar] [CrossRef]
- Florenzano, P.; Hartley, I.R.; Jimenez, M.; Roszko, K.; Gafni, R.I.; Collins, M.T. Tumor-Induced Osteomalacia. Calcif. Tissue Int. 2021, 108, 128–142. [Google Scholar] [CrossRef]
- Bosman, A.; Palermo, A.; Vanderhulst, J.; De Beur, S.M.J.; Fukumoto, S.; Minisola, S.; Xia, W.; Body, J.J.; Zillikens, M.C. Tumor-Induced Osteomalacia: A Systematic Clinical Review of 895 Cases. Calcif. Tissue Int. 2022, 111, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsen, B.; Smith, C.D.; Minisola, S. Epidemiology of Tumor-Induced Osteomalacia in Denmark. Calcif. Tissue Int. 2021, 109, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Savage, C.R.; Zimmer, L.A. Oncogenic osteomalacia from pterygopalatine fossa mass. J. Laryngol. Otol. 2009, 123, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Santa Cruz, D. Phosphaturic mesenchymal tumors. A polymorphous group causing osteomalacia or rickets. Cancer 1987, 59, 1442–1454. [Google Scholar] [CrossRef]
- Viscasillas, G.; Maiz, J.; Lao, X.; Zschaeck, C.; Sanz, J.J. Oncogenic osteomalacia due to phosphaturic mesenchymal tumour in infratemporal fossa. Acta Otorrinolaringol. Esp. 2010, 61, 392–394. [Google Scholar] [CrossRef]
- Ogose, A.; Hotta, T.; Emura, I.; Hatano, H.; Inoue, Y.; Umezu, H.; Endo, N. Recurrent malignant variant of phosphaturic mesenchymal tumor with oncogenic osteomalacia. Skeletal. Radiol. 2001, 30, 99–103. [Google Scholar] [CrossRef]
- Fatani, H.A.; Sunbuli, M.; Lai, S.Y.; Bell, D. Phosphaturic mesenchymal tumor: A report of 6 patients treated at a single institution and comparison with reported series. Ann. Diagn. Pathol. 2013, 17, 319–321. [Google Scholar] [CrossRef]
- Uramoto, N.; Furukawa, M.; Yoshizaki, T. Malignant phosphaturic mesenchymal tumor, mixed connective tissue variant of the tongue. Auris Nasus Larynx 2009, 36, 104–105. [Google Scholar] [CrossRef]
- Hannan, F.M.; Nesbit, M.A.; Turner, J.J.; Stacey, J.M.; Cianferotti, L.; Christie, P.T.; Conigrave, A.D.; Whyte, M.P.; Thakker, R.V. Comparison of human chromosome 19q13 and syntenic region on mouse chromosome 7 reveals absence, in man, of 11.6 Mb containing four mouse calcium-sensing receptor-related sequences: Relevance to familial benign hypocalciuric hypercalcaemia type 3. Eur. J. Hum. Genet. 2010, 18, 442–447. [Google Scholar] [CrossRef]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef]
- Atiakshin, D.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Tryptase as a polyfunctional component of mast cells. Histochem. Cell Biol. 2018, 149, 461–477. [Google Scholar] [CrossRef]
- Ribatti, D.; Tamma, R.; Crivellato, E. The dual role of mast cells in tumor fate. Cancer Lett. 2018, 433, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Derakhshani, A.; Vahidian, F.; Alihasanzadeh, M.; Mokhtarzadeh, A.; Lotfi Nezhad, P.; Baradaran, B. Mast cells: A double-edged sword in cancer. Immunol. Lett. 2019, 209, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Kostin, A.; Buchwalow, I.; Samoilova, V.; Tiemann, M. Protease Profile of Tumor-Associated Mast Cells in Melanoma. Int. J. Mol. Sci. 2022, 23, 8930. [Google Scholar] [CrossRef] [PubMed]
- Elieh Ali Komi, D.; Jalili, A. The emerging role of mast cells in skin cancers: Involved cellular and molecular mechanisms. Int. J. Dermatol. 2022, 61, 792–803. [Google Scholar] [CrossRef]
- Sinnamon, M.J.; Carter, K.J.; Sims, L.P.; Lafleur, B.; Fingleton, B.; Matrisian, L.M. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis 2008, 29, 880–886. [Google Scholar] [CrossRef]
- Varricchi, G.; de Paulis, A.; Marone, G.; Galli, S.J. Future Needs in Mast Cell Biology. Int. J. Mol. Sci. 2019, 20, 4397. [Google Scholar] [CrossRef]
- Jachetti, E.; Cancila, V.; Rigoni, A.; Bongiovanni, L.; Cappetti, B.; Belmonte, B.; Enriquez, C.; Casalini, P.; Ostano, P.; Frossi, B.; et al. Cross-Talk between Myeloid-Derived Suppressor Cells and Mast Cells Mediates Tumor-Specific Immunosuppression in Prostate Cancer. Cancer Immunol. Res. 2018, 6, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Carroll-Portillo, A.; Cannon, J.L.; te Riet, J.; Holmes, A.; Kawakami, Y.; Kawakami, T.; Cambi, A.; Lidke, D.S. Mast cells and dendritic cells form synapses that facilitate antigen transfer for T cell activation. J. Cell Biol. 2015, 210, 851–864. [Google Scholar] [CrossRef]
- Portales-Cervantes, L.; Dawod, B.; Marshall, J.S. Mast Cells and Natural Killer Cells-A Potentially Critical Interaction. Viruses 2019, 11, 514. [Google Scholar] [CrossRef]
- Atiakshin, D.; Kostin, A.; Trotsenko, I.; Samoilova, V.; Buchwalow, I.; Tiemann, M. Carboxypeptidase A3-A Key Component of the Protease Phenotype of Mast Cells. Cells 2022, 11, 570. [Google Scholar] [CrossRef]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef]
- Mekori, Y.A. The mastocyte: The “other” inflammatory cell in immunopathogenesis. J. Allergy Clin. Immunol. 2004, 114, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Breitling, S.; Hui, Z.; Zabini, D.; Hu, Y.; Hoffmann, J.; Goldenberg, N.M.; Tabuchi, A.; Buelow, R.; Dos Santos, C.; Kuebler, W.M. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L710–L721. [Google Scholar] [CrossRef] [PubMed]
- Atiakshin, D.; Buchwalow, I.; Tiemann, M. Mast cell chymase: Morphofunctional characteristics. Histochem. Cell Biol. 2019, 152, 253–269. [Google Scholar] [CrossRef] [PubMed]
- May, M.; Oheim, R.; Bovy, L.; Doess, A.; Maessen, D.; Neukirch, B.; Norris, R.; Williams, A.; Abrahamsen, B. Epidemiology of Tumor-Induced Osteomalacia in Germany Based on Real World Data. Calcif. Tissue Int. 2023, 113, 630–639. [Google Scholar] [CrossRef] [PubMed]

| Antibodies | Host | Catalogue Number | Dilution | Source |
|---|---|---|---|---|
| Tryptase | Mouse monoclonal | ab2378 | 1:4000 | AbCam, Cambridge, UK |
| Chymase | Mouse monoclonal | ab2377 | 1:5000 | AbCam, Cambridge, UK |
| Anti-FGF 23 | Goat Polyclonal | ab56326 | 1:1000 | AbCam, Cambridge, UK |
| CPA3 | Rabbit Polyclonal | ab251696 | 1:500 | AbCam, Cambridge, UK |
| CD3 | Rabbit monoclonal | ab16669 | 1:150 | AbCam, Cambridge, UK |
| CD8 | Rabbit monoclonal | ab101500 | 1:100 | AbCam, Cambridge, UK |
| CD31 | Rabbit monoclonal | ab182981 | 1:2000 | AbCam, Cambridge, UK |
| CD14 | Rabbit monoclonal | EPR3653 | RTU | Cell Marque, Rocklin, CA, USA |
| CD68 | Mouse monoclonal | ab955 | 1:3000 | AbCam, Cambridge, UK |
| CD138 | Mouse monoclonal | B-A38 | RTU | Cell Marque, Rocklin, CA, USA |
| CD163 | Rabbit monoclonal | ab182422 | 1:500 | AbCam, Cambridge, UK |
| Ki67 | Mouse monoclonal | ab279653 | 1:1000 | AbCam, Cambridge, UK |
| HEXAb | Rabbit monoclonal | ab140649 | 1:200 | AbCam, Cambridge, UK |
| CD4 | Rabbit Multiclonal | ab288724 | 1:1000 | AbCam, Cambridge, UK |
| CD20 | Rabbit monoclonal | ab166865 | 1:200 | AbCam, Cambridge, UK |
| CD38 | Rabbit monoclonal | ab108403 | 1:500 | AbCam, Cambridge, UK |
| Vimentin | Rabbit monoclonal | ab92547 | 1:500 | AbCam, Cambridge, UK |
| αSMA | Mouse monoclonal | ab7817 | 1:5000 | AbCam, Cambridge, UK |
| Antibodies and Other Reagents | Source | Dilution | Label |
|---|---|---|---|
| Goat anti-mouse IgG Ab (#ab97035) | AbCam, Cambridge, UK | 1/300 | Cy3 |
| Goat anti-rabbit IgG Ab (#ab150077) | AbCam, Cambridge, UK | 1/300 | Alexa Fluor 488 |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor® 555) (#ab150078) | AbCam, Cambridge, UK | 1/300 | Alexa Fluor 555 |
| Goat Anti-Mouse IgG H&L (Alexa Fluor® 555) preadsorbed (#ab150118) | AbCam, Cambridge, UK | 1/300 | Alexa Fluor 555 |
| Goat Anti-Mouse IgG H&L (Alexa Fluor® 594) (#ab150116) | AbCam, Cambridge, UK | 1/200 | Alexa Fluor 594 |
| Goat Anti-Mouse IgG H&L (Alexa Fluor® 647) (#ab150115) | AbCam, Cambridge, UK | 1/200 | Alexa Fluor 647 |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor® 647) (#ab150079) | AbCam, Cambridge, UK | 1/200 | Alexa Fluor 647 |
| DAPI FP1501001KT | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | DAPI |
| 1X Plus Auto Amplification Diluent FP1609 | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | w/o |
| Opal Polymer HRP Ms + Rb Akoya Biosciences ARH1001EA | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | HRP |
| Antibody Diluent/Block buffer Akoya Biosciences ARD1001EA | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | w/o |
| Secondary antibodies conjugated with horseradish peroxidase FP1500001KT | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 480 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 520 Reagent (#FP1487001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 520 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 540 Reagent (#FP1494001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 540 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 570 Reagent (#FP1488001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 570 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 620 Reagent (#FP1495001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 620 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 650 Reagent (#FP1496001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 650 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 690 Reagent (#FP1497001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 690 |
| Secondary antibodies conjugated with horseradish peroxidase Opal 780 Reagent Pack (FP1501001KT) | Akoya Biosciences, Marlborough, MA, USA | ready-to-use | Opal 780 |
| AmpliStainTM anti-Mouse 1-Step HRP (#AS-M1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| AmpliStainTM anti-Rabbit 1-Step HRP (#AS-R1-HRP) | SDT GmbH, Baesweiler, Germany | ready-to-use | HRP |
| 4′,6-diamidino-2-phenylindole (DAPI, #D9542-5MG) | Sigma, Hamburg, Germany | 5 µg/mL | w/o |
| VECTASHIELD® Mounting Medium (#H-1000) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | w/o |
| DAB Peroxidase Substrate Kit (#SK-4100) | Vector Laboratories, Burlingame, CA, USA | ready-to-use | DAB |
| Toluidine blue (Biovitrum, #07-002) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Giemsa solution (Biovitrum, #21-023) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Silver impregnation (Biovitrum, #21-026) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Weigert–Van Gieson (Biovitrum, #21-020) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Picro Sirius Red Stain Kit (Connective Tissue Stain) (#ab150681) | AbCam, Cambridge, United Kingdom | ready-to-use | w/o |
| Mayer’s Haematoxylin (Biovitrum, #05-002) | ErgoProduction LLC, Saint Petersburg, Russia | ready-to-use | w/o |
| Design Challenge | Detection Targets | Label | Counterstaining of Nuclei |
|---|---|---|---|
| Profile of MCs specific proteases | Tryptase + Chymase | Alexa Fluor 488 and Alexa Fluor 647 | DAPI (Sigma, Hamburg, Germany) |
| Tryptase + CPA3 | |||
| Chymase + CPA3 | |||
| Tryptase + Chymase + CPA3 | OPAL 480, 540 and 690 | DAPI (Akoya Biosciences, Marlborough, MA, USA) | |
| Interaction of MCs with atypical cells | Tryptase + FGF23 | Alexa Fluor 488 and Alexa Fluor 647 | DAPI (Sigma, Hamburg, Germany) |
| Immune landscape of the tumour microenvironment | Tryptase + CD3 | Alexa Fluor 488 and Cy3 | |
| Tryptase + CD4 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD8 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD14 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD20 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD38 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD68 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD138 | Alexa Fluor 488 and Cy3 | ||
| Tryptase + CD163 | Alexa Fluor 488 and Cy3 | ||
| Stromal landscape of the tumour microenvironment | Tryptase + CD31 | Alexa Fluor 488 and Cy3 | |
| Tryptase + aSMA | Alexa Fluor 488 and Cy3 | ||
| Proliferative activity | Tryptase + Ki67 | Alexa Fluor 488 and Cy3 | |
| Immune and stromal landscape | Tryptase + CD3+ CD8+ CD14 + CD68+ CD163 + CD31+ aSMA | OPAL 480, OPAL 520, OPAL 540, OPAL 570, OPAL 620, OPAL 650, OPAL 690, OPAL 780 | DAPI (Akoya Biosciences, Marlborough, MA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostin, A.; Lyundup, A.; Alekhnovich, A.; Prikhodko, A.; Patsap, O.; Gronskaia, S.; Belaya, Z.; Lesnyak, O.; Melnichenko, G.; Mokrysheva, N.; et al. Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23. Med. Sci. 2025, 13, 195. https://doi.org/10.3390/medsci13030195
Kostin A, Lyundup A, Alekhnovich A, Prikhodko A, Patsap O, Gronskaia S, Belaya Z, Lesnyak O, Melnichenko G, Mokrysheva N, et al. Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23. Medical Sciences. 2025; 13(3):195. https://doi.org/10.3390/medsci13030195
Chicago/Turabian StyleKostin, Andrey, Alexei Lyundup, Alexander Alekhnovich, Aleksandra Prikhodko, Olga Patsap, Sofia Gronskaia, Zhanna Belaya, Olga Lesnyak, Galina Melnichenko, Natalia Mokrysheva, and et al. 2025. "Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23" Medical Sciences 13, no. 3: 195. https://doi.org/10.3390/medsci13030195
APA StyleKostin, A., Lyundup, A., Alekhnovich, A., Prikhodko, A., Patsap, O., Gronskaia, S., Belaya, Z., Lesnyak, O., Melnichenko, G., Mokrysheva, N., Buchwalow, I., Tiemann, M., & Atiakshin, D. (2025). Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23. Medical Sciences, 13(3), 195. https://doi.org/10.3390/medsci13030195






