Proteome Differences in Smooth Muscle Cells from Diabetic and Non-Diabetic Abdominal Aortic Aneurysm Patients Reveal Metformin-Induced Mechanisms
Abstract
1. Introduction
2. Methods
2.1. Patient Samples
2.1.1. Patient Population
2.1.2. Aortic Biopsy and Cell Culture
2.1.3. Plasma Samples
2.2. Proteomics Analysis
2.2.1. SMC Proteomics Analysis
SMC Sample Preparation
SMC Peptide Digestion and Mass Spectrometry
SMC Protein Quantification
SMC Proteomics Data Analysis
Data Availability
2.2.2. Tissue Proteomics Analysis
2.3. Experiments to Test the Effect of Metformin on SMCs
2.3.1. Metformin Dosage
2.3.2. RNA Isolation and Quantitative Polymerase Chain Reaction
2.3.3. Western Blotting
2.3.4. Immunofluorescence
2.3.5. Pentosidine Measurement
2.3.6. Baseline Measurement of Oxygen Consumption and Extracellular Acidification
2.3.7. L-Lactate Assay
2.3.8. NQO1 Activity Assay Kit
2.3.9. Statistics
3. Results
3.1. Clinical Characteristics
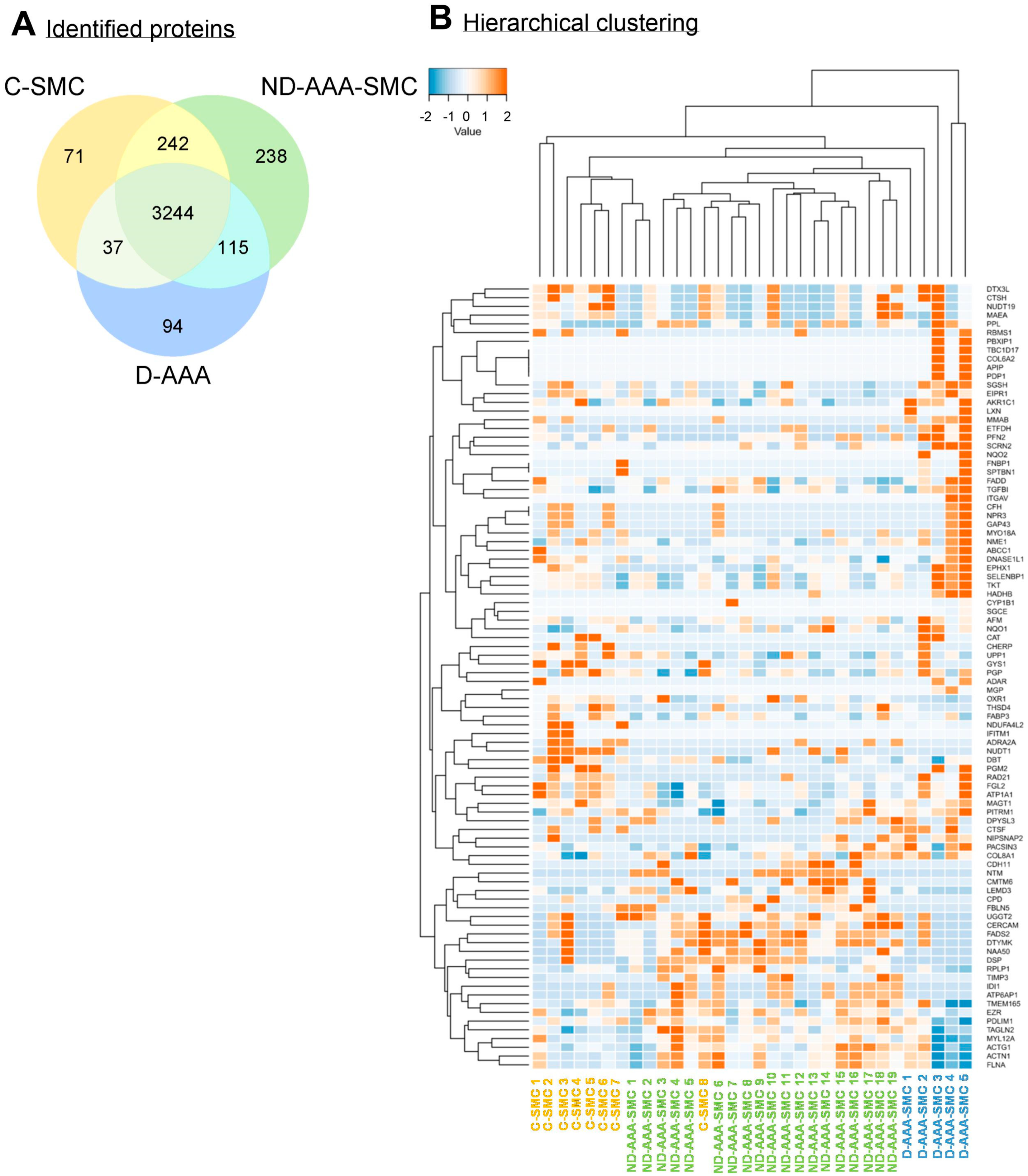
3.2. Differences in Proteomic Profile Between SMCs from Controls, Non-Diabetic and Diabetic AAA Patients
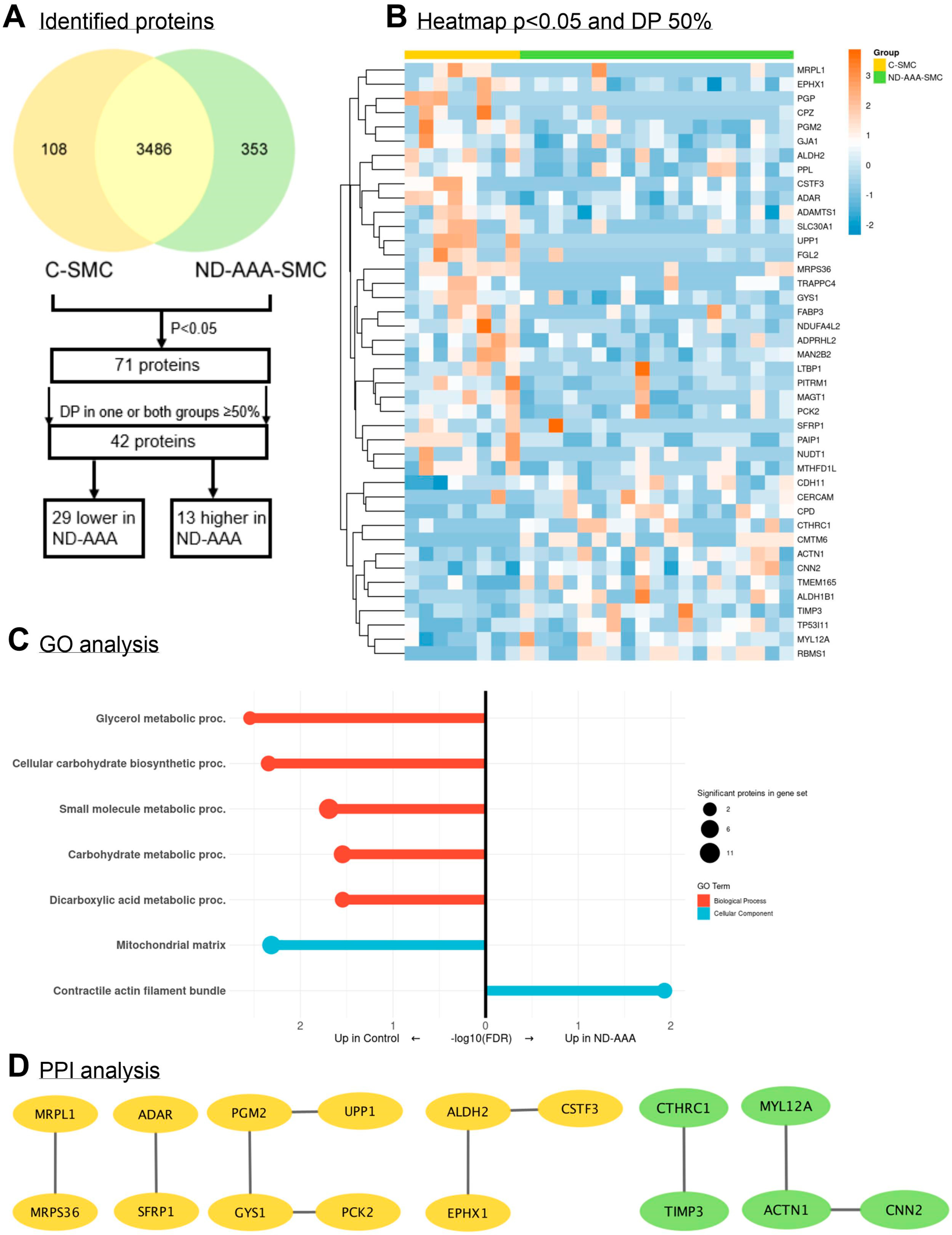
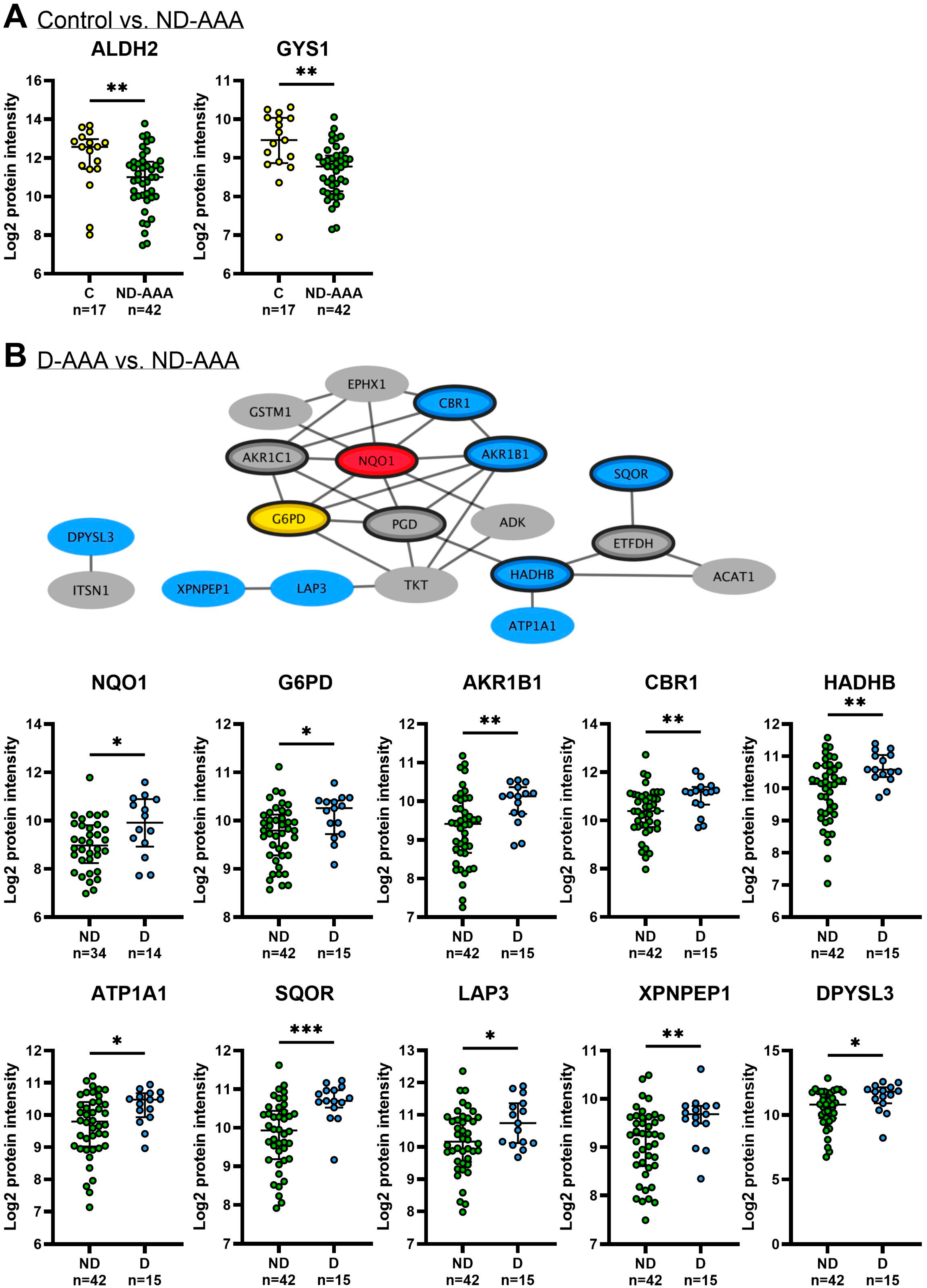
3.3. Differences in Proteomic Profile Between SMCs from Non-Diabetic AAA Patients and Aortic Controls
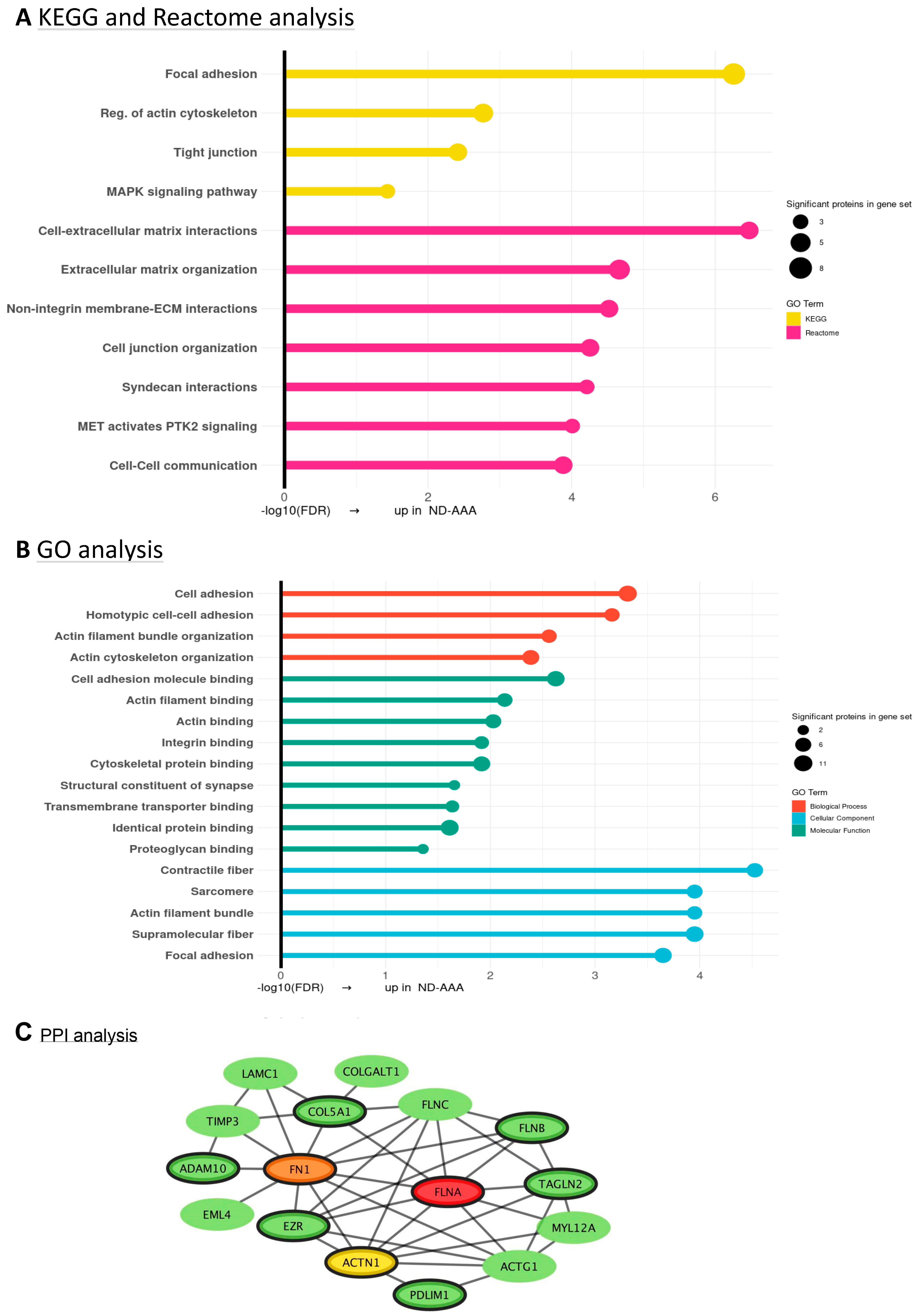
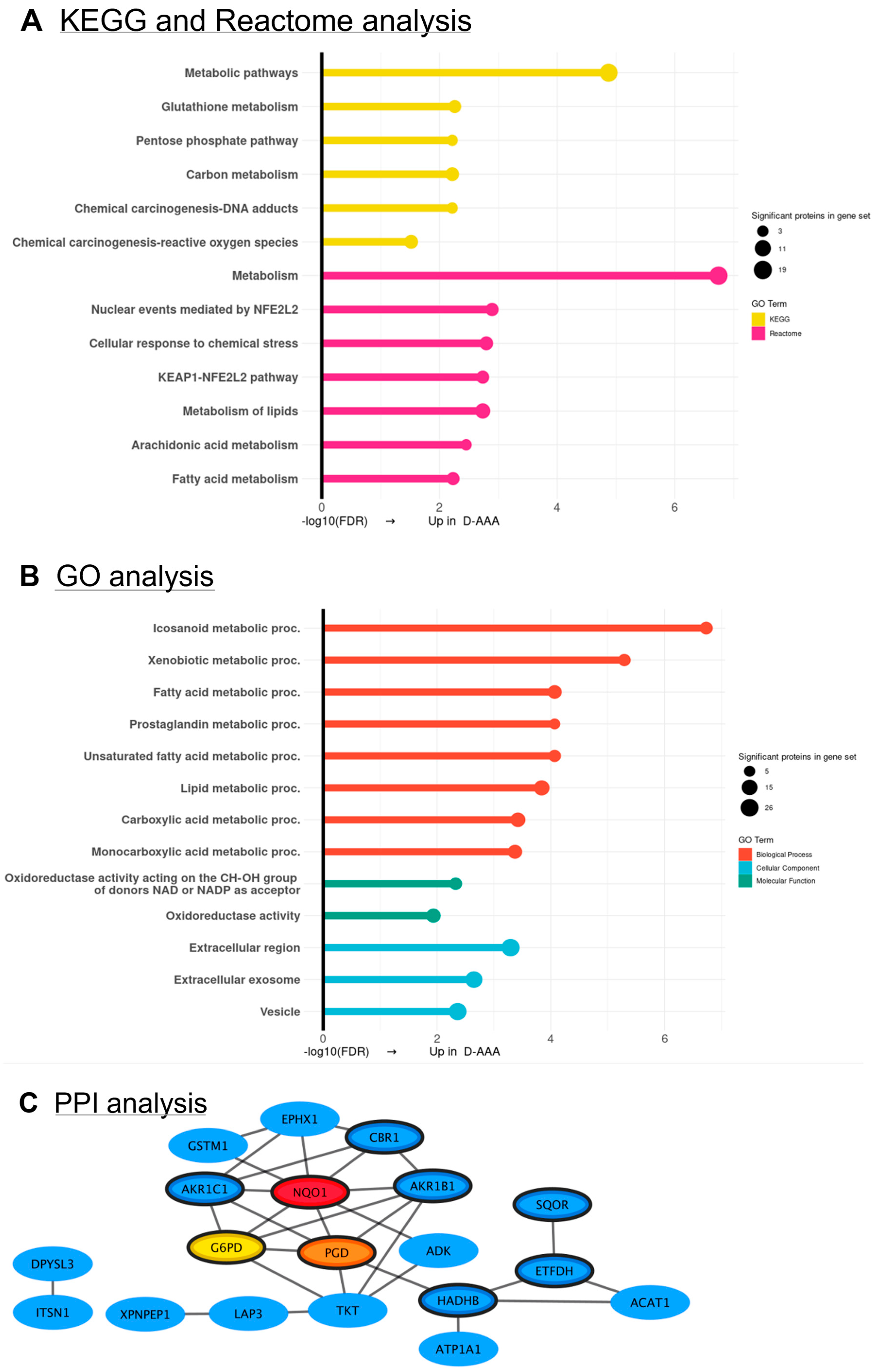
3.4. Differences in Proteomic Profile Between SMCs from Diabetic and Non-Diabetic AAA Patients

3.5. Validation of SMC Proteomics Findings in Aortic Tissue
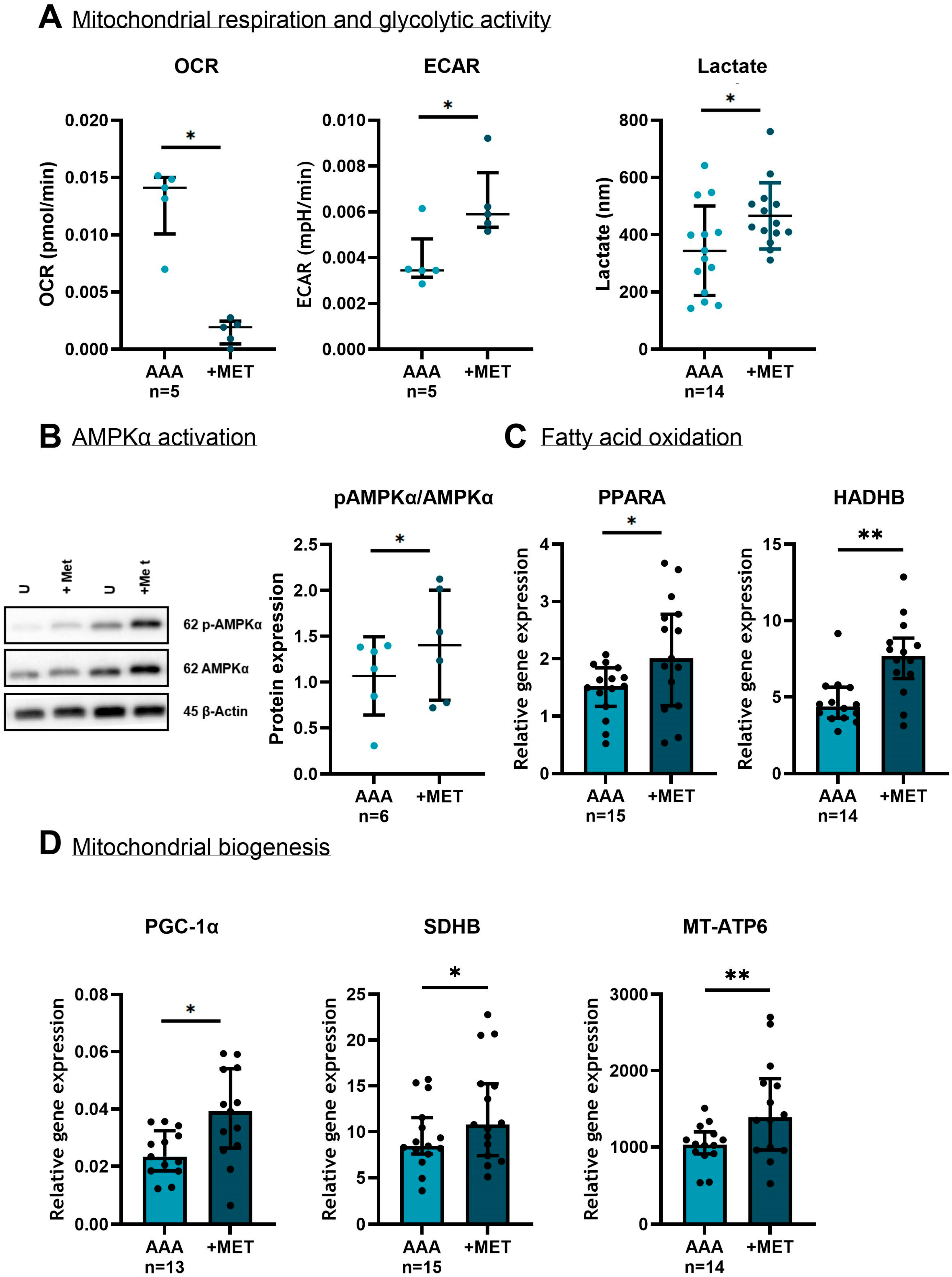
3.6. The Effect of Metformin on SMCs in Vitro
3.6.1. The Effect of Metformin on Cytoskeletal and Extracellular Matrix Markers
he Effect of Metformin on Mitochondria and the Oxidative Stress Defense Mechanism

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations/Nomenclature
| AAA | Abdominal aortic aneurysm |
| ACTG1 | Actin Gamma-1 |
| ACTN1 | Actinin Alpha 1 |
| ADAM10 | A Disintegrin and Metalloproteinase 10 |
| AGEs | Advanced Glycation End Products |
| AKR | Aldo-Keto Reductases |
| ALDH2 | Aldehyde Dehydrogenase 2 Family Member |
| AMPK | AMP-Activated Protein Kinase |
| ARE | Antioxidant response elements |
| C | Non-pathological controls (postmortem heart-beating kidney donors) |
| CAT | Catalase |
| CBR1 | Carbonyl Reductase 1 |
| CNN2 | Calponin 2 |
| COL1A1 | Collagen Type I Alpha 1 |
| COL5A1 | Collagen Type V Alpha-1 |
| COLGALT1 | Collagen Beta(1-O)Galactosyltransferase 1 |
| CTHRC1 | Collagen Triple Helix Repeat Containing 1 |
| D-AAA | diabetic AAA patients |
| ECAR | Extracellular acidification rate |
| ECM | Extracellular matrix |
| EML4 | Echinoderm Microtubule-Associated Protein-Like 4 |
| EPHX1 | Epoxide Hydrolase 1 |
| EVAR | Endovascular aneurysm repair |
| EZR | Ezrin |
| FDR | False Discovery Rate |
| FLNA | Filamin A |
| FLNB | Filamin B |
| FLNC | Filamin C |
| FN1 | Fibronectin 1 |
| G6PD | Glucose-6-Phosphate Dehydrogenase |
| GO | Gene Ontology |
| GSTM1 | Glutathione-S-Transferase M1 |
| GYS1 | Glycogen Synthase 1 |
| H2O2 | Hydrogen Peroxide |
| HADHB | Hydroxyacyl-CoA Dehydrogenase Trifunctional Multienzyme Complex Subunit Beta |
| KEAP1 | Kelch-Like ECH-Associated Protein 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LAMC1 | Laminin Subunit Gamma-1 |
| mGPDH | Mitochondrial glycerol-3-phosphate dehydrogenase |
| MMP | Matrix metalloproteinase |
| MT-ATP6 | Mitochondrially Encoded ATP Synthase Membrane Subunit 6 |
| MTP | Mitochondrial Trifunctional Protein |
| MYL12A | Myosin Light Chain 12A |
| ND-AAA | Non-diabetic AAA patients |
| NQO1 | NAD(P)H: Quinone Oxidoreductase 1 |
| NRF2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| O2− | Superoxide anion |
| OCR | Oxygen consumption rate |
| PCK2 | Phosphoenolpyruvate Carboxykinase 2 |
| PDLIM1 | PDZ and LIM Domain Protein 1 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1-Alpha |
| PGD | 6-Phosphogluconate Dehydrogenase |
| PGM2 | Phosphoglucomutase 2 |
| PPARA | Peroxisome Proliferator-Activated Receptor Alpha |
| PPI | Protein–protein interaction |
| PRKG1 | Protein Kinase cGMP-Dependent 1 |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| RT-qPCR | Real-time quantitative Polymerase Chain Reaction |
| SDHB | Succinate Dehydrogenase Complex Subunit B |
| sMaF | Small Maf proteins |
| SMC | Smooth muscle cell |
| SOD2 | Superoxide Dismutase 2 |
| T2D | Type 2 diabetes |
| TAGLN2 | Transgelin-2 |
| TBP | TATA box binding protein |
| TFA | Trifluoroacetic acid |
| TGFBI | Growth Factor Beta-Induced Protein |
| TIMP3 | Tissue Inhibitor of Metalloproteinase 3 |
| TKT | Transketolase |
References
- Yang, L.; Shen, L.; Gao, P.; Li, G.; He, Y.; Wang, M.; Zhou, H.; Yuan, H.; Jin, X.; Wu, X. Effect of AMPK signal pathway on pathogenesis of abdominal aortic aneurysms. Oncotarget 2017, 8, 92827–92840. [Google Scholar] [CrossRef]
- Jessula, S.; Cote, C.L.; Cooper, M.; McDougall, G.; Kivell, M.; Kim, Y.; Tansley, G.; Casey, P.; Smith, M.; Herman, C. Dying to Get There: Patients Who Reside at Increased Distance from Tertiary Center Experience Increased Mortality Following Abdominal Aortic Aneurysm Rupture. Ann. Vasc. Surg. 2023, 91, 135–144. [Google Scholar] [CrossRef]
- Golledge, J.; Moxon, J.; Pinchbeck, J.; Anderson, G.; Rowbotham, S.; Jenkins, J.; Bourke, M.; Bourke, B.; Dear, A.; Buckenham, T.; et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Brit J. Surg. 2017, 104, 1486–1493. [Google Scholar] [CrossRef]
- Wanhainen, A.; Van Herzeele, I.; Bastos Goncalves, F.; Bellmunt Montoya, S.; Berard, X.; Boyle, J.R.; D’Oria, M.; Prendes, C.F.; Karkos, C.D.; Kazimierczak, A.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2024 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 192–331. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.S.S.; Krasniqi, L.; Obel, L.M.; Kavaliunaite, E.; Liisberg, M.; Lindholt, J.S. Exploring Drug Re-Purposing for Treatment of Abdominal Aortic Aneurysms: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2024, 67, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Itoga, N.K.; Rothenberg, K.A.; Suarez, P.; Ho, T.V.; Mell, M.W.; Xu, B.; Curtin, C.M.; Dalman, R.L. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J. Vasc. Surg. 2019, 69, 710–716.e713. [Google Scholar] [CrossRef] [PubMed]
- van Merrienboer, T.A.R.; Warlich, V.; Holewijn, S.; Driessen, W.; Yeung, K.K.; Reijnen, M. The Impact of Diabetes Mellitus and Metformin Use on Outcomes After Endovascular Aneurysm Repair. J. Clin. Med. 2025, 14, 295. [Google Scholar] [CrossRef]
- van Merrienboer, T.A.R.; Rombouts, K.B.; Bogunovic, N.; Mieremet, A.; Meekel, J.P.; Balm, R.; de Waard, V.; Yeung, K.K. Metformin Improves the Function of Abdominal Aortic Aneurysm Patient Derived Aortic Smooth Muscle Cells. Eur. J. Vasc. Endovasc. Surg. 2024, 69, 485–495. [Google Scholar] [CrossRef]
- Summerhill, V.I.; Sukhorukov, V.N.; Eid, A.H.; Nedosugova, L.V.; Sobenin, I.A.; Orekhov, A.N. Pathophysiological Aspects of the Development of Abdominal Aortic Aneurysm with a Special Focus on Mitochondrial Dysfunction and Genetic Associations. Biomol. Concepts 2021, 12, 55–67. [Google Scholar] [CrossRef]
- Kazaleh, M.; Gioscia-Ryan, R.; Ailawadi, G.; Salmon, M. Oxidative Stress and the Pathogenesis of Aortic Aneurysms. Biomedicines 2023, 12, 3. [Google Scholar] [CrossRef]
- Xiong, W.; Mactaggart, J.; Knispel, R.; Worth, J.; Zhu, Z.; Li, Y.; Sun, Y.; Baxter, B.T.; Johanning, J. Inhibition of reactive oxygen species attenuates aneurysm formation in a murine model. Atherosclerosis 2009, 202, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Oller, J.; Gabande-Rodriguez, E.; Ruiz-Rodriguez, M.J.; Desdin-Mico, G.; Aranda, J.F.; Rodrigues-Diez, R.; Ballesteros-Martinez, C.; Blanco, E.M.; Roldan-Montero, R.; Acuna, P.; et al. Extracellular Tuning of Mitochondrial Respiration Leads to Aortic Aneurysm. Circulation 2021, 143, 2091–2109. [Google Scholar] [CrossRef]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Henneman, A.A.; Knol, J.C.; Pham, T.V.; Piersma, S.R.; Jimenez, C.R.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. Insight in the (Phospho)proteome of Vascular Smooth Muscle Cells Derived From Patients With Abdominal Aortic Aneurysm Reveals Novel Disease Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2226–2243. [Google Scholar] [CrossRef]
- Pham, T.V.; Piersma, S.R.; Warmoes, M.; Jimenez, C.R. On the beta-binomial model for analysis of spectral count data in label-free tandem mass spectrometry-based proteomics. Bioinformatics 2010, 26, 363–369. [Google Scholar] [CrossRef]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. jvenn: An interactive Venn diagram viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef]
- Ge, S.X.; Jung, D.M.; Yao, R.A. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics 2020, 36, 2628–2629. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.D.; Chen, M.J.; Huang, X.H.; Zhang, G.C.; Zeng, L.; Zhang, G.S.; Wu, S.J.; Wang, Y.W. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. CytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; Garcia-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Zougman, A.; Selby, P.J.; Banks, R.E. Suspension trapping (STrap) sample preparation method for bottom-up proteomics analysis. Proteomics 2014, 14, 1006-1000. [Google Scholar] [CrossRef]
- Thanou, E.; Koopmans, F.; Pita-Illobre, D.; Klaassen, R.V.; Ozer, B.; Charalampopoulos, I.; Smit, A.B.; Li, K.W. Suspension TRAPping Filter (sTRAP) Sample Preparation for Quantitative Proteomics in the Low microg Input Range Using a Plasmid DNA Micro-Spin Column: Analysis of the Hippocampus from the 5xFAD Alzheimer’s Disease Mouse Model. Cells 2023, 12, 1242. [Google Scholar] [CrossRef]
- Demichev, V.; Messner, C.B.; Vernardis, S.I.; Lilley, K.S.; Ralser, M. DIA-NN: Neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. [Google Scholar] [CrossRef]
- Pham, T.V.; Henneman, A.A.; Jimenez, C.R. iq: An R package to estimate relative protein abundances from ion quantification in DIA-MS-based proteomics. Bioinformatics 2020, 36, 2611–2613. [Google Scholar] [CrossRef]
- Tsai, S.H.; Hsu, L.A.; Tsai, H.Y.; Yeh, Y.H.; Lu, C.Y.; Chen, P.C.; Wang, J.C.; Chiu, Y.L.; Lin, C.Y.; Hsu, Y.J. Aldehyde dehydrogenase 2 protects against abdominal aortic aneurysm formation by reducing reactive oxygen species, vascular inflammation, and apoptosis of vascular smooth muscle cells. FASEB J. 2020, 34, 9498–9511. [Google Scholar] [CrossRef] [PubMed]
- Gautheron, J.; Jéru, I. The Multifaceted Role of Epoxide Hydrolases in Human Health and Disease. Int. J. Mol. Sci. 2021, 22, 13. [Google Scholar] [CrossRef]
- Annabi, B.; Shedid, D.; Ghosn, P.; Kenigsberg, R.L.; Desrosiers, R.R.; Bojanowski, M.W.; Beaulieu, E.; Nassif, E.; Moumdjian, R.; Beliveau, R. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J. Vasc. Surg. 2002, 35, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Carrell, T.W.G.; Burnand, K.G.; Wells, G.M.A.; Clements, J.M.; Smith, A. Stromelysin-1 (matrix metalloproteinase-3) and tissue inhibitor of metalloproteinase-3 are overexpressed in the wall of abdominal aortic aneurysms. Circulation 2002, 105, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Fan, D.; Kandalam, V.; Lee, J.; Das, S.K.; Wang, X.; Baldwin, T.A.; Oudit, G.Y.; Kassiri, Z. Loss of Timp3 gene leads to abdominal aortic aneurysm formation in response to angiotensin II. J. Biol. Chem. 2012, 287, 44083–44096. [Google Scholar] [CrossRef]
- Cao, M.; Ke, D.; Zhou, H. The role and molecular mechanism of CTHRC1 in fibrosis. Life Sci. 2024, 350, 122745. [Google Scholar] [CrossRef]
- Modrego, J.; Lopez-Farre, A.J.; Martinez-Lopez, I.; Muela, M.; Macaya, C.; Serrano, J.; Monux, G. Expression of cytoskeleton and energetic metabolism-related proteins at human abdominal aortic aneurysm sites. J. Vasc. Surg. 2012, 55, 1124–1133. [Google Scholar] [CrossRef]
- Gabel, G.; Northoff, B.H.; Balboa, A.; Becirovic-Agic, M.; Petri, M.; Busch, A.; Maegdefessel, L.; Mahlmann, A.; Ludwig, S.; Teupser, D.; et al. Parallel Murine and Human Aortic Wall Genomics Reveals Metabolic Reprogramming as Key Driver of Abdominal Aortic Aneurysm Progression. J. Am. Heart Assoc. 2021, 10, e020231. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Guo, D.; Hostetler, E.; Marin, I.; Pinard, A.C.; Cecchi, A.C. Update on the genetic risk for thoracic aortic aneurysms and acute aortic dissections: Implications for clinical care. J. Cardiovasc. Surg. 2021, 62, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Milewicz, D.M.; Guo, D.C.; Tran-Fadulu, V.; Lafont, A.L.; Papke, C.L.; Inamoto, S.; Kwartler, C.S.; Pannu, H. Genetic basis of thoracic aortic aneurysms and dissections: Focus on smooth muscle cell contractile dysfunction. Annu. Rev. Genomics Hum. Genet. 2008, 9, 283–302. [Google Scholar] [CrossRef]
- Zheng, Y.; Ma, H.; Yan, Y.; Ye, P.; Yu, W.; Lin, S.; Chen, S.L. Deficiency of filamin A in smooth muscle cells protects against hypoxia-mediated pulmonary hypertension in mice. Int. J. Mol. Med. 2023, 51, 22. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Deng, E.S.; Yamada, J.M.; Choudhury, S.; Scotellaro, J.; Kelley, L.; Isselbacher, E.; Lindsay, M.E.; Walsh, C.A.; Doan, R.N. Contributions of Germline and Somatic Mosaic Genetics to Thoracic Aortic Aneurysms in Nonsyndromic Individuals. J. Am. Heart Assoc. 2024, 13, e033232. [Google Scholar] [CrossRef]
- Pinard, A.; Jones, G.T.; Milewicz, D.M. Genetics of Thoracic and Abdominal Aortic Diseases Aneurysms, Dissections, and Ruptures. Circ. Res. 2019, 124, 588–606. [Google Scholar] [CrossRef]
- Jain, M.; Chauhan, A.K. Role of Integrins in Modulating Smooth Muscle Cell Plasticity and Vascular Remodeling: From Expression to Therapeutic Implications. Cells 2022, 11, 646. [Google Scholar] [CrossRef]
- Qiu, R.F.; Chen, S.X.; Gao, P.X.; Luo, K.; Feng, X.D.; Yuan, H.; Wu, X.J.; Li, G. ADAM10 attenuates the development of abdominal aortic aneurysms in a mouse model. Mol. Med. Rep. 2021, 24, 774. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yu, B.; Li, Z.Z.; Chen, Y.H.; Sun, Y.; Wang, D.W. Variants Cause Aortic Dissection by Activating TGF-β-Signaling Pathway. J. Am. Heart Assoc. 2021, 10, e019276. [Google Scholar] [CrossRef]
- Hamann, B.; Klimova, A.; Klotz, F.; Frank, F.; Janichen, C.; Kapalla, M.; Sabarstinski, P.; Wolk, S.; Morawietz, H.; Poitz, D.M.; et al. Regulation of CD163 Receptor in Patients with Abdominal Aortic Aneurysm and Associations with Antioxidant Enzymes HO-1 and NQO1. Antioxidants 2023, 12, 947. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Song, N.; Yu, J.E.; Ji, E.; Choi, K.H.; Lee, S. Hydrogen sulfide inhibits gene expression associated with aortic valve degeneration by inducing NRF2-related pro-autophagy effect in human aortic valve interstitial cells. Mol. Cell Biochem. 2024, 479, 2653–2662. [Google Scholar] [CrossRef]
- Kruger, A.; Gruning, N.M.; Wamelink, M.M.; Kerick, M.; Kirpy, A.; Parkhomchuk, D.; Bluemlein, K.; Schweiger, M.R.; Soldatov, A.; Lehrach, H.; et al. The pentose phosphate pathway is a metabolic redox sensor and regulates transcription during the antioxidant response. Antioxid. Redox Signal 2011, 15, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Heiss, E.H.; Schachner, D.; Zimmermann, K.; Dirsch, V.M. Glucose availability is a decisive factor for Nrf2-mediated gene expression. Redox Biol. 2013, 1, 359–365. [Google Scholar] [CrossRef]
- Dattani, N.; Sayers, R.D.; Bown, M.J. Diabetes mellitus and abdominal aortic aneurysms: A review of the mechanisms underlying the negative relationship. Diab. Vasc. Dis. Res. 2018, 15, 367–374. [Google Scholar] [CrossRef] [PubMed]
- van der Pluijm, I.; Burger, J.; van Heijningen, P.M.; Ijpma, A.; van Vliet, N.; Milanese, C.; Schoonderwoerd, K.; Sluiter, W.; Ringuette, L.J.; Dekkers, D.H.W.; et al. Decreased mitochondrial respiration in aneurysmal aortas of Fibulin-4 mutant mice is linked to PGC1A regulation. Cardiovasc. Res. 2018, 114, 1776–1793. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Klarin, D.; Levin, M.G.; Spin, J.M.; Rhee, Y.H.; Deng, A.; Headley, C.A.; Tsao, N.L.; Gellatly, C.; Zuber, V.; et al. Genome-wide association meta-analysis identifies risk loci for abdominal aortic aneurysm and highlights PCSK9 as a therapeutic target. Nat. Genet. 2023, 55, 1831–1842. [Google Scholar] [CrossRef]
- Averill-Bates, D. Reactive oxygen species and cell signaling. Review. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119573. [Google Scholar] [CrossRef]
- De Waard, V. Created in Biorender. Available online: https://BioRender.com/q50d341 (accessed on 5 September 2025).
- Tavris, B.S.; Peters, A.S.; Bockler, D.; Dihlmann, S. Mitochondrial Dysfunction and Increased DNA Damage in Vascular Smooth Muscle Cells of Abdominal Aortic Aneurysm (AAA-SMC). Oxid. Med. Cell Longev. 2023, 2023, 6237960. [Google Scholar] [CrossRef]
- Oller, J.; Gabande-Rodriguez, E.; Roldan-Montero, R.; Ruiz-Rodriguez, M.J.; Redondo, J.M.; Martin-Ventura, J.L.; Mittelbrunn, M. Rewiring Vascular Metabolism Prevents Sudden Death due to Aortic Ruptures-Brief Report. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 462–469. [Google Scholar] [CrossRef]
- Yi, M.; Cruz Cisneros, L.; Cho, E.J.; Alexander, M.; Kimelman, F.A.; Swentek, L.; Ferrey, A.; Tantisattamo, E.; Ichii, H. Nrf2 Pathway and Oxidative Stress as a Common Target for Treatment of Diabetes and Its Comorbidities. Int. J. Mol. Sci. 2024, 25, 821. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Bao, L.; Zhu, P.; Wang, S. Effect of metformin on the epigenetic age of peripheral blood in patients with diabetes mellitus. Front. Genet. 2022, 13, 955835. [Google Scholar] [CrossRef] [PubMed]
- Koole, D.; van Herwaarden, J.A.; Schalkwijk, C.G.; Lafeber, F.; Vink, A.; Smeets, M.B.; Pasterkamp, G.; Moll, F.L. A potential role for glycated cross-links in abdominal aortic aneurysm disease. J. Vasc. Surg. 2017, 65, 1493–1503.e3. [Google Scholar] [CrossRef] [PubMed]
- Foretz, M.; Guigas, B.; Viollet, B. Metformin: Update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023, 19, 460–476. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Gabrielson, M.; Vorkapic, E.; Folkesson, M.; Welander, M.; Matussek, A.; Dimberg, J.; Lanne, T.; Skogberg, J.; Wagsater, D. Altered PPARgamma Coactivator-1 Alpha Expression in Abdominal Aortic Aneurysm: Possible Effects on Mitochondrial Biogenesis. J. Vasc. Res. 2016, 53, 17–26. [Google Scholar] [CrossRef]
- Petsouki, E.; Cabrera, S.N.S.; Heiss, E.H. AMPK and NRF2: Interactive players in the same team for cellular homeostasis? Free Radic. Biol. Med. 2022, 190, 75–93. [Google Scholar] [CrossRef]
- Song, H.Y.; Xu, T.; Feng, X.F.; Lai, Y.X.; Yang, Y.; Zheng, H.; He, X.; Wei, G.Q.; Liao, W.J.; Liao, Y.L.; et al. Itaconate prevents abdominal aortic aneurysm formation through inhibiting inflammation via activation of Nrf2. Ebiomedicine 2020, 57, 102832. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, A.; Werner, E.; Grochot-Przeczek, A.; Kloska, D.; Hajduk, K.; Neumayer, C.; Jozkowicz, A.; Piechota-Polanczyk, A. Simvastatin Attenuates Abdominal Aortic Aneurysm Formation Favoured by Lack of Nrf2 Transcriptional Activity. Oxid. Med. Cell Longev. 2020, 2020, 6340190. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.H.; Morimoto, K.; Yu, J.; Bao, W.L.; Okita, Y.; Okada, K. Endogenous superoxide dismutase activation by oral administration of riboflavin reduces abdominal aortic aneurysm formation in rats. J. Vasc. Surg. 2016, 64, 737–745. [Google Scholar] [CrossRef]
- Parastatidis, I.; Weiss, D.; Joseph, G.; Taylor, W.R. Overexpression of catalase in vascular smooth muscle cells prevents the formation of abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2389–2396. [Google Scholar] [CrossRef]
- He, X.; Deng, J.; Yu, X.J.; Yang, S.; Yang, Y.; Zang, W.J. Activation of M3AChR (Type 3 Muscarinic Acetylcholine Receptor) and Nrf2 (Nuclear Factor Erythroid 2-Related Factor 2) Signaling by Choline Alleviates Vascular Smooth Muscle Cell Phenotypic Switching and Vascular Remodeling. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2649–2664. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Li, C.L.; Huang, X.J.; Chen, G.A.; Huang, X.R.; Song, F.E.; Zhou, Y.; Liu, X.C.; Zhou, X.K.; Meng, J.X.; et al. Single-cell RNA sequencing reveals that NRF2 regulates vascular smooth muscle cell phenotypic switching in abdominal aortic aneurysm. FASEB J. 2024, 38, e23707. [Google Scholar] [CrossRef]
- Wanhainen, A.; Dalman, R.L. Update on Ongoing Randomised Controlled Trials Evaluating the Protective Effect of Metformin on Abdominal Aortic Aneurysm Progression. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 6–8. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 8) | ND-AAA (n = 19) | D-AAA (n = 5) * | p Value C vs. ND-AAA vs. D-AAA | p Value ND-AAA vs. D-AAA | |
| Age (years) | 52.4 ± 16.7 | 72.1 ± 9.1 | 75.0 ± 7.8 | <0.001 | 0.520 |
| Male | 3 (42.9) | 12 (66.7) | 2 (40) | 0.893 | 1.000 |
| Aneurysm size (mm) | N/A | 76.2 ± 19.9 | 65.0 ± 10.1 | N/A | 0.113 |
| Rupture | N/A | 3 (16.7) | 1 (20.0) | N/A | 1.000 |
| Current smoking | N/A | 6 (35.3) | 0 (0.0) | N/A | 0.266 |
| Hypertension † | N/A | 7 (43.8) | 5 (100) | N/A | 0.045 |
| Previous vascular surgery ‡ | N/A | 6 (37.5) | 3 (60) | N/A | 0.611 |
| Renal dysfunction | N/A | 1 (6.3) | 4 (80) | N/A | 0.004 |
| BMI | N/A | 26.2 ± 4.1 | 29.2 ± 6.3 | N/A | 0.211 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Merrienboer, T.A.R.; Rombouts, K.B.; van Wijk, A.C.W.A.; Knol, J.C.; Pham, T.V.; Piersma, S.R.; Jimenez, C.R.; Balm, R.; Yeung, K.K.; de Waard, V. Proteome Differences in Smooth Muscle Cells from Diabetic and Non-Diabetic Abdominal Aortic Aneurysm Patients Reveal Metformin-Induced Mechanisms. Med. Sci. 2025, 13, 184. https://doi.org/10.3390/medsci13030184
van Merrienboer TAR, Rombouts KB, van Wijk ACWA, Knol JC, Pham TV, Piersma SR, Jimenez CR, Balm R, Yeung KK, de Waard V. Proteome Differences in Smooth Muscle Cells from Diabetic and Non-Diabetic Abdominal Aortic Aneurysm Patients Reveal Metformin-Induced Mechanisms. Medical Sciences. 2025; 13(3):184. https://doi.org/10.3390/medsci13030184
Chicago/Turabian Stylevan Merrienboer, Tara A. R., Karlijn B. Rombouts, Albert C. W. A. van Wijk, Jaco C. Knol, Thang V. Pham, Sander R. Piersma, Connie R. Jimenez, Ron Balm, Kak K. Yeung, and Vivian de Waard. 2025. "Proteome Differences in Smooth Muscle Cells from Diabetic and Non-Diabetic Abdominal Aortic Aneurysm Patients Reveal Metformin-Induced Mechanisms" Medical Sciences 13, no. 3: 184. https://doi.org/10.3390/medsci13030184
APA Stylevan Merrienboer, T. A. R., Rombouts, K. B., van Wijk, A. C. W. A., Knol, J. C., Pham, T. V., Piersma, S. R., Jimenez, C. R., Balm, R., Yeung, K. K., & de Waard, V. (2025). Proteome Differences in Smooth Muscle Cells from Diabetic and Non-Diabetic Abdominal Aortic Aneurysm Patients Reveal Metformin-Induced Mechanisms. Medical Sciences, 13(3), 184. https://doi.org/10.3390/medsci13030184







