Obesity and Pregnancy: Impact on Childbirth Timing, Delivery Mode, and Maternal Recovery: An Update
Abstract
1. Introduction
2. Materials and Methods
3. Maternal Obesity and Pregnancy Complications
3.1. Pathophysiology of Obesity During Pregnancy
3.2. Chronic Inflammation and Placental Dysfunction
3.3. Insulin Resistance, Dyslipidemia, and Gestational Diabetes
3.4. Renin–Angiotensin System Dysregulation in Pregnancy
3.5. Cardiovascular and Hemodynamic Stress in Obese Pregnancies
3.6. Microbiome Dysbiosis and Inflammation
4. The Impact of Obesity on Childbirth Timing
4.1. Mode of Delivery in Obese Women
4.2. Anesthesia and Surgical Considerations
5. Fetal and Neonatal Outcomes
5.1. Impact on Fetal Development
5.2. Neonatal Health at Birth and Long-Term Implications
6. Maternal Progress and Recovery
6.1. Postpartum Recovery Challenges
6.2. Breastfeeding Challenges
6.3. Public Health Implications and Clinical Recommendations
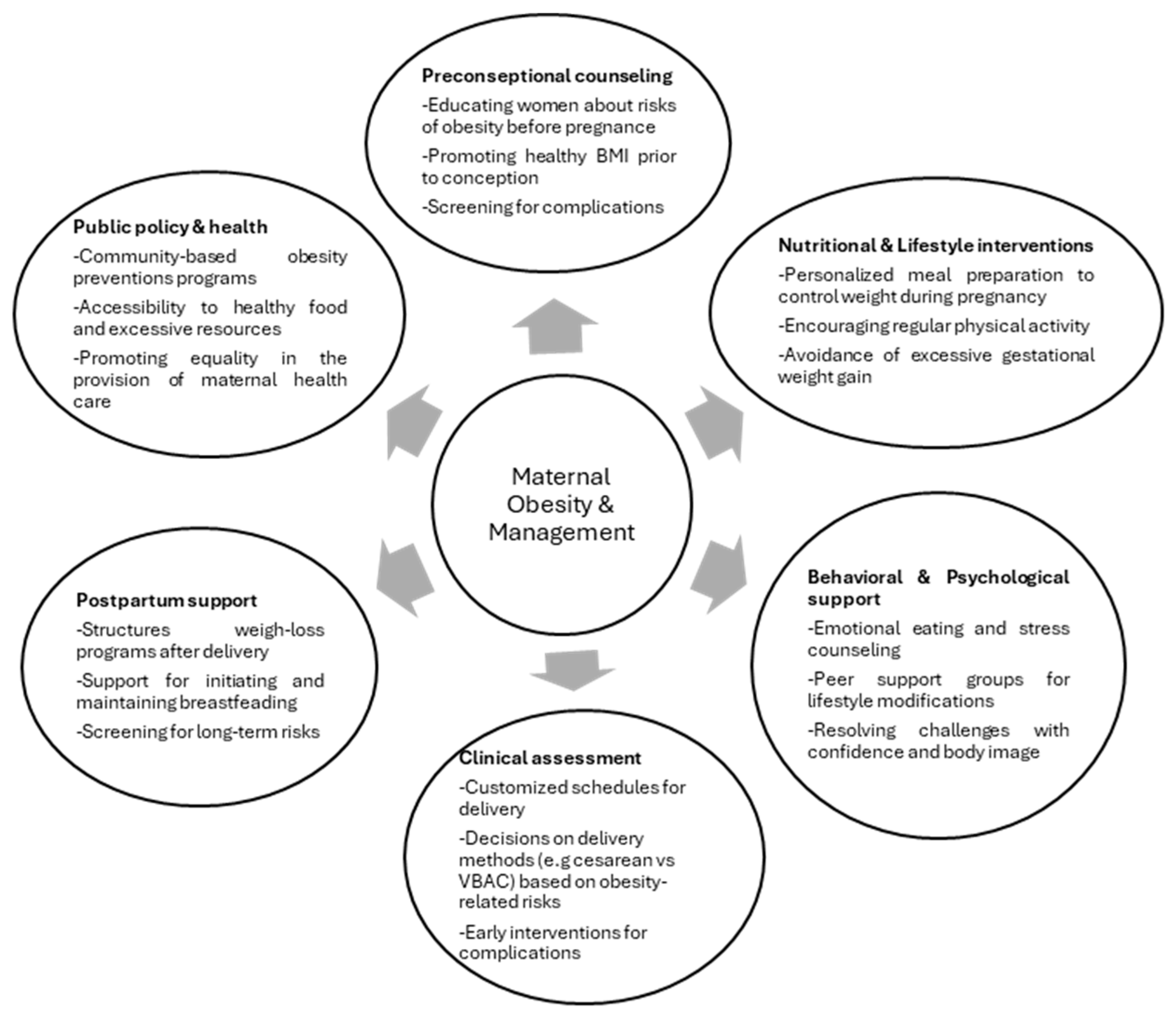
7. Preventive Strategies and Recommendations
7.1. Lifestyle Modifications for Weight Management
7.2. Recommendations for Clinicians and Healthcare Providers
7.3. Policies on Timing and Mode of Delivery
7.4. Postpartum Support for Weight Management and Mental Health
8. Discussion
9. Research Gaps and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GDM | Gestational Diabetes Mellitus |
| NAFLD | Non-Alcoholic Fatty Liver Disease |
| GWG | Gestational Weight Gain |
| BMI | Body Mass Index |
| WHO | World Health Organization |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| CRP | C-Reactive Protein |
| ROS | Reactive Oxygen Species |
| IUGR | Intrauterine Growth Restriction |
| RAS | Renin–Angiotensin System |
| PIH | Pregnancy-Induced Hypertension |
| VBAC | Vaginal Birth After Cesarean |
| LPS | Lipopolysaccharide |
| FATP | Fatty Acid Transport Protein |
| GLUT1 | Glucose Transporter 1 |
| IRS-1 | Insulin Receptor Substrate-1 |
| Ang II | Angiotensin II |
| LDL | Low-Density Lipoprotein |
| VLDL | Very-Low-Density Lipoprotein |
| TGs | Triglycerides |
| FFAs | Free Fatty Acids |
| MMP | Matrix Metalloproteinase |
| PROM | Premature Rupture of Membranes |
| PIS | Perinatal Ischemic Stroke |
| RSA | Recurrent Spontaneous Abortion |
| PCOS | Polycystic Ovary Syndrome |
| SSIs | Surgical Site Infections |
| DVT | Deep Vein Thrombosis |
| mHealth | Mobile Health |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor Gamma |
| NO | Nitric Oxide |
| PPROM | Preterm Premature Rupture of Membranes |
| FIGO | International Federation of Gynecology and Obstetrics |
References
- Poniedziałek-Czajkowska, E.; Mierzyński, R.; Leszczyńska-Gorzelak, B. Preeclampsia and Obesity—The Preventive Role of Exercise. Int. J. Environ. Res. Public Health 2023, 20, 1267. [Google Scholar] [CrossRef]
- Berti, C.; Elahi, S.; Catalano, P.; Bhutta, Z.A.; Krawinkel, M.B.; Parisi, F.; Agostoni, C.; Cetin, I.; Hanson, M. Obesity, Pregnancy and the Social Contract with Today’s Adolescents. Nutrients 2022, 14, 3550. [Google Scholar] [CrossRef]
- Flenady, V.; Koopmans, L.; Middleton, P.; Frøen, J.F.; Smith, G.C.; Gibbons, K.; Coory, M.; Gordon, A.; Ellwood, D.; McIntyre, H.D.; et al. Major Risk Factors for Stillbirth in High-Income Countries: A Systematic Review and Meta-Analysis. Lancet 2011, 377, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Victora, C.G.; Walker, S.P.; Bhutta, Z.A.; Christian, P.; De Onis, M.; Ezzati, M.; Grantham-Mcgregor, S.; Katz, J.; Martorell, R.; et al. Maternal and Child Undernutrition and Overweight in Low-Income and Middle-Income Countries. Lancet 2013, 382, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Tekin-Guler, T.; Koc, N.; Kara-Uzun, A.; Fisunoglu, M. The Association of Pre-Pregnancy Obesity and Breast Milk Fatty Acids Composition and the Relationship of Postpartum Maternal Diet, Breast Milk Fatty Acids and Infant Growth. Breastfeed. Med. 2023, 18, 475–482. [Google Scholar] [CrossRef]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The Role of Endocrine Disruptors Bisphenols and Phthalates in Obesity: Current Evidence, Perspectives and Controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef]
- Navaee, M.; Kashanian, M.; Kabir, A.; Zamaninour, N.; Chamari, M.; Pazouki, A. Maternal and Fetal/Neonatal Outcomes in Pregnancy, Delivery and Postpartum Following Bariatric Surgery and Comparison with Pregnant Women with Obesity: A Study Protocol for a Prospective Cohort. Reprod. Health 2024, 21, 8. [Google Scholar] [CrossRef]
- Stamilio, D.M.; Scifres, C.M. Extreme Obesity and Postcesarean Maternal Complications. Obstet. Gynecol. 2014, 124, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Conner, S.N.; Verticchio, J.C.; Tuuli, M.G.; Odibo, A.O.; Macones, G.A.; Cahill, A.G. Maternal Obesity and Risk of Postcesarean Wound Complications. Am. J. Perinatol. 2014, 31, 299–303. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of Maternal Obesity on the Long-Term Health of Offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Moll, U.; Olsson, H.; Landin-Olsson, M. Women with a Predisposition for Diabetes Have an Increased Risk of Pregnancy Complications, Especially in Combination with Pregestational Overweight. BMC Pregnancy Childbirth 2020, 20, 74. [Google Scholar] [CrossRef]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The Impact of Maternal Obesity on Maternal and Fetal Health. Rev. Obstet. Gynecol. 2008, 1, 170. [Google Scholar] [CrossRef]
- de Siqueira, C.D.; Borges, L.; Dal Mora, T.; Saleh, N.A.; Alves, E.S.; Wopereis, S.; Mendes, B.G.; de Moraes, A.C.R.; Hatanaka, E.; Filippin-Monteiro, F.B. Early Postnatal Effects of Maternal Obesity on Breast Milk Composition and Breastfeeding Outcomes. Clin. Nutr. ESPEN 2025, 65, 365–374. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Maternal High-Fat Diet and Offspring Hypertension. Int. J. Mol. Sci. 2022, 23, 8179. [Google Scholar] [CrossRef]
- Poornima, I.G.; Indaram, M.; Ross, J.D.; Agarwala, A.; Wild, R.A. Hyperlipidemia and Risk for Preclampsia. J. Clin. Lipidol. 2022, 16, 253–260. [Google Scholar] [CrossRef]
- Preda, A.; Preda, S.D.; Mota, M.; Iliescu, D.G.; Zorila, L.G.; Comanescu, A.C.; Mitrea, A.; Clenciu, D.; Mota, E.; Vladu, I.M. Dyslipidemia in Pregnancy: A Systematic Review of Molecular Alterations and Clinical Implications. Biomedicines 2024, 12, 2252. [Google Scholar] [CrossRef]
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef]
- Huifen, Z.; Yaping, X.; Meijing, Z.; Huibin, H.; Chunhong, L.; Fengfeng, H.; Yaping, Z. Effects of Moderate-Intensity Resistance Exercise on Blood Glucose and Pregnancy Outcome in Patients with Gestational Diabetes Mellitus: A Randomized Controlled Trial. J. Diabetes Complicat. 2022, 36, 108186. [Google Scholar] [CrossRef] [PubMed]
- Uebel, K.; Pusch, K.; Gedrich, K.; Schneider, K.T.M.; Hauner, H.; Bader, B.L. Effect of Maternal Obesity with and without Gestational Diabetes on Offspring Subcutaneous and Preperitoneal Adipose Tissue Development from Birth up to Year-1. BMC Pregnancy Childbirth 2014, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and Maternal Obesity: Epidemiology and Health Consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, A.; Vilariño-García, T.; Guadix, P.; Dueñas, J.L.; Sánchez-Margalet, V. Leptin and Nutrition in Gestational Diabetes. Nutrients 2020, 12, 1970. [Google Scholar] [CrossRef]
- Pheiffer, C.; Dias, S.; Jack, B.; Malaza, N.; Adam, S. Adiponectin as a Potential Biomarker for Pregnancy Disorders. Int. J. Mol. Sci. 2021, 22, 1326. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental Function in Maternal Obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Yang, X.; Hu, R.; Shi, M.; Wang, L.; Yan, J.; Gong, J.; Zhang, Q.; He, J.; Wu, S. Placental Malfunction, Fetal Survival and Development Caused by Sow Metabolic Disorder: The Impact of Maternal Oxidative Stress. Antioxidants 2023, 12, 360. [Google Scholar] [CrossRef]
- Bokor, S.; Csölle, I.; Felső, R.; Vass, R.A.; Funke, S.; Ertl, T.; Molnár, D. Dietary Nutrients during Gestation Cause Obesity and Related Metabolic Changes by Altering DNA Methylation in the Offspring. Front. Endocrinol. 2024, 15, 1287255. [Google Scholar] [CrossRef]
- Sheldon, R.E.; Mashayamombe, C.; Shi, S.Q.; Garfield, R.E.; Shmygol, A.; Blanks, A.M.; Van Den Berg, H.A. Alterations in Gap Junction Connexin43/Connexin45 Ratio Mediate a Transition from Quiescence to Excitation in a Mathematical Model of the Myometrium. J. R. Soc. Interface 2014, 11, 20140726. [Google Scholar] [CrossRef]
- Jin, X.; Perrella, S.L.; Lai, C.T.; Taylor, N.L.; Geddes, D.T. Causes of Low Milk Supply: The Roles of Estrogens, Progesterone, and Related External Factors. Adv. Nutr. 2023, 15, 100129. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling Pathways in Obesity: Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2022, 7, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.; Wang, J.; Leng, Y.; Li, L.; Wang, H. Role of Obesity in Female Reproduction. Int. J. Med. Sci. 2023, 20, 366–375. [Google Scholar] [CrossRef]
- Wang, J.; Liao, B.; Wang, C.; Zhong, O.; Lei, X.; Yang, Y. Effects of Antioxidant Supplementation on Metabolic Disorders in Obese Patients from Randomized Clinical Controls: A Meta-Analysis and Systematic Review. Oxid. Med. Cell. Longev. 2022, 2022, 7255413. [Google Scholar] [CrossRef]
- Leca, B.M.; Lagojda, L.; Kite, C.; Karteris, E.; Kassi, E.; Randeva, H.S.; Kyrou, I. Maternal Obesity and Metabolic (Dysfunction) Associated Fatty Liver Disease in Pregnancy: A Comprehensive Narrative Review. Expert Rev. Endocrinol. Metab. 2024, 19, 335–348. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Luo, C.; Huang, J.; Li, C.; Liu, Z.; Liu, F. Gestational Diabetes Mellitus and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. BMJ 2022, 377, e067946. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.; Poulsen, C.W.; Kampmann, U.; Smedegaard, S.B.; Ovesen, P.G.; Fuglsang, J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients 2020, 12, 3050. [Google Scholar] [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ 2017, 356, j1. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, Y.; Zang, H.; Cong, X.; Shen, Q.; Chen, L.; Chen, X. Lipid Metabolism in Pregnancy Women with Hypothyroidism and Potential Influence on Pregnancy Outcome. J. Lipids 2024, 2024, 5589492. [Google Scholar] [CrossRef]
- Albrecht, M.; Worthmann, A.; Heeren, J.; Diemert, A.; Arck, P.C. Maternal Lipids in Overweight and Obesity: Implications for Pregnancy Outcomes and Offspring’s Body Composition. Semin. Immunopathol. 2025, 47, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Formisano, E.; Proietti, E.; Perrone, G.; Demarco, V.; Galoppi, P.; Stefanutti, C.; Pisciotta, L. Characteristics, Physiopathology and Management of Dyslipidemias in Pregnancy: A Narrative Review. Nutrients 2024, 16, 2927. [Google Scholar] [CrossRef]
- Qu, H.; Khalil, R.A. Vascular Mechanisms and Molecular Targets in Hypertensive Pregnancy and Preeclampsia. Rev. Integr. Cardiovasc. Physiol. Pathophysiol. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, 661–681. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming. Int. J. Mol. Sci. 2024, 25, 3298. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; He, H.; Zhao, K. Thrombophilic Gene Polymorphisms and Recurrent Pregnancy Loss: A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2023, 40, 1533–1558. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Sharma, V.; Kaur, D.; Purkait, P. Angiotensin-Converting Enzyme (ACE) Insertion/Deletion (I/D) Polymorphism as a Conjoint Regulator of Coagulation, Fibrinolytic, and RAAS Pathway in Infertility and Associated Pregnancy Complications. JRAAS—J. Renin-Angiotensin-Aldosterone Syst. 2022, 2022, 1695769. [Google Scholar] [CrossRef]
- Veille, J.C.; Hanson, R. Obesity, Pregnancy, and Left Ventricular Functioning during the Third Trimester. Am. J. Obs. Gynecol. 1994, 171, 980–983. [Google Scholar] [CrossRef]
- Ferrari, B.; Peyvandi, F. How I Treat Thrombotic Thrombocytopenic Purpura in Pregnancy. Blood 2020, 136, 2125–2132. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and Consequences of Endothelial Cell Senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Torres-Torres, J.; Espino-y-Sosa, S.; Martinez-Portilla, R.; Borboa-Olivares, H.; Estrada-Gutierrez, G.; Acevedo-Gallegos, S.; Ruiz-Ramirez, E.; Velasco-Espin, M.; Cerda-Flores, P.; Ramirez-Gonzalez, A.; et al. A Narrative Review on the Pathophysiology of Preeclampsia. Int. J. Mol. Sci. 2024, 25, 7569. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, M.; Celewicz, Z.; Szczuko, M. Short-Chain Fatty Acids, Maternal Microbiota and Metabolism in Pregnancy. Nutrients 2021, 13, 1244. [Google Scholar] [CrossRef]
- Arya, S.; Hansen, K.R.; Peck, J.D.; Wild, R.A. Metabolic Syndrome in Obesity: Treatment Success and Adverse Pregnancy Outcomes with Ovulation Induction in Polycystic Ovary Syndrome. Am. J. Obstet. Gynecol. 2021, 225, 280.e1–280.e11. [Google Scholar] [CrossRef]
- Koletzko, B.; Godfrey, K.M.; Poston, L.; Szajewska, H.; Van Goudoever, J.B.; De Waard, M.; Brands, B.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M.; et al. Nutrition during Pregnancy, Lactation and Early Childhood and Its Implications for Maternal and Long-Term Child Health: The Early Nutrition Project Recommendations. Ann. Nutr. Metab. 2019, 74, 93–106. [Google Scholar] [CrossRef]
- Reed, J.; Case, S.; Rijhsinghani, A. Maternal Obesity: Perinatal Implications. SAGE Open Med. 2023, 11, 20503121231176128. [Google Scholar] [CrossRef]
- Grieger, J.A.; Hutchesson, M.J.; Cooray, S.D.; Bahri Khomami, M.; Zaman, S.; Segan, L.; Teede, H.; Moran, L.J. A Review of Maternal Overweight and Obesity and Its Impact on Cardiometabolic Outcomes during Pregnancy and Postpartum. Ther. Adv. Reprod. Health 2021, 15, 263349412098654. [Google Scholar] [CrossRef]
- Pinheiro, T.V.; Goldani, M.Z. Maternal Pre-Pregnancy Overweight/Obesity and Gestational Diabetes Interaction on Delayed Breastfeeding Initiation. PLoS ONE 2018, 13, e0194879. [Google Scholar] [CrossRef]
- Froń, A.; Orczyk-Pawiłowicz, M. Understanding the Immunological Quality of Breast Milk in Maternal Overweight and Obesity. Nutrients 2023, 15, 5016. [Google Scholar] [CrossRef]
- Wen, L.M.; Simpson, J.M.; Baur, L.A.; Rissel, C.; Flood, V.M. Family Functioning and Obesity Risk Behaviors: Implications for Early Obesity Intervention. Obesity 2011, 19, 1252–1258. [Google Scholar] [CrossRef]
- Hautakangas, T.; Uotila, J.; Kontiainen, J.; Huhtala, H.; Palomäki, O. Impact of Obesity on Uterine Contractile Activity during Labour: A Blinded Analysis of a Randomised Controlled Trial Cohort. BJOG 2022, 129, 1790–1797. [Google Scholar] [CrossRef] [PubMed]
- Langley-Evans, S.C.; Pearce, J.; Ellis, S. Overweight, Obesity and Excessive Weight Gain in Pregnancy as Risk Factors for Adverse Pregnancy Outcomes: A Narrative Review. J. Hum. Nutr. Diet. 2022, 35, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.D.; Shi, Z.; Lambrecht, N.J.; Jiang, Y.; Wang, J.; Burmeister, M.; Li, M.; Lozoff, B. Maternal Overweight and Obesity during Pregnancy Are Associated with Neonatal, but Not Maternal, Hepcidin Concentrations. J. Nutr. 2021, 151, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Sauder, K.A.; Gamalski, K.; DeRoeck, J.; Vasquez, F.P.; Dabelea, D.; Glueck, D.H.; Catenacci, V.A.; Fabbri, S.; Ritchie, N.D. A Pre-Conception Clinical Trial to Reduce Intergenerational Obesity and Diabetes Risks: The NDPP-NextGen Trial Protocol. Contemp. Clin. Trials 2023, 133, 107305. [Google Scholar] [CrossRef]
- Kissler, K.; Hurt, K.J. The Pathophysiology of Labor Dystocia: Theme with Variations. Reprod. Sci. 2023, 30, 729–742. [Google Scholar] [CrossRef]
- Kim, S.T. Anesthetic Management of Obese and Morbidly Obese Parturients. Anesth. Pain Med. 2021, 16, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Wawer, A.A.; Hodyl, N.A.; Fairweather-Tait, S.; Froessler, B. Are Pregnant Women Who Are Living with Overweight or Obesity at Greater Risk of Developing Iron Deficiency/Anaemia? Nutrients 2021, 13, 1572. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wu, H.; Chen, Q.; Tang, T.; Li, J.; An, H.; Zhu, S.; Han, L.; Sun, H.; Ge, J.; et al. Maternal Obesity Induces the Meiotic Defects and Epigenetic Alterations During Fetal Oocyte Development. Adv. Sci. 2024, 11, e2309184. [Google Scholar] [CrossRef] [PubMed]
- Khammarnia, M.; Ansari-Moghaddam, A.; Kakhki, F.G.; Clark, C.C.T.; Barahouei, F.B. Maternal Macronutrient and Energy Intake during Pregnancy: A Systematic Review and Meta-Analysis. BMC Public Health 2024, 24, 478. [Google Scholar] [CrossRef]
- Nagl, M.; Linde, K.; Stepan, H.; Kersting, A. Obesity and Anxiety during Pregnancy and Postpartum: A Systematic Review. J. Affect. Disord. 2015, 186, 293–305. [Google Scholar] [CrossRef]
- de Souza Lima, B.; Sanches, A.P.V.; Ferreira, M.S.; de Oliveira, J.L.; Cleal, J.K.; Ignacio-Souza, L. Maternal-Placental Axis and Its Impact on Fetal Outcomes, Metabolism, and Development. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166855. [Google Scholar] [CrossRef]
- Harmancıoğlu, B.; Kabaran, S. Maternal High Fat Diets: Impacts on Offspring Obesity and Epigenetic Hypothalamic Programming. Front. Genet. 2023, 14, 1158089. [Google Scholar] [CrossRef]
- Shrestha, A.; Prowak, M.; Berlandi-Short, V.M.; Garay, J.; Ramalingam, L. Maternal Obesity: A Focus on Maternal Interventions to Improve Health of Offspring. Front. Cardiovasc. Med. 2021, 8, 696812. [Google Scholar] [CrossRef]
- Broughton, D.E.; Moley, K.H. Obesity and Female Infertility: Potential Mediators of Obesity’s Impact. Fertil. Steril. 2017, 107, 840–847. [Google Scholar] [CrossRef]
- Alsheef, M.A.; Alabbad, A.M.; Albassam, R.A.; Alarfaj, R.M.; Zaidi, A.R.Z.; Al-Arfaj, O.; Abu-Shaheen, A. Pregnancy and Venous Thromboembolism: Risk Factors, Trends, Management, and Mortality. Biomed. Res. Int. 2020, 2020, 4071892. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, C.; He, Q.; Liao, S.; Luo, B. Effects of MHealth-Based Lifestyle Interventions on Gestational Diabetes Mellitus in Pregnant Women With Overweight and Obesity: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2024, 12, e49373. [Google Scholar] [CrossRef]
- Monaco-Brown, M.; Lawrence, D.A. Obesity and Maternal-Placental-Fetal Immunology and Health. Front. Pediatr. 2022, 10, 859885. [Google Scholar] [CrossRef]
- Duttaroy, A.K.; Basak, S. Maternal Fatty Acid Metabolism in Pregnancy and Its Consequences in the Feto-Placental Development. Front. Physiol. 2022, 12, 787848. [Google Scholar] [CrossRef]
- Gaudet, L.; Ferraro, Z.M.; Wen, S.W.; Walker, M. Maternal Obesity and Occurrence of Fetal Macrosomia: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2014, 2014, 640291. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Molecular Links and Clinical Effects of Inflammation and Metabolic Background on Ischemic Stroke: An Update Review. J. Clin. Med. 2024, 13, 7515. [Google Scholar] [CrossRef]
- Hasanpour-Segherlou, Z.; Butler, A.A.; Candelario-Jalil, E.; Hoh, B.L. Role of the Unique Secreted Peptide Adropin in Various Physiological and Disease States. Biomolecules 2024, 14, 1613. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental Origins of Metabolic Diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Fruh, S.M. Obesity: Risk Factors, Complications, and Strategies for Sustainable Long-Term Weight Management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Moussa, H.N.; Alrais, M.A.; Leon, M.G.; Abbas, E.L.; Sibai, B.M. Obesity Epidemic: Impact from Preconception to Postpartum. Future Sci. OA 2016, 2, FSO137. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Li, L.; Chen, W.; Liu, Z.B.; Ma, L.; Gao, X.X.; He, J.L.; Wang, H.; Zhao, M.; Yang, Y.Y.; et al. Pre-Pregnancy Underweight and Obesity Are Positively Associated with Small-for-Gestational-Age Infants in a Chinese Population. Sci. Rep. 2019, 9, 15544. [Google Scholar] [CrossRef]
- Hoek, A.; Wang, Z.; van Oers, A.M.; Groen, H.; Cantineau, A.E.P. Effects of Preconception Weight Loss after Lifestyle Intervention on Fertility Outcomes and Pregnancy Complications. Fertil. Steril. 2022, 118, 456–462. [Google Scholar] [CrossRef]
- Poobalan, A.S.; Aucott, L.S.; Precious, E.; Crombie, I.K.; Smith, W.C.S. Weight Loss Interventions in Young People (18 to 25 Year Olds): A Systematic Review. Obes. Rev. 2010, 11, 580–592. [Google Scholar] [CrossRef] [PubMed]
- McAuliffe, F.M.; Killeen, S.L.; Jacob, C.M.; Hanson, M.A.; Hadar, E.; McIntyre, H.D.; Kapur, A.; Kihara, A.B.; Ma, R.C.; Divakar, H.; et al. Management of Prepregnancy, Pregnancy, and Postpartum Obesity from the FIGO Pregnancy and Non-Communicable Diseases Committee: A FIGO (International Federation of Gynecology and Obstetrics) Guideline. Int. J. Gynecol. Obstet. 2020, 151 (Suppl. 1), 16–36. [Google Scholar] [CrossRef]
- Grobman, W.A. The Role of Labor Induction in Modern Obstetrics. Am. J. Obs. Gynecol. 2024, 230, S662–S668. [Google Scholar] [CrossRef]
- Wen, C.P.; Wai, J.P.M.; Tsai, M.K.; Yang, Y.C.; Cheng, T.Y.D.; Lee, M.C.; Chan, H.T.; Tsao, C.K.; Tsai, S.P.; Wu, X. Minimum Amount of Physical Activity for Reduced Mortality and Extended Life Expectancy: A Prospective Cohort Study. Lancet 2011, 378, 1244–1253. [Google Scholar] [CrossRef]
- Lassi, Z.S.; Dean, S.V.; Mallick, D.; Bhutta, Z.A. Preconception Care: Delivery Strategies and Packages for Care. Reprod. Health 2014, 11, 1–17. [Google Scholar] [CrossRef]
- Blomberg, M. Maternal and Neonatal Outcomes among Obese Women with Weight Gain below the New Institute of Medicine Recommendations. Obstet. Gynecol. 2011, 117, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Kim, S.Y.; Schmid, C.H.; Dietz, P.M.; Callaghan, W.M.; Lau, J.; Curtis, K.M. Maternal Obesity and Risk of Cesarean Delivery: A Meta-Analysis. Obes. Rev. 2007, 8, 385–394. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Versace, V.; Mohebbi, M.; Lim, S.; Janus, E.; Dunbar, J. The Effect of a Diabetes Prevention Program on Dietary Quality in Women with Previous Gestational Diabetes. BMC Womens Health 2019, 19, 88. [Google Scholar] [CrossRef]
- Phelan, S.; Hagobian, T.; Brannen, A.; Hatley, K.E.; Schaffner, A.; Muñoz-Christian, K.; Tate, D.F. Effect of an Internet-Based Program on Weight Loss for Low-Income Postpartum Women: A Randomized Clinical Trial. JAMA 2017, 317, 2381. [Google Scholar] [CrossRef] [PubMed]
- Anne Gilmore, L.; Klempel, M.C.; Martin, C.K.; Myers, C.A.; Burton, J.H.; Sutton, E.F.; Redman, L.M. Personalized Mobile Health Intervention for Health and Weight Loss in Postpartum Women Receiving Women, Infants, and Children Benefit: A Randomized Controlled Pilot Study. J. Womens Health 2017, 26, 719–724. [Google Scholar] [CrossRef]
- Molyneaux, E.; Poston, L.; Ashurst-Williams, S.; Howard, L.M. Obesity and Mental Disorders during Pregnancy and Postpartum: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2014, 123, 857–867. [Google Scholar] [CrossRef]
- Su, K.P.; Matsuoka, Y.; Pae, C.U. Omega-3 Polyunsaturated Fatty Acids in Prevention of Mood and Anxiety Disorders. Clin. Psychopharmacol. Neurosci. 2015, 13, 129. [Google Scholar] [CrossRef]
- Slykerman, R.F.; Hood, F.; Wickens, K.; Thompson, J.M.D.; Barthow, C.; Murphy, R.; Kang, J.; Rowden, J.; Stone, P.; Crane, J.; et al. Effect of Lactobacillus rhamnosus HN001 in Pregnancy on Postpartum Symptoms of Depression and Anxiety: A Randomised Double-Blind Placebo-Controlled Trial. EBioMedicine 2017, 24, 159–165. [Google Scholar] [CrossRef]
- Denizli, M.; Capitano, M.L.; Kua, K.L. Maternal Obesity and the Impact of Associated Early-Life Inflammation on Long-Term Health of Offspring. Front. Cell. Infect. Microbiol. 2022, 12, 940937. [Google Scholar] [CrossRef]
- Reichetzeder, C. Overweight and Obesity in Pregnancy: Their Impact on Epigenetics. Eur. J. Clin. Nutr. 2021, 75, 1710–1722. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Felix, J.F.; Duijts, L.; Jaddoe, V.W.V. Childhood Consequences of Maternal Obesity and Excessive Weight Gain during Pregnancy. Acta Obs. Gynecol. Scand. 2014, 93, 1085–1089. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Diagnosis and Classification of Diabetes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47, S20–S42. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. Reducing the Risk of Thrombosis and Embolism During Pregnancy and the Puerperium (Green-Top Guideline No. 37a). RCOG. Available online: https://www.rcog.org.uk/guidance/browse-all-guidance/green-top-guidelines/reducing-the-risk-of-thrombosis-and-embolism-during-pregnancy-and-the-puerperium-green-top-guideline-no-37a/ (accessed on 19 May 2025).
- Heslehurst, N.; Simpson, H.; Ells, L.J.; Rankin, J.; Wilkinson, J.; Lang, R.; Brown, T.J.; Summerbell, C.D. The Impact of Maternal BMI Status on Pregnancy Outcomes with Immediate Short-Term Obstetric Resource Implications: A Meta-Analysis. Obes. Rev. 2008, 9, 635–683. [Google Scholar] [CrossRef]
| Molecular Mechanism | Key Regulators | Impact on Pregnancy | Outcome |
|---|---|---|---|
| Chronic Inflammation | TNF-a, IL-6, CRP | Endothelial dysfunction, Oxidative stress, Reduced NO generation | Preeclampsia, GDM |
| Hormonal Dysregulation | Leptin, adiponectin | Appetite dysregulation, Impaired glucose metabolism | Excessive GWG, GDM |
| Placental Dysfunction | GLUT1, FATP, ROS | Altered nutrient transport, Trophoblast dysfunction | Macrosomia, Fetal growth restriction, Stillbirth |
| Epigenetic Modifications | DNA methylation, histone modifications, miRNAs | Altered gene expression in metabolic pathways | Long-term risks, Childhood obesity, Type 2 diabetes |
| Uterine Contractility and Labor | Oxytocin receptors, connexin 43 | Reduced contractility, Delayed labor onset | Prolonged labor, Cesarean delivery |
| Postpartum Recovery Challenges | MMPs, macrophages, prolactin | Impaired wound healing, Reduced lactation | Delayed recovery, Breastfeeding difficulties |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerede, A.; Danavasi, M.; Stavros, S.; Potiris, A.; Zikopoulos, A.; Moustakli, E.; Skentou, C.; Domali, E.; Nikolettos, N.; Eleftheriades, M. Obesity and Pregnancy: Impact on Childbirth Timing, Delivery Mode, and Maternal Recovery: An Update. Med. Sci. 2025, 13, 182. https://doi.org/10.3390/medsci13030182
Gerede A, Danavasi M, Stavros S, Potiris A, Zikopoulos A, Moustakli E, Skentou C, Domali E, Nikolettos N, Eleftheriades M. Obesity and Pregnancy: Impact on Childbirth Timing, Delivery Mode, and Maternal Recovery: An Update. Medical Sciences. 2025; 13(3):182. https://doi.org/10.3390/medsci13030182
Chicago/Turabian StyleGerede, Angeliki, Maria Danavasi, Sofoklis Stavros, Anastasios Potiris, Athanasios Zikopoulos, Efthalia Moustakli, Charikleia Skentou, Ekaterini Domali, Nikolaos Nikolettos, and Makarios Eleftheriades. 2025. "Obesity and Pregnancy: Impact on Childbirth Timing, Delivery Mode, and Maternal Recovery: An Update" Medical Sciences 13, no. 3: 182. https://doi.org/10.3390/medsci13030182
APA StyleGerede, A., Danavasi, M., Stavros, S., Potiris, A., Zikopoulos, A., Moustakli, E., Skentou, C., Domali, E., Nikolettos, N., & Eleftheriades, M. (2025). Obesity and Pregnancy: Impact on Childbirth Timing, Delivery Mode, and Maternal Recovery: An Update. Medical Sciences, 13(3), 182. https://doi.org/10.3390/medsci13030182








