Abstract
Emerging evidence suggests that vitamin D and dipeptidyl peptidase-4 (DPP-4) inhibitors exert synergistic immunomodulatory, anti-inflammatory and antioxidant actions. Moreover, intervention studies showed that combination therapy based on the concomitant use of vitamin D and DPP-4 inhibitors (VIDPP-4i) may preserve beta-cell function in patients with type 1 diabetes mellitus (T1D) and latent autoimmune diabetes in adults (LADA). These effects are particularly relevant in the context of beta-cell replacement strategies, whose long-term efficacy can be hampered by various factors, such as immune-mediated graft rejection, inadequate vascularization, hypoxia, trauma-induced cell apoptosis, fibrosis, host immune response, and recurrence of autoimmunity. Based on preclinical and clinical studies conducted in the fields of autoimmune diabetes and solid organ/cell transplantation, the present narrative review aims to describe the rationale behind the investigation of VIDPP-4i combination therapy as an adjuvant treatment strategy to enhance the efficacy of novel beta-cell replacement therapies for T1D. In this regard, we discuss the potential immune and metabolic mechanisms through which vitamin D and DPP-4 inhibitors can promote the long-term function and survival of transplanted islets in patients with T1D receiving various types of beta-cell replacement therapies, including therapeutic approaches using encapsulated stem cell-derived beta cells.
1. Introduction: Beta-Cell Replacement Therapies for Type 1 Diabetes
Type 1 diabetes mellitus (T1D or T1DM) is an organ-specific autoimmune disease characterized by the gradual, immune-mediated destruction of insulin-secreting pancreatic beta cells, which ultimately leads to lifelong dependence on exogenous insulin [1]. Currently, there is still no definitive biological cure for T1D. Nevertheless, after a century of almost exclusive reliance on insulin therapy for the treatment of T1D, novel immunotherapies are now focused on immunomodulation for patients with pre-symptomatic disease (stages 1 and 2 T1D) and for patients with newly diagnosed (symptomatic) disease (stage 3 T1D) [2]. On the other hand, novel beta-cell replacement strategies aim at restoring pancreatic beta-cell mass and function in patients with new-onset and established T1D [3]. Islet transplantation has proven effective in restoring endogenous insulin secretion and hypoglycemia awareness in T1D patients [4]. Yet, organ donor shortage, risk of graft rejection, and the need for chronic immunosuppression restrict the adoption of islet transplantation to a limited subset of patients with T1D [5,6]. Another major challenge in clinical islet transplantation is the considerable islet cell loss occurring early and late following intrahepatic islet transplantation due to various factors, such as the instant blood-mediated inflammatory reaction (IBMIR), pronounced host autoimmune and alloimmune responses, as well as beta-cell toxicity induced by immunosuppressants [7]. Therefore, different strategies are under study to circumvent these detrimental events, such as the investigation of alternative transplantation sites, as well as the transplantation of stem cell-derived insulin-producing beta cells [3,7].
Over the past few years, remarkable advancements in stem cell biology, tissue engineering, nanotechnology, and gene editing have driven a paradigm shift in the field of beta-cell replacement therapies for T1D [5,8]. The biotechnology company ViaCyte—acquired by Vertex Pharmaceuticals in 2022—has contributed to the development of the investigational stem cell-derived, fully differentiated pancreatic islet cell therapies Zimislecel (formerly known as VX-880) and VX-264. Zimislecel is an investigational allogeneic stem cell-derived, fully differentiated, insulin-secreting islet cell therapy, which is delivered through an infusion into the hepatic portal vein and requires the chronic use of immunosuppressants to prevent the immune-mediated rejection of islet cells [9,10]. The recently published phase 1–2 VX-880-101 FORWARD study investigated the safety and efficacy profile of Zimislecel in adults with T1D who had impaired awareness of hypoglycemia and experienced repeated episodes of severe hypoglycemia [11]. Authors assessed Zimislecel at a half dose (0.4 × 109 cells) in part A of the study and at a full dose (0.8 × 109 cells) in parts B and C of the study. Zimislecel was administered through a single infusion into the portal vein over a period of 30–60 min. Study participants had a mean duration of diabetes of 22.8 years. Furthermore, all study participants received glucocorticoid-free immunosuppressive therapy. Overall, 14 participants (2 participants in part A; 12 participants in parts B and C) were included in the analyses, as they completed at least 12 months of follow-up. At baseline, fasting C-peptide (a biomarker of endogenous insulin secretion) was not detectable in all study participants. All participants exhibited engraftment and islet function after Zimislecel infusion, as documented by detectable fasting and mixed-meal tolerance test (MMTT)-stimulated serum C-peptide levels. Remarkably, 10 of the 12 participants (83%) in parts B and C of the study exhibited insulin independence at day 365. Moreover, all 12 participants enrolled in parts B and C of the study remained free of severe hypoglycemic episodes, showed a glycated hemoglobin (HbA1c) value lower than 7%, and exhibited more than 70% of the time spent in the target glucose range (70–180 mg/dL) at day 365. No serious adverse events were considered by investigators to be related (or possibly related) to Zimislecel [11].
On the other hand, VX-264 is an investigational cell therapy in which allogeneic human stem cell-derived islets are encapsulated in a channel array device designed to shield the islet cells from the attack mediated by the immune system [12]. However, Vertex recently announced that the phase 1/2 VX-264 study (based on the investigation of the fully differentiated pancreatic islet cell therapy encapsulated within a proprietary immunoprotective device) did not meet the efficacy endpoint [10]. VX-264 was generally safe and well-tolerated, but the increases in stimulated C-peptide were not observed at levels required to provide benefit [10]. Thus, VX-264, which was originally designed to be surgically implanted without the need for immunosuppression to protect the islet cells [12], will not be advancing further in clinical trials [10].
A major limitation of the current encapsulation devices containing stem cell-derived pancreatic beta cells is the partial post-transplant loss of beta cells caused by various factors, such as inadequate vascularization, hypoxia, trauma-induced cell apoptosis, fibrosis, and host immune response, which can reduce the long-term efficacy of such therapeutic strategies [13]. In particular, inadequate vascularization represents a major challenge in islet encapsulation, with functional vasculature around an islet implant ensuring proper oxygen and nutrient supply, removal of metabolic waste, and rapid insulin release kinetics [14]. Hence, there is a strong demand for novel approaches aimed at promoting and maintaining proper vascularization of the islet cell implants, ensuring adequate supply of oxygen and nutrients to beta cells, and preventing the dysfunction of the transplanted islets. Moreover, it should be highlighted that T1D is often accompanied by many abnormalities other than the loss and dysfunction of beta cells, including the dysfunction of glucagon-secreting pancreatic alpha cells [15], histological abnormalities of the exocrine pancreas [16], as well as increased serum activity of the enzyme dipeptidyl peptidase-4 (DPP-4) [17], among others.
In light of the aforementioned considerations, safe and cost-effective approaches to mitigate the risk of failure of beta-cell replacement strategies are highly required. Based on preclinical and clinical studies conducted in the field of autoimmune diseases and solid organ and cell transplantation, the present narrative review aims to describe the rationale behind the investigation of combination therapy with vitamin D plus DPP-4 inhibitors (DPP-4i) [18] as a potential adjuvant treatment strategy to enhance the efficacy of novel beta-cell replacement therapies for T1D.
2. Impact of Vitamin D on Inflammation, Autoimmunity, and Type 1 Diabetes
Vitamin D has been shown to exert immunomodulatory and anti-inflammatory actions besides its known role in the regulation of calcium-phosphorus homeostasis and bone metabolism [19]. Remarkably, vitamin D receptor (VDR) has been detected in distinct immune cells, such as monocytes, professional antigen-presenting cells (APCs: dendritic cells, macrophages, B cells), T cells, neutrophils, and microglia [20,21,22,23,24,25,26,27]. Moreover, both murine and human APCs express 25-hydroxyvitamin D(3)-1α-hydroxylase [28,29], which is the enzyme responsible for the conversion of 25-hydroxyvitamin D3 (a.k.a. calcifediol) into the biologically active form of vitamin D3 called 1,25-dihydroxyvitamin D3 (a.k.a. calcitriol) [19].
Besides serving as vitamin D targets, immune cells also act as local sources of vitamin D production [30]. It has been shown that various immune cells—such as monocytes, macrophages, dendritic cells, T cells, and B cells—express vitamin D-activating enzymes [20,28,29,31,32,33]. Moreover, it has been documented that calcitriol inhibits the production of pro-inflammatory cytokines by monocytes and macrophages [34], reduces the T-cell stimulatory capacity of macrophages [35], stimulates the transition of macrophages from a pro-inflammatory state (M1 or “classically activated” macrophages) to an anti-inflammatory state (M2 or “alternatively activated” macrophages) [36], renders the dendritic cells more tolerogenic [37], promotes the development of regulatory T cells (Tregs) [38], and favors the transition of T cells from an “effector” phenotype to a “regulatory” (anti-inflammatory) phenotype by enhancing the T-helper (Th) 2 cell development and decreasing the differentiation of Th1 and Th17 cells [39,40,41]. Moreover, a systematic review and meta-analysis of clinical trials found that vitamin D supplementation has the potential to exert antioxidant actions [42].
Additionally, preclinical evidence indicates that vitamin D can regulate insulin synthesis and secretion from pancreatic beta cells [43]. Human pancreatic beta cells express both 25-hydroxyvitamin D(3)-1α-hydroxylase [44,45] and VDR [46]. Moreover, a vitamin D response element (VDRE) has been detected in the human insulin receptor gene promoter [47]. Wei et al. [48] described a VDR-dependent transcriptional program sustaining beta-cell survival through anti-inflammatory responses. Morró et al. [49] demonstrated that transgenic mice with VDR overexpression in pancreatic beta cells are protected against the development of streptozotocin-induced diabetes and show reduced islet inflammation and preserved beta-cell mass. In addition, evidence suggests that vitamin D may promote insulin secretion and enhance insulin sensitivity. In fact, mice lacking a functional VDR show an altered insulin secretory capacity [43]. Bourlon et al. [50] found that calcitriol stimulates insulin biosynthesis and promotes the conversion of proinsulin to insulin in the rat pancreatic islets. Accordingly, calcitriol administration has been shown to increase insulin secretion in vitamin D-deficient rats [51]. Some observational studies documented an inverse correlation between serum 25-hydroxyvitamin D [25(OH)D] levels and insulin resistance [52,53], although this finding was not confirmed by other studies [54,55]. Indeed, a study conducted on subjects with overweight/obesity found that neither plasma 25(OH)D3 concentrations nor plasma 1,25-dihydroxyvitamin D3 concentrations were associated with hepatic, adipose tissue, and peripheral insulin sensitivity [55]. Interestingly, a 6-month randomized, placebo-controlled trial demonstrated that high-dose vitamin D3 supplementation (5000 IU/day) significantly increased peripheral insulin sensitivity and beta-cell function in individuals with newly diagnosed type 2 diabetes (T2D) or at high risk of diabetes [56].
Hypovitaminosis D has been increasingly recognized as a risk factor for various autoimmune diseases, including T1D [19,57,58]. Studies suggested that vitamin D intake and higher serum vitamin D levels during infancy and early childhood may decrease the risk of T1D development later in life [58,59,60]. Hence, vitamin D supplementation has been suggested as a valid tool for the prevention and treatment of different autoimmune diseases, including T1D [57,58,61,62].
Numerous preclinical studies involving non-obese diabetic (NOD) mice—a well-established animal model of human T1D [63]—documented that calcitriol and its analogs can prevent the development or counteract the progression of insulitis and autoimmune diabetes [64,65,66,67,68]. Several observational studies demonstrated that patients with new-onset and long-standing T1D exhibit significantly lower serum vitamin D levels as compared to healthy controls [69,70,71,72,73,74,75,76,77,78]. Randomized controlled trials conducted in patients with new-onset T1D showed that the administration of cholecalciferol (a.k.a. vitamin D3) increased the percentage and the suppressive capacity of Tregs [79,80]. Recently, a post hoc secondary analysis of the POSEIDON trial documented that serum 25(OH)D levels are directly associated with fasting serum C-peptide in youth and adults with recent-onset T1D [81]. This study also documented that fasting serum C-peptide levels were significantly lower in subjects with hypovitaminosis D than in subjects with sufficient serum 25(OH)D levels [81]. These findings suggested that low serum 25(OH)D levels may be associated with more aggressive beta-cell autoimmunity and lower preservation of beta-cell mass and function in subjects with recent-onset T1D. Another study documented that the administration of calcifediol for 12 months—designed to attain serum 25(OH)D values greater than 50 ng/mL—was associated with reduced peripheral blood mononuclear cell reactivity against proinsulin and glutamic acid decarboxylase 65 (GAD65) upon 25(OH)D3 replenishment, along with stable fasting C-peptide levels [82]. In keeping with these findings, we previously described the case of a 22-year-old man with recent-onset T1D who received calcifediol soon after the disease diagnosis and exhibited a prolonged duration (31 months) of the clinical remission phase of T1D [83]. A 12-month randomized controlled trial conducted in youth with new-onset T1D demonstrated that vitamin D2 (a.k.a. ergocalciferol; dose: 50,000 IU/week for 2 months, and then once every 2 weeks for 10 months) significantly reduced serum tumor necrosis factor (TNF)-alpha concentration and the rates of increase in HbA1c and insulin dose-adjusted HbA1c (IDAA1c) [84]. These findings suggested that ergocalciferol (a.k.a. vitamin D2) may preserve residual beta-cell function and sustain partial clinical remission of T1D. A post hoc secondary analysis of the aforementioned trial [85] found that vitamin D2 significantly decreased fasting proinsulin-to-C-peptide ratio—a biomarker of beta-cell endoplasmic reticulum stress [86]—and slowed the decrease in the area under the curve (AUC) of C-peptide in youth with newly diagnosed T1D [85]. Moreover, it is important to highlight that vitamin D supplementation has proven beneficial in autoimmune diseases other than T1D. Recently, a parallel, double-blind, randomized placebo-controlled clinical trial demonstrated that 24-month high-dose vitamin D3 administration (at a dose of 100,000 IU every 2 weeks) significantly decreased disease activity in adults with clinically isolated syndrome and early relapsing-remitting multiple sclerosis [87].
Vitamin D also appears to be involved in wound healing. In mice with streptozotocin-induced diabetes mellitus, 1,25-dihydroxyvitamin D3 has been shown to accelerate diabetic wound healing by alleviating inflammation, improving vascular endothelial dysfunction, and stimulating angiogenesis via the upregulated expression of angiogenic factors, such as Vascular Endothelial Growth Factor (VEGF) [88]. Furthermore, 1,25-dihydroxyvitamin D3 has been shown to accelerate wound healing by suppressing endoplasmic reticulum stress in mice with streptozotocin-induced diabetes mellitus [89]. Notably, a study conducted on ex vivo human skin explants and primary cell culture models documented that 1,25-dihydroxyvitamin D3 increases early wound closure rate by accelerating keratinocyte migration and promoting re-epithelialization, and reduces extracellular matrix remodeling by inhibiting fibroblast migration and fibroblast transition into profibrotic myofibroblasts [90]. These findings indicate that optimizing vitamin D status may favor wound healing while minimizing excessive scarring and fibrosis.
A recent meta-analysis of randomized controlled trials evaluating the impact of vitamin D on diabetic foot ulcers showed that vitamin D supplementation can promote diabetic foot ulcer healing by improving glucose control and attenuating oxidative stress and inflammation [91]. Notably, a randomized, double-blind clinical trial recently showed that the improvement in the healing of diabetic foot ulcers occurred particularly when a high cholecalciferol dose (amounting to a daily oral intake of 170 μg) was used [92].
3. Role of Vitamin D in Solid Organ and Cell Transplantation
Preclinical studies conducted in animal models of allogeneic and syngeneic islet transplantation showed that vitamin D and its analogs promote islet graft survival and prevent or delay recurrence of autoimmunity and allograft rejection [93,94,95,96,97,98,99]. Furthermore, observational studies documented that low serum vitamin D levels can worsen clinical outcomes in patients undergoing solid organ transplantation. Fotros et al. [100] showed that vitamin D deficiency during the pre-transplant period is significantly associated with an increased risk of acute cellular rejection in patients with cirrhosis undergoing liver transplantation. In a retrospective study conducted on lung transplant recipients, Ki et al. [101] found that subjects with vitamin D deficiency exhibit a greater incidence of post-transplant pneumonia and overall mortality as compared to subjects with sufficient serum vitamin D levels. Koimtzis et al. [102] documented that vitamin D deficiency is associated with adverse short-term and long-term outcomes after kidney transplantation, including greater incidence of acute rejection episodes, worse graft function, greater incidence of proteinuria, viral infections, and lower overall graft and patient survival rates. Additionally, vitamin D deficiency is highly prevalent in patients undergoing hematopoietic stem cell transplantation and may influence the risk of developing chronic graft-versus-host disease (GVHD) in this population [103].
4. Impact of DPP-4/CD26 and DPP-4 Inhibitors on Inflammation, Autoimmunity, and Type 1 Diabetes
The enzyme DPP-4 (a.k.a. CD26 or cluster of differentiation 26) is a multifunctional cell surface antigen expressed in various cells and tissues, including endothelia, kidney, lung, liver, intestine, pancreatic duct and islet cells, fibroblasts, and immune cells, such as dendritic cells, monocytes, macrophages, activated B cells, T cells, and activated natural killer (NK) cells [104,105,106]. Besides its enzymatic activity, DPP-4 serves as a binding protein and as a ligand for various extracellular molecules [107]. DPP-4 is a type II transmembrane, homodimeric glycoprotein anchored to the cell membrane by its signal peptide [104], although it also exists in a soluble circulating form, which is released from the cell membrane into the bloodstream and accounts for a significant proportion of DPP-4 activity in the human serum [104,108]. DPP-4 mediates the cleavage and inactivation of the insulinotropic (gut-derived) hormones GLP-1 (Glucagon-like peptide 1) and GIP (Glucose-dependent insulinotropic polypeptide), which are also known as “incretins” [109,110]. DPP-4/CD26 also acts as a potent co-stimulatory factor involved in T-cell activation and proliferation [111]. Moreover, approximately 50% of human B lymphocytes express DPP-4/CD26 upon their activation [104,112].
DPP-4 inhibitors (DPP-4i) are oral glucose-lowering medications approved for the treatment of T2D due to their ability to improve glucose control by extending the half-life and biological activity of endogenous GLP-1 and GIP [113,114]. However, evidence suggests that DPP-4i exert pleiotropic effects beyond their glucose-lowering actions. Notably, studies suggested that DPP-4i exert in vitro and in vivo anti-inflammatory and immunomodulatory actions [115,116,117,118,119,120,121], which could be leveraged for the treatment of various chronic inflammatory and autoimmune diseases. In this regard, we previously documented that the DPP-4 inhibitor sitagliptin inhibits human peripheral blood mononuclear cell proliferation in a dose-dependent manner and reduces Th1/Th17 cell differentiation in vitro [122]. A study conducted on human isolated pancreatic islets demonstrated that the DPP-4 inhibitor linagliptin improves beta-cell function and survival, reduces oxidative stress, and counteracts glucotoxicity, lipotoxicity and cytokine-induced toxicity [118].
Additional evidence suggests that DPP-4/CD26 plays a role in the pathogenesis of various chronic fibrotic diseases, such as lung fibrosis, liver cirrhosis, cardiac fibrosis, kidney fibrosis, and systemic sclerosis [123]. Indeed, the inhibition of DPP-4/CD26 has been shown to reduce fibrotic changes and to modulate the profibrotic tissue microenvironment within affected organs in chronic fibrotic diseases [123]. Interestingly, it has been shown that DPP-4/CD26 affects periwound inflammation, extracellular matrix secretion, re-epithelialization, and skin fibrosis [124]. Selective inhibition of fibroblast- and keratinocyte-derived DPP-4/CD26 has been proposed as a therapeutic tool to inhibit skin fibrosis and prevent proliferative scarring and keloid scar formation [124]. In this regard, preclinical studies have shown that DPP-4i improve wound healing and reduce scar formation by promoting angiogenesis, epithelialization, epithelial-mesenchymal transition, and bone marrow-derived mesenchymal progenitor cell population recruitment to wounds [125,126,127,128].
Marfella et al. [129] showed that the DPP-4 inhibitor vildagliptin (administered at the dose of 50 mg twice daily) may accelerate the healing of chronic foot ulcers in patients with T2D by enhancing angiogenesis (as evidenced by increased ulcer capillary density and VEGF expression levels) and reducing the levels of nitrotyrosine (a marker of oxidative stress) within the ulcer specimens. Accordingly, a randomized, double-blind, placebo-controlled trial conducted on 50 adults with T2D and diabetic foot ulcers showed that 12-week vildagliptin therapy (at a daily dose of 100 mg)—in addition to standard of care for diabetic foot ulcers—led to an estimated 35% increase in diabetic foot ulcer healing capacity as compared to placebo combined with the standard of care for diabetic foot ulcers [130]. Furthermore, DPP-4i may exert proangiogenic actions in pancreatic islets. Indeed, in a study conducted on diabetic mice undergoing the implantation of mouse or porcine donor islets under the kidney capsule, the use of sitagliptin increased the mean insulin content of islet grafts and beta-cell proliferation, induced islet vascularization via the VEGF-A/VEGF receptor (VEGFR)-2 signaling pathway, and markedly increased endothelial cell proliferation and microvessel density [131].
Clinical trials have demonstrated that DPP-4i exert beneficial effects in T1D patients. An open-label, parallel-group, randomized controlled trial conducted in 46 adolescents with T1D and diabetic nephropathy recently showed that the use of sitagliptin (administered orally, at a daily dose of 50 mg) as an add-on therapy to the advanced hybrid closed-loop (AHCL) system MiniMed™ 780G (Medtronic, Northridge, CA, USA) was accompanied by a significant decrease in urinary albumin-to-creatinine ratio, 2-h postprandial glucose levels, mean sensor glucose levels, glucose management indicator (GMI) values, coefficient of variation of sensor glucose (a marker of glycemic variability), and total daily dose of insulin, along with a significant increase in time in range (TIR) 3.9-10.0 mmol/L (70–180 mg/dL) and insulin-to-carbohydrate ratio [132]. In this trial, sitagliptin use was safe and well-tolerated, and there were no reported episodes of severe hypoglycemia or diabetic ketoacidosis (DKA) [132]. It is important to note that diabetic nephropathy is associated with an upregulation of DPP-4 expression, suggesting the role of DPP-4 as a potential therapeutic target for the management of this condition [133].
A previously published randomized clinical trial showed that vildagliptin administration (at a daily dose of 50 mg) with iftar meal (together with pre-meal insulin iftar bolus) for the entire month of Ramadan (4 weeks) significantly mitigated postprandial hyperglycemia, increased TIR 70–180 mg/dL, and reduced glycemic variability among Egyptian adolescents and young adults with T1D who were using the MiniMed™ 780G AHCL system (Medtronic, Northridge, CA, USA) [134]. Moreover, there were no reported episodes of severe hypoglycemia or DKA in the study [134].
Another randomized controlled trial conducted in 60 adolescents with T1D and non-alcoholic steatohepatitis (NASH) found that 6-month administration of vildagliptin (administered at a daily dose of 50 mg) as an add-on to insulin therapy improved glycemic control and dyslipidemia, decreased matrix metalloproteinase-14 (MMP-14) levels, and reduced liver stiffness and carotid intima-media thickness (CIMT) [135].
Importantly, the 2-year, two-arm, multicenter, randomized, open-label clinical trial called “PRE1BRAZIL” will investigate the efficacy of the DPP-4i alogliptin (at a dose of 25 mg/day) in delaying the progression of stage 2 T1D (pre-symptomatic T1D with dysglycemia) to stage 3 T1D (clinically symptomatic T1D) in Brazilian patients (aged 18 to 35 years) [136].
5. Role of DPP-4i in Solid Organ and Cell Transplantation
The use of DPP-4i has been associated with beneficial clinical outcomes in patients undergoing solid organ and hematopoietic stem cell transplantation.
Ergin et al. [137] conducted a retrospective study on 26 patients who developed noninsulin-dependent hyperglycemia at least one year after pancreas transplantation. Authors showed that the time to insulin requirement was significantly longer among patients (n = 11) who received sitagliptin shortly after the occurrence of hyperglycemia, as compared to patients (n = 15) who did not receive any oral or non-insulin injectable glucose-lowering medications until insulin was clearly required to manage hyperglycemia [137]. These results suggested that early treatment of hyperglycemia can prolong insulin-free graft function after pancreas transplantation. Another retrospective cohort study conducted on 312 patients (of whom 234 with T1D) who underwent pancreas transplantation suggested that post-transplant DPP-4i administration may improve clinical outcomes (including beta-cell function) in this population [138]. Among the 312 study participants, 147 (47%) had received DPP-4i for more than 30 days (DPP-4i group), while 165 (53%) had either not received DPP-4i (n = 137) or had received DPP-4i for less than 30 days (n = 28) (non-DPP-4i group) following pancreas transplantation. Authors documented that patients who used DPP-4i had significantly higher serum C-peptide levels for up to 24 months after transplantation. During the 15-year follow-up period, patients in the DPP-4i group, as compared to those in the non-DPP-4i group, exhibited a significantly higher overall and death-censored pancreas graft survival [138].
In NOD mice, DPP-4 inhibition before and after islet transplantation can reduce the detrimental effects of beta-cell autoimmunity and prolong islet graft survival, partly by reducing the homing of CD4+ T cells to pancreatic beta cells [139]. An uncontrolled, open-label study conducted on 8 islet transplant recipients with evidence of early graft insufficiency showed that 6-month combination therapy with sitagliptin (at a dose of 100 mg/day) plus the proton pump inhibitor pantoprazole (at a dose of 40 mg twice daily) restored insulin independence in one quarter of the study participants (n = 2), although this beneficial effect was not sustained when treatment was withdrawn [140].
In the context of transplantation, DPP-4i may also counteract the detrimental effects of immunosuppressive drugs on pancreatic islets. Indeed, a study conducted in rats found that DPP-4 inhibition alleviated the pancreatic islet dysfunction induced by the immunosuppressant tacrolimus by exerting antioxidant and antiapoptotic actions through the enhancement of GLP-1 receptor signaling [141]. These findings are highly relevant, since post-transplant diabetes mellitus is a common post-transplant complication caused by immunosuppressive drugs [142]. In a 3-month pilot study conducted on 15 kidney transplant recipients who had been diagnosed with new-onset diabetes after transplantation (NODAT) and were treated with sitagliptin (at a standard daily dose of 100 mg, with dosing adjusted according to the estimated glomerular filtration rate) in addition to low-dose tacrolimus (target levels: 2–4 ng/mL) and sirolimus (target levels: 4–6 ng/mL), no significant changes in estimated glomerular filtration rate (eGFR) or in tacrolimus and sirolimus drug levels and doses were observed over the course of the study [143]. Mean HbA1c values significantly decreased from 7.2% (at baseline) to 6.7%, while no patient discontinued sitagliptin due to side effects [143]. Yamada et al. [144] retrospectively reviewed the records of patients who underwent lung transplantation and analyzed data regarding subjects with diabetes mellitus at 6 months post-transplantation. Of 102 patients with diabetes mellitus, 29 were treated with DPP-4i. The 5-year overall survival rates were 77.0% and 44.3%, while the 5-year chronic lung allograft dysfunction (CLAD)-free survival rates were 77.8% and 49.1% in patients who were treated with DPP-4i and in patients who were not treated with DPP-4i, respectively. Moreover, DPP-4/CD26 expression was detected in the CLAD grafts of patients who were not treated with DPP-4i [144]. Similarly, a study conducted on 221 lung transplant recipients documented that CLAD was absent in 34 patients who were treated with sitagliptin (as compared to a CLAD incidence of 18% in subjects who did not use sitagliptin; p = 0.02) [145]. The 5-year survival was significantly higher (80% vs. 58%) and the incidence of acute cellular rejection was significantly lower (7% vs. 35%) in patients who used sitagliptin as compared to patients who did not use DPP-4i. Remarkably, immunohistochemical analysis of lung biopsies revealed CD26 expression in perifibrotic areas of CLAD lesions [145].
Furthermore, a growing body of evidence has shown that DPP-4i may also represent a valid therapeutic tool against acute GVHD. In a phase 2, non-randomized clinical trial conducted by Farag et al. [146] on 36 patients, high-dose sitagliptin (administered at a dose of 600 mg every 12 h from the day before transplantation through post-transplant day 14), in combination with sirolimus and tacrolimus, reduced the incidence of grade II–IV acute GVHD by day 100 following myeloablative allogeneic hematopoietic stem cell transplantation. No toxic effects were related to sitagliptin by the investigators [146]. Bacigalupo et al. [147] showed that the anti-CD26 monoclonal antibody begelomab induced over 60% responses among patients with steroid refractory acute GVHD, thereby suggesting that CD26+ T cells may participate in the GVHD-related tissue damage. A prospective, multicenter, open-label, randomized controlled trial conducted on 191 patients receiving alternative donor transplantation showed that sitagliptin (combined with conventional prophylaxis) led to a significant reduction of grade II–IV acute GVHD [148]. Study participants with hematologic malignancies who underwent their first allogeneic hematopoietic stem cell transplantation in first or second complete remission state received busulfan and cyclophosphamide myeloablative conditioning regimen followed by alternative donor transplantation. Sitagliptin was administered at high doses (600 mg every 12 h from day −1 to +14) in addition to a conventional prophylaxis regimen including a calcineurin inhibitor (cyclosporin A/tacrolimus), methotrexate, mycophenolate mofetil, and anti-thymocyte globulin. By day +100, subjects in the sitagliptin group, as compared to subjects in the control group (who received only conventional prophylaxis), exhibited a significantly lower cumulative incidence rate of grade II–IV acute GVHD (15.1% vs. 28.6%, respectively; p = 0.019). Overall, the regimen including sitagliptin was well-tolerated, and no significant differences were reported between the sitagliptin group and the control group in terms of Epstein-Barr virus reactivation, cytomegalovirus reactivation, and complications related to the transplant [148]. These findings suggested that DPP-4 inhibition may serve as a potential immunomodulatory strategy for the prophylaxis of acute GVHD.
6. Vitamin D and DPP-4i Combination Therapy (VIDPP-4i) in Autoimmune Diabetes and Its Potential as an Adjuvant Treatment Strategy to Improve the Outcomes of Novel Beta-Cell Replacement Therapies
Both clinical and preclinical studies demonstrated that the combined administration of vitamin D and DPP-4i—which we refer to as VIDPP-4i—is associated with more pronounced anti-inflammatory, antioxidant and immunomodulatory effects as compared to the single use of vitamin D or DPP-4i [18,149,150,151], thus suggesting that vitamin D and DPP-4i exert synergistic anti-inflammatory, antioxidant and immunomodulatory actions.
Interestingly, a study showed that VIDPP-4i (based on the co-administration of vitamin D3 and linagliptin) rescued spermatogenesis and testicular steroidogenesis in rats exposed to cisplatin by reducing endoplasmic reticulum stress and activation of nuclear factor kappa B (NF-κB)/inducible nitric oxide synthase (iNOS) [151]. Noteworthy, a recent in silico computational study found that vitamin D3 is also capable of inhibiting DPP-4 [152]. This study also found that combination therapy with the DPP-4 inhibitor vildagliptin (at a daily dose of 10 mg/kg) and vitamin D3 (at a daily dose of 10 µg/kg) considerably elevated serum GLP-1 levels (as compared to vildagliptin alone) in rats with metabolic syndrome [152]. On the other hand, clinical studies conducted in subjects with T2D documented that the use of DPP-4i, as compared to other antidiabetic medications, is associated with a better vitamin D status [153,154]. Interestingly, it has been shown that vitamin D and DPP4i exert synergistic anti-inflammatory actions in patients with T2D by upregulating FOXP3 and interleukin (IL)-37 and by reducing the production of pro-inflammatory cytokines, such as IL-17 and interferon (IFN)-γ [149].
The abovementioned findings may partly explain the beneficial effects (with respect to the preservation of residual beta-cell function) deriving from the use of adjuvant VIDPP-4i combination therapy in individuals with autoimmune diabetes, including T1D and latent autoimmune diabetes in adults (LADA) [18,155,156]. We first documented a substantially prolonged clinical remission phase (4-year clinical remission), decreased glutamic acid decarboxylase antibody (GADA) titers, and preserved beta-cell function in two patients with recent-onset T1D who received VIDPP-4i [sitagliptin (at a daily dose of 100 mg) plus vitamin D3 (at a daily dose of 5000 IU)] in addition to insulin therapy [157]. In a subsequent retrospective study involving 46 patients with recent-onset T1D, co-administration of insulin therapy plus vitamin D3 (at a daily dose of 2000–5000 IU) and sitagliptin (at a daily dose of 50–100 mg), as compared to insulin therapy alone, was associated with a greater frequency and prolonged duration of the clinical remission phase, with 14.8% of patients maintaining insulin independence at 24 months from initiation of VIDPP-4i combination therapy [158]. Similarly, Rapti et al. [159] documented that 2-year combination therapy with metformin (dose: 850 mg twice daily), sitagliptin (dose: 50 mg twice daily), and vitamin D3 (dose: 2000 IU/day) led to normalization of HbA1c values and negativization of GADA in a 31-year-old man with LADA.
A multicenter, randomized, controlled trial investigated the role of 24-month saxagliptin plus vitamin D combination therapy (saxagliptin: 5 mg/day; vitamin D3: 2000 IU/day) as an add-on to conventional therapy in patients with adult-onset autoimmune T1D [155]. As compared to patients who received conventional therapy alone, patients who received saxagliptin plus vitamin D combination therapy—particularly those with higher GADA levels—exhibited a significantly smaller reduction from baseline in the 2-h MMTT C-peptide AUC values [155]. Another multicenter, randomized controlled trial conducted in 60 patients with LADA showed that 12-month co-administration of vitamin D3 and saxagliptin (at daily doses of 2000 IU/day and 5 mg, respectively), in addition to conventional therapy, was associated with stabilization of fasting C-peptide, 2-h postprandial C-peptide and C-peptide index values, and was accompanied by a significant reduction in GADA titers as compared to baseline [156]. In the aforementioned studies [155,156,157,158,159], the co-administration of vitamin D3 and DPP-4i appeared to be safe and well-tolerated. Table 1 summarizes the main clinical studies investigating the role of adjuvant combination therapy with vitamin D and DPP-4i (VIDPP-4i) in patients with autoimmune diabetes (T1D and LADA).

Table 1.
Summary of the main clinical studies investigating the role of adjuvant combination therapy with vitamin D and DPP-4i (VIDPP-4i) in patients with autoimmune diabetes (T1D and LADA).
Based on the aforementioned preliminary findings, larger randomized controlled trials are warranted to investigate the long-term efficacy of VIDPP-4i combination therapy in patients with T1D and LADA, as well as its potential role as an adjuvant combination therapy aimed at promoting successful long-term outcomes of novel beta-cell replacement strategies for the treatment of T1D. These trials would also help establish the long-term safety of VIDPP-4i combination therapy in patients with autoimmune diabetes across different age groups, particularly regarding side effects that may result from the concomitant use of DPP-4i and vitamin D (such as upper respiratory tract infections, nasopharyngitis, hypoglycemia, hypercalcemia, and hypercalciuria) [160,161].
Another aspect that needs clarification concerns the most proper doses of vitamin D and DPP-4i that should be investigated in clinical studies on beta-cell replacement therapies for T1D. With regard to vitamin D supplementation, high doses are likely to be more effective than low doses in determining the achievement of serum 25(OH)D levels between 40 and 60 ng/mL, which appear to be associated with more pronounced immunomodulatory and anti-inflammatory effects of vitamin D [162]. These remarks regarding intervention dosage may also apply to DPP-4i. In fact, it is interesting to note that single doses of sitagliptin substantially and dose-dependently inhibit plasma DPP-4 activity, with approximately 80% or greater inhibition of DPP-4 activity occurring at a dose of 50 mg (or greater) over 12 h, and at a dose of 100 mg (or greater) over 24 h [163]. Accordingly, high-dose sitagliptin therapy has been associated with beneficial clinical outcomes in patients who underwent allogeneic hematopoietic stem cell transplantation [146,148]. It has also been shown that addition of sitagliptin to insulin therapy may attenuate the progression of carotid atherosclerosis in patients with T2D in a dose-dependent manner [164]. Moreover, the immunomodulatory and anti-inflammatory properties of DPP-4i could be partly driven by the DPP-4 inhibition-mediated increases in circulating concentrations of GIP and GLP-1. Indeed, a recent study conducted in mice receiving cardiac or islet allotransplantation found that GLP-1 receptor can act as a T cell-negative co-stimulatory molecule, and GLP-1 receptor signaling decreases T lymphocyte graft infiltration, alleviates alloimmune response, and prolongs allograft survival [165]. Other preclinical studies conducted in mice demonstrated that GLP-1 receptor agonists can reduce aberrant immune responses and systemic inflammation by acting on GLP-1 receptor in the central nervous system [166,167]. GLP-1 receptor agonism and GIP receptor agonism can also reduce gut inflammation in mice [168,169]. A systematic review and meta-analysis of randomized controlled trials documented that GLP-1 receptor agonists can lead to significant reductions in biomarkers of oxidative stress and inflammation [170]. Furthermore, preliminary clinical evidence has shown that both GLP-1 receptor agonists and the dual GIP/GLP-1 receptor agonist tirzepatide may effectively improve glycemic control, prevent excess weight gain, and preserve residual beta-cell function in subjects with T1D across different disease stages [171].
An innovative biomimetic pancreas composed of alpha cells and beta cells differentiated from human induced pluripotent stem cells embedded within a biofunctional matrix with glucose-responsive nanoparticles that encapsulate a GLP-1 analog has recently yielded promising results in C57BL/6 male mice with streptozotocin-induced diabetes mellitus, leading to lower blood glucose levels and higher survival rates of transplanted animals [172]. Thus, novel encapsulation strategies for customized drug delivery may be investigated for the administration of vitamin D [173] and DPP-4i [174] in the context of pancreatic islet transplantation. Indeed, such strategies may maximize the anti-inflammatory, antioxidant, immunomodulatory, and insulinotropic effects of VIDPP-4i within the transplanted pancreatic islets. Figure 1 illustrates the potential mechanisms of actions through which VIDPP-4i combination therapy may promote successful outcomes of novel beta-cell replacement therapies.
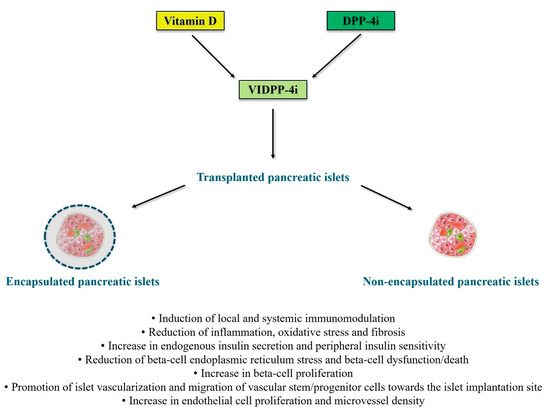
Figure 1.
Potential mechanisms of actions through which vitamin D and DPP-4 inhibitor (VIDPP-4i) adjuvant combination therapy may promote successful outcomes of novel beta-cell replacement therapies for type 1 diabetes (particularly long-term islet graft survival and prevention of autoimmunity recurrence and allograft rejection). Figure 1 was created with images adapted from Servier Medical Art licensed under the Creative Commons Attribution 4.0 International License (CC BY 4.0) [URL: https://smart.servier.com/—accessed on 8 July 2025]. The main references for the information presented in the figure are the following: [34,35,36,37,38,39,40,41,42,43,48,49,50,51,56,64,65,66,67,68,79,80,81,82,84,85,88,89,90,91,115,116,117,118,119,120,121,122,125,126,127,128,129,131,151]. Abbreviations: DPP-4i, dipeptidyl peptidase-4 inhibitors; VIDPP-4i, vitamin D and DPP-4 inhibitor combination therapy.
7. Conclusions
The long-term efficacy of novel beta-cell replacement strategies can be hampered by various factors, such as immune-mediated graft rejection, inadequate vascularization, hypoxia, trauma-induced cell apoptosis, fibrosis, host immune response, and recurrence of autoimmunity. Based on the existing literature in the fields of autoimmune diabetes and solid organ/cell transplantation, the co-administration of vitamin D and DPP-4i (VIDPP-4i adjuvant combination therapy) has the potential to promote and maintain long-term successful outcomes of novel beta-cell replacement therapies for T1D by exerting anti-inflammatory, antioxidant, immunomodulatory, insulinotropic, and insulin-sensitizing effects. In particular, VIDPP-4i may promote the long-term function and survival of transplanted (encapsulated or non-encapsulated) beta cells (including stem cell-derived beta cells) by counteracting beta-cell autoimmunity, favoring immune tolerance, promoting local and systemic immunomodulation, mitigating beta-cell dysfunction/death, and reducing glucotoxicity, lipotoxicity and cytokine-induced toxicity. Additionally, VIDPP-4i may contribute to reduce immunosuppression-related toxicity and to promote proper vascularization of the transplanted islets through the stimulation of angiogenesis and migration of vascular stem/progenitor cells towards the islet transplant site. Therefore, mechanistic studies and randomized controlled trials are warranted to confirm these hypotheses and to define the safety and efficacy profile of VIDPP-4i in T1D patients receiving various types of beta-cell replacement therapies, including those based on the use of encapsulated stem cell-derived beta cells.
Author Contributions
Conceptualization, M.M.P.; writing—original draft preparation, M.M.P., F.M.M.P., B.U.N., and M.I.; writing—review and editing, B.F.D.S., N.P., D.D.-M., and C.R.; supervision, M.M.P., C.R., and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Figure 1 was created with images adapted from Servier Medical Art licensed under the Creative Commons Attribution 4.0 International License (CC BY 4.0) [URL: https://smart.servier.com/—accessed on 8 July 2025].
Conflicts of Interest
CR serves as Scientific Advisor and Consultant for Vertex Pharmaceuticals (Boston, MA, USA) and Novo Nordisk (Bagsværd, Denmark). The other authors declare no conflicts of interest.
References
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Pinheiro, F.M.M.; Garo, M.L.; Pastore, D.; Pacifici, F.; Ricordi, C.; Della-Morte, D.; Infante, M. Prevention and treatment of type 1 diabetes: In search of the ideal combination therapy targeting multiple immunometabolic pathways. Metab. Target Organ Damage 2024, 4, 19. [Google Scholar] [CrossRef]
- Lanzoni, G.; Ricordi, C. Transplantation of stem cell-derived pancreatic islet cells. Nat. Rev. Endocrinol. 2021, 17, 7–8. [Google Scholar] [CrossRef]
- Rickels, M.R.; Robertson, R.P. Pancreatic Islet Transplantation in Humans: Recent Progress and Future Directions. Endocr. Rev. 2019, 40, 631–668. [Google Scholar] [CrossRef]
- Piemonti, L. The Last Mile in Beta-Cell Replacement Therapy for Type 1 Diabetes: Time to Grow Up. Transpl. Int. 2025, 38, 14565. [Google Scholar] [CrossRef]
- Altabas, V.; Bulum, T. Current Challenges in Pancreas and Islet Transplantation: A Scoping Review. Biomedicines 2024, 12, 2853. [Google Scholar] [CrossRef]
- Gamble, A.; Pepper, A.R.; Bruni, A.; Shapiro, A.M.J. The journey of islet cell transplantation and future development. Islets 2018, 10, 80–94. [Google Scholar] [CrossRef]
- Ernst, A.U.; Bowers, D.T.; Wang, L.H.; Shariati, K.; Plesser, M.D.; Brown, N.K.; Mehrabyan, T.; Ma, M. Nanotechnology in cell replacement therapies for type 1 diabetes. Adv. Drug Deliv. Rev. 2019, 139, 116–138. [Google Scholar] [CrossRef]
- Press Release (21 June 2024)—Vertex Announces Positive Results from Ongoing Phase 1/2 Study of VX-880 for the Treatment of Type 1 Diabetes Presented at the American Diabetes Association 84th Scientific Sessions. Available online: https://news.vrtx.com/news-releases/news-release-details/vertex-announces-positive-results-ongoing-phase-12-study-vx-880 (accessed on 8 July 2025).
- Press Release (28 March 2025)—Vertex Announces Program Updates for Type 1 Diabetes Portfolio. Available online: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-program-updates-type-1-diabetes-portfolio (accessed on 8 July 2025).
- Reichman, T.W.; Markmann, J.F.; Odorico, J.; Witkowski, P.; Fung, J.J.; Wijkstrom, M.; Kandeel, F.; de Koning, E.J.P.; Peters, A.L.; Mathieu, C.; et al. Stem Cell-Derived, Fully Differentiated Islets for Type 1 Diabetes. N. Engl. J. Med. 2025; online ahead of print. [Google Scholar]
- Press Release (9 March 2023)—Vertex Announces FDA Clearance of Investigational New Drug Application for VX-264, a Novel Encapsulated Cell Therapy for the Treatment of Type 1 Diabetes. Available online: https://investors.vrtx.com/news-releases/news-release-details/vertex-announces-fda-clearance-investigational-new-drug (accessed on 8 July 2025).
- Yitayew, M.Y.; Luginina, M.; Tabrizian, M. Advances in the Use of Biologics and Biomaterials toward the Improvement of Pancreatic Islet Graft Survival in Type 1 Diabetes. Adv. NanoBiomed. Res. 2024, 4, 2300097. [Google Scholar] [CrossRef]
- Bowers, D.T.; Song, W.; Wang, L.H.; Ma, M. Engineering the vasculature for islet transplantation. Acta Biomater. 2019, 95, 131–151. [Google Scholar] [CrossRef]
- Guo, K.; Tian, Q.; Yang, L.; Zhou, Z. The Role of Glucagon in Glycemic Variability in Type 1 Diabetes: A Narrative Review. Diabetes Metab. Syndr. Obes. 2021, 14, 4865–4873. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.; Rodriguez-Calvo, T.; Battaglia, M. Abnormalities of the Exocrine Pancreas in Type 1 Diabetes. Curr. Diab. Rep. 2015, 15, 79. [Google Scholar] [CrossRef]
- Osawa, S.; Kawamori, D.; Katakami, N.; Takahara, M.; Sakamoto, F.; Katsura, T.; Yasuda, T.; Kaneto, H.; Matsuhisa, M.; Matsuoka, T.A.; et al. Significant elevation of serum dipeptidyl peptidase-4 activity in young-adult type 1 diabetes. Diabetes Res. Clin. Pract. 2016, 113, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.; Pinheiro, F.M.M.; Diniz, S.N.; Fabbri, A.; Infante, M. Combination of vitamin D and dipeptidyl peptidase-4 inhibitors (VIDPP-4i) as an immunomodulation therapy for autoimmune diabetes. Int. Immunopharmacol. 2021, 95, 107518. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Infante, M.; Calanchini, M.; Mammi, C.; Fabbri, A. Vitamin D: Not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat. Weight Disord. 2017, 22, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Arora, J. Two lineages of immune cells that differentially express the vitamin D receptor. J. Steroid Biochem. Mol. Biol. 2023, 228, 106253. [Google Scholar] [CrossRef]
- Takahashi, K.; Nakayama, Y.; Horiuchi, H.; Ohta, T.; Komoriya, K.; Ohmori, H.; Kamimura, T. Human neutrophils express messenger RNA of vitamin D receptor and respond to 1alpha,25-dihydroxyvitamin D3. Immunopharmacol. Immunotoxicol. 2002, 24, 335–347. [Google Scholar] [CrossRef]
- Athanassiou, L.; Mavragani, C.P.; Koutsilieris, M. The Immunomodulatory Properties of Vitamin D. Mediterr. J. Rheumatol. 2022, 33, 7–13. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G.; Giarratana, N.; Roncari, A.; Amuchastegui, S.; Daniel, K.C.; Uskokovic, M. Dendritic cells as key targets for immunomodulation by Vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 437–441. [Google Scholar] [CrossRef]
- Baeke, F.; Korf, H.; Overbergh, L.; van Etten, E.; Verstuyf, A.; Gysemans, C.; Mathieu, C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J. Steroid Biochem. Mol. Biol. 2010, 121, 221–227. [Google Scholar] [CrossRef]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 alters microglia immune activation by an IL-10 dependent SOCS3 mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef]
- Lue, L.F.; Kuo, Y.M.; Beach, T.; Walker, D.G. Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol. Neurobiol. 2010, 41, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Overbergh, L.; Decallonne, B.; Valckx, D.; Verstuyf, A.; Depovere, J.; Laureys, J.; Rutgeerts, O.; Saint-Arnaud, R.; Bouillon, R.; Mathieu, C. Identification and immune regulation of 25-hydroxyvitamin D-1-alpha-hydroxylase in murine macrophages. Clin. Exp. Immunol. 2000, 120, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef]
- Monkawa, T.; Yoshida, T.; Hayashi, M.; Saruta, T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000, 58, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Kongsbak, M.; von Essen, M.R.; Levring, T.B.; Schjerling, P.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Geisler, C. Vitamin D-binding protein controls T cell responses to Vitamin D. BMC Immunol. 2014, 15, 35. [Google Scholar] [CrossRef]
- Fritsche, J.; Mondal, K.; Ehrnsperger, A.; Andreesen, R.; Kreutz, M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood 2003, 102, 3314–3316. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Korf, H.; Wenes, M.; Stijlemans, B.; Takiishi, T.; Robert, S.; Miani, M.; Eizirik, D.L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology 2012, 217, 1292–1300. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, M.; Guo, Y.; Song, Z.; Liu, B. 1,25-Dihydroxyvitamin D3 Promotes High Glucose-Induced M1 Macrophage Switching to M2 via the VDR-PPARγ Signaling Pathway. Biomed Res. Int. 2015, 2015, 157834. [Google Scholar]
- Ferreira, G.B.; van Etten, E.; Verstuyf, A.; Waer, M.; Overbergh, L.; Gysemans, C.; Mathieu, C. 1,25-Dihydroxyvitamin D3 alters murine dendritic cell behaviour in vitro and in vivo. Diabetes Metab. Res. Rev. 2011, 27, 933–941. [Google Scholar] [CrossRef]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef]
- Overbergh, L.; Decallonne, B.; Waer, M.; Rutgeerts, O.; Valckx, D.; Casteels, K.M.; Laureys, J.; Bouillon, R.; Mathieu, C. 1alpha,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543). Diabetes 2000, 49, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef]
- Bouillon, R.; Lieben, L.; Mathieu, C.; Verstuyf, A.; Carmeliet, G. Vitamin D action: Lessons from VDR and Cyp27b1 null mice. Pediatr. Endocrinol. Rev. 2013, 10 (Suppl. S2), 354–366. [Google Scholar] [PubMed]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki Hagiagha, A.; Heshmati, J. The effect of vitamin D supplementation on oxidative stress parameters: A systematic review and meta-analysis of clinical trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Zeitz, U.; Weber, K.; Soegiarto, D.W.; Wolf, E.; Balling, R.; Erben, R.G. Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J. 2003, 17, 509–511. [Google Scholar] [CrossRef] [PubMed]
- Bland, R.; Markovic, D.; Hills, C.E.; Hughes, S.V.; Chan, S.L.; Squires, P.E.; Hewison, M. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 121–125. [Google Scholar] [CrossRef]
- Zehnder, D.; Bland, R.; Williams, M.C.; McNinch, R.W.; Howie, A.J.; Stewart, P.M.; Hewison, M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 2001, 86, 888–894. [Google Scholar]
- Johnson, J.A.; Grande, J.P.; Roche, P.C.; Kumar, R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am. J. Physiol. 1994, 267 Pt 1, E356–E360. [Google Scholar] [CrossRef]
- Maestro, B.; Dávila, N.; Carranza, M.C.; Calle, C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J. Steroid Biochem. Mol. Biol. 2003, 84, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yoshihara, E.; He, N.; Hah, N.; Fan, W.; Pinto, A.F.M.; Huddy, T.; Wang, Y.; Ross, B.; Estepa, G.; et al. Vitamin D Switches BAF Complexes to Protect β Cells. Cell 2018, 173, 1135–1149.e15. [Google Scholar] [CrossRef]
- Morró, M.; Vilà, L.; Franckhauser, S.; Mallol, C.; Elias, G.; Ferré, T.; Molas, M.; Casana, E.; Rodó, J.; Pujol, A.; et al. Vitamin D Receptor Overexpression in β-Cells Ameliorates Diabetes in Mice. Diabetes 2020, 69, 927–939. [Google Scholar] [CrossRef]
- Bourlon, P.M.; Billaudel, B.; Faure-Dussert, A. Influence of vitamin D3 deficiency and 1,25 dihydroxyvitamin D3 on de novo insulin biosynthesis in the islets of the rat endocrine pancreas. J. Endocrinol. 1999, 160, 87–95. [Google Scholar] [CrossRef]
- Clark, S.A.; Stumpf, W.E.; Sar, M. Effect of 1,25 dihydroxyvitamin D3 on insulin secretion. Diabetes 1981, 30, 382–386. [Google Scholar] [CrossRef]
- Lei, X.; Zhou, Q.; Wang, Y.; Fu, S.; Li, Z.; Chen, Q. Serum and supplemental vitamin D levels and insulin resistance in T2DM populations: A meta-analysis and systematic review. Sci. Rep. 2023, 13, 12343. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Luo, Y.; Shen, Y.; Zhao, Y.; Cao, W.; Cao, J.; Xu, L.; Kong, L. Associations between hypovitaminosis D, adiposity indices and insulin resistance in adolescents: Mediation analyses from NHANES 2011–2018. Nutr. Diabetes 2025, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Marques-Vidal, P.; Vollenweider, P.; Guessous, I.; Henry, H.; Boulat, O.; Waeber, G.; Jornayvaz, F.R. Serum Vitamin D Concentrations Are Not Associated with Insulin Resistance in Swiss Adults. J. Nutr. 2015, 145, 2117–2122. [Google Scholar] [CrossRef]
- Pramono, A.; Jocken, J.W.E.; Essers, Y.P.G.; Goossens, G.H.; Blaak, E.E. Vitamin D and Tissue-Specific Insulin Sensitivity in Humans with Overweight/Obesity. J. Clin. Endocrinol. Metab. 2019, 104, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, P.; Weisnagel, S.J.; Caron, A.Z.; Julien, A.S.; Morisset, A.S.; Carreau, A.M.; Poirier, J.; Tchernof, A.; Robitaille, J.; Bergeron, J.; et al. Effects of 6-month vitamin D supplementation on insulin sensitivity and secretion: A randomised, placebo-controlled trial. Eur. J. Endocrinol. 2019, 181, 287–299. [Google Scholar] [CrossRef]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2016, 7, 697. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Sanchez, J.; Clare-Salzler, M.J.; Padilla, N.; Fuenmayor, V.; Chavez, C.; Alvarez, A.; Baidal, D.; Alejandro, R.; et al. Influence of Vitamin D on Islet Autoimmunity and Beta-Cell Function in Type 1 Diabetes. Nutrients 2019, 11, 2185. [Google Scholar] [CrossRef]
- Miettinen, M.E.; Niinistö, S.; Erlund, I.; Cuthbertson, D.; Nucci, A.M.; Honkanen, J.; Vaarala, O.; Hyöty, H.; Krischer, J.P.; Knip, M.; et al. Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: The TRIGR nested case-control ancillary study. Diabetologia 2020, 63, 780–787. [Google Scholar] [CrossRef]
- Hyppönen, E.; Läärä, E.; Reunanen, A.; Järvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef] [PubMed]
- Kachapati, K.; Adams, D.; Bednar, K.; Ridgway, W.M. The non-obese diabetic (NOD) mouse as a model of human type 1 diabetes. Methods Mol. Biol. 2012, 933, 3–16. [Google Scholar]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Prevention of autoimmune diabetes in NOD mice by 1,25 dihydroxyvitamin D3. Diabetologia 1994, 37, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Waer, M.; Casteels, K.; Laureys, J.; Bouillon, R. Prevention of type I diabetes in NOD mice by nonhypercalcemic doses of a new structural analog of 1,25-dihydroxyvitamin D3, KH1060. Endocrinology 1995, 136, 866–872. [Google Scholar] [CrossRef]
- Mathieu, C.; Laureys, J.; Sobis, H.; Vandeputte, M.; Waer, M.; Bouillon, R. 1,25-Dihydroxyvitamin D3 prevents insulitis in NOD mice. Diabetes 1992, 41, 1491–1495. [Google Scholar] [CrossRef]
- Casteels, K.M.; Mathieu, C.; Waer, M.; Valckx, D.; Overbergh, L.; Laureys, J.M.; Bouillon, R. Prevention of type I diabetes in nonobese diabetic mice by late intervention with nonhypercalcemic analogs of 1,25-dihydroxyvitamin D3 in combination with a short induction course of cyclosporin A. Endocrinology 1998, 139, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Giarratana, N.; Smiroldo, S.; Uskokovic, M.; Adorini, L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 2002, 51, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Pozzilli, P.; Manfrini, S.; Crinò, A.; Picardi, A.; Leomanni, C.; Cherubini, V.; Valente, L.; Khazrai, M.; Visalli, N.; IMDIAB Group. Low levels of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 in patients with newly diagnosed type 1 diabetes. Horm. Metab. Res. 2005, 37, 680–683. [Google Scholar] [CrossRef]
- Cadario, F.; Prodam, F.; Savastio, S.; Monzani, A.; Balafrej, A.; Bellomo, G.; Bona, G. Vitamin D status and type 1 diabetes in children: Evaluation according to latitude and skin color. Minerva Pediatr. 2015, 67, 263–267. [Google Scholar]
- Littorin, B.; Blom, P.; Schölin, A.; Arnqvist, H.J.; Blohmé, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjörnsdottir, S.; Nyström, L.; et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Borkar, V.V.; Verma, S.; Bhalla, A.K. Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes. Pediatr. Diabetes 2010, 11, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Rasoul, M.A.; Al-Mahdi, M.; Al-Kandari, H.; Dhaunsi, G.S.; Haider, M.Z. Low serum vitamin-D status is associated with high prevalence and early onset of type-1 diabetes mellitus in Kuwaiti children. BMC Pediatr. 2016, 16, 95. [Google Scholar] [CrossRef]
- Federico, G.; Genoni, A.; Puggioni, A.; Saba, A.; Gallo, D.; Randazzo, E.; Salvatoni, A.; Toniolo, A. Vitamin D status, enterovirus infection, and type 1 diabetes in Italian children/adolescents. Pediatr. Diabetes 2018, 19, 923–929. [Google Scholar] [CrossRef]
- Daga, R.A.; Laway, B.A.; Shah, Z.A.; Mir, S.A.; Kotwal, S.K.; Zargar, A.H. High prevalence of vitamin D deficiency among newly diagnosed youth-onset diabetes mellitus in north India. Arq. Bras. Endocrinol. Metabol. 2012, 56, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Al-Attas, O.S.; Alokail, M.S.; Alkharfy, K.M.; Yakout, S.M.; Aljohani, N.J.; Al Fawaz, H.; Al-Ajlan, A.S.; Sheshah, E.S.; Al-Yousef, M.; et al. Lower vitamin D status is more common among Saudi adults with diabetes mellitus type 1 than in non-diabetics. BMC Public Health 2014, 14, 153. [Google Scholar] [CrossRef]
- Al-Zubeidi, H.; Leon-Chi, L.; Newfield, R.S. Low vitamin D level in pediatric patients with new onset type 1 diabetes is common, especially if in ketoacidosis. Pediatr. Diabetes 2016, 17, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Greer, R.M.; Portelli, S.L.; Hung, B.S.; Cleghorn, G.J.; McMahon, S.K.; Batch, J.A.; Conwell, L.S. Serum vitamin D levels are lower in Australian children and adolescents with type 1 diabetes than in children without diabetes. Pediatr. Diabetes 2013, 14, 31–41. [Google Scholar] [CrossRef]
- Gabbay, M.A.; Sato, M.N.; Finazzo, C.; Duarte, A.J.; Dib, S.A. Effect of cholecalciferol as adjunctive therapy with insulin on protective immunologic profile and decline of residual β-cell function in new-onset type 1 diabetes mellitus. Arch. Pediatr. Adolesc. Med. 2012, 166, 601–607. [Google Scholar] [CrossRef]
- Treiber, G.; Prietl, B.; Fröhlich-Reiterer, E.; Lechner, E.; Ribitsch, A.; Fritsch, M.; Rami-Merhar, B.; Steigleder-Schweiger, C.; Graninger, W.; Borkenstein, M.; et al. Cholecalciferol supplementation improves suppressive capacity of regulatory T-cells in young patients with new-onset type 1 diabetes mellitus—A randomized clinical trial. Clin. Immunol. 2015, 161, 217–224. [Google Scholar] [CrossRef]
- Baidal, D.A.; Alvarez, A.M.; Padilla, N.; Sanchez, J.; Lanzoni, G.; Alejandro, R.; Ricordi, C. 25(OH) Vitamin D Levels and Severity of Type 1 Diabetes in Youth and Adults with Recent-Onset Disease. J. Endocr. Soc. 2025, 9, bvaf061. [Google Scholar] [CrossRef]
- Federico, G.; Focosi, D.; Marchi, B.; Randazzo, E.; De Donno, M.; Vierucci, F.; Bugliani, M.; Campi, F.; Scatena, F.; Saggese, G.; et al. Administering 25-hydroxyvitamin D3 in vitamin D-deficient young type 1A diabetic patients reduces reactivity against islet autoantigens. Clin. Nutr. 2014, 33, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Vitiello, L.; Fabbri, A.; Ricordi, C.; Padilla, N.; Pacifici, F.; Perna, P.D.; Passeri, M.; Della-Morte, D.; Caprio, M.; et al. Prolonged clinical remission of type 1 diabetes sustained by calcifediol and low-dose basal insulin: A case report. Immunotherapy 2023, 15, 1009–1019. [Google Scholar] [CrossRef]
- Nwosu, B.U.; Parajuli, S.; Jasmin, G.; Fleshman, J.; Sharma, R.B.; Alonso, L.C.; Lee, A.F.; Barton, B.A. Ergocalciferol in New-onset Type 1 Diabetes: A Randomized Controlled Trial. J. Endocr. Soc. 2022, 6, bvab179. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, B.U.; Parajuli, S.; Sharma, R.B.; Lee, A.F. Effect of Ergocalciferol on β-Cell Function in New-Onset Type 1 Diabetes: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2024, 7, e241155. [Google Scholar] [CrossRef]
- Sims, E.K.; Chaudhry, Z.; Watkins, R.; Syed, F.; Blum, J.; Ouyang, F.; Perkins, S.M.; Mirmira, R.G.; Sosenko, J.; DiMeglio, L.A.; et al. Elevations in the Fasting Serum Proinsulin-to-C-Peptide Ratio Precede the Onset of Type 1 Diabetes. Diabetes Care 2016, 39, 1519–1526. [Google Scholar] [CrossRef]
- Thouvenot, E.; Laplaud, D.; Lebrun-Frenay, C.; Derache, N.; Le Page, E.; Maillart, E.; Froment-Tilikete, C.; Castelnovo, G.; Casez, O.; Coustans, M.; et al. High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial. JAMA 2025, 333, 1413–1422. [Google Scholar] [CrossRef]
- Ma, Y.; Gong, Y.; Wu, Y.; Zhao, Q.; Fu, R.; Zhang, X.; Li, Y.; Zhi, X. 1,25(OH)2D3 improves diabetic wound healing by modulating inflammation and promoting angiogenesis. J. Steroid Biochem. Mol. Biol. 2024, 239, 106477. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.F.; Das, S.K.; Li, M.Q. Vitamin D Ameliorates Impaired Wound Healing in Streptozotocin-Induced Diabetic Mice by Suppressing Endoplasmic Reticulum Stress. J. Diabetes Res. 2018, 2018, 1757925. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.Q.; Riches-Suman, K.; Graham, A.M.; Mahajan, A.L.; Thornton, M.J. Divergent effects of vitamin D3 on human dermal fibroblasts and keratinocytes in wound repair: Implications for therapeutic targeting in tissue remodelling and scarring. J. Plast. Reconstr. Aesthet. Surg. 2025, 105, 323–335. [Google Scholar] [CrossRef]
- Wu, X.; Zeng, J.; Ye, X.; Peng, M.; Lan, Y.; Zhang, S.; Li, H. Effects of vitamin D supplementation on diabetic foot ulcer healing: A meta-analysis. Postgrad. Med. J. 2025, 101, 100–107. [Google Scholar] [CrossRef]
- Halschou-Jensen, P.M.; Sauer, J.; Bouchelouche, P.; Fabrin, J.; Brorson, S.; Ohrt-Nissen, S. Improved Healing of Diabetic Foot Ulcers After High-dose Vitamin D: A Randomized Double-blinded Clinical Trial. Int. J. Low. Extrem. Wounds 2023, 22, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Infante, M.; Ricordi, C.; Padilla, N.; Alvarez, A.; Linetsky, E.; Lanzoni, G.; Mattina, A.; Bertuzzi, F.; Fabbri, A.; Baidal, D.; et al. The Role of Vitamin D and Omega-3 PUFAs in Islet Transplantation. Nutrients 2019, 11, 2937. [Google Scholar] [CrossRef]
- Baeke, F.; Van Belle, T.L.; Takiishi, T.; Ding, L.; Korf, H.; Laureys, J.; Gysemans, C.; Mathieu, C. Low doses of anti-CD3, ciclosporin A and the vitamin D analogue, TX527, synergise to delay recurrence of autoimmune diabetes in an islet-transplanted NOD mouse model of diabetes. Diabetologia 2012, 55, 2723–2732. [Google Scholar] [CrossRef]
- Jiao, Z.Z.; Li, Y.; Fan, P.; Guo, J.; Xue, W.J.; Ding, X.M.; Tian, X.H.; Feng, X.S.; Zheng, J.; Tian, P.X.; et al. 1,25(OH)2D3 prolongs islet graft survival by inflammatory inhibition. Transplant. Proc. 2014, 46, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, C.; Waer, M.; Laureys, J.; Rutgeerts, O.; Bouillon, R. Activated form of vitamin D [1,25(OH)2D3] and its analogs are dose-reducing agents for cyclosporine in vitro and in vivo. Transplant. Proc. 1994, 26, 3048–3049. [Google Scholar]
- Mathieu, C.; Laureys, J.; Waer, M.; Bouillon, R. Prevention of autoimmune destruction of transplanted islets in spontaneously diabetic NOD mice by KH1060, a 20-epi analog of vitamin D: Synergy with cyclosporine. Transplant. Proc. 1994, 26, 3128–3129. [Google Scholar]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.; Adorini, L. Transplantation tolerance by 1,25-dihydroxyvitamin D(3)-induced costimulation blockade. Transplant. Proc. 2001, 33, 219–220. [Google Scholar] [CrossRef]
- Gregori, S.; Casorati, M.; Amuchastegui, S.; Smiroldo, S.; Davalli, A.M.; Adorini, L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J. Immunol. 2001, 167, 1945–1953. [Google Scholar] [CrossRef]
- Fotros, D.; Sohouli, M.; Yari, Z.; Sakhdari, H.; Shafiekhani, M.; Nikoupour, H.; Jafarzadeh, M.A.; Jafari, K.; Afiatjoo, S.S.; Fatemi, S.A.; et al. Vitamin D status as a predictor for liver transplant outcomes. Sci. Rep. 2023, 13, 21018. [Google Scholar] [CrossRef]
- Ki, M.S.; Kim, N.E.; Woo, A.; Kim, S.Y.; Kim, Y.S.; Kim, H.E.; Lee, J.G.; Paik, H.C.; Park, M.S. Post-Transplant Vitamin D Deficiency in Lung Transplant Recipients: Impact on Outcomes and Prognosis. Transpl. Int. 2024, 37, 13313. [Google Scholar] [CrossRef]
- Koimtzis, G.; Stefanopoulos, L.; Brooker, V.; Geropoulos, G.; Chalklin, C.G.; Gupta, S.; Carrington-Windo, E.; Papaioannou, M.; Papavramidis, T.S. The Role of Vitamin D in Kidney Transplantation Outcomes: A Systematic Review. Life 2022, 12, 1664. [Google Scholar] [CrossRef]
- Mancin, S.; Cangelosi, G.; Matteucci, S.; Palomares, S.M.; Parozzi, M.; Sandri, E.; Sguanci, M.; Piredda, M. The Role of Vitamin D in Hematopoietic Stem Cell Transplantation: Implications for Graft-versus-Host Disease—A Narrative Review. Nutrients 2024, 16, 2976. [Google Scholar] [CrossRef] [PubMed]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Augstein, P.; Naselli, G.; Loudovaris, T.; Hawthorne, W.J.; Campbell, P.; Bandala-Sanchez, E.; Rogers, K.; Heinke, P.; Thomas, H.E.; Kay, T.W.; et al. Localization of dipeptidyl peptidase-4 (CD26) to human pancreatic ducts and islet alpha cells. Diabetes Res. Clin. Pract. 2015, 110, 291–300. [Google Scholar] [CrossRef]
- Soare, A.; Györfi, H.A.; Matei, A.E.; Dees, C.; Rauber, S.; Wohlfahrt, T.; Chen, C.W.; Ludolph, I.; Horch, R.E.; Bäuerle, T.; et al. Dipeptidylpeptidase 4 as a Marker of Activated Fibroblasts and a Potential Target for the Treatment of Fibrosis in Systemic Sclerosis. Arthritis. Rheumatol. 2020, 72, 137–149. [Google Scholar] [CrossRef]
- Deacon, C.F. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes. Front. Endocrinol. 2019, 10, 80. [Google Scholar]
- Mulvihill, E.E.; Drucker, D.J. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014, 35, 992–1019. [Google Scholar] [CrossRef]
- Deacon, C.F. Circulation and degradation of GIP and GLP-1. Horm. Metab. Res. 2004, 36, 761–765. [Google Scholar] [CrossRef]
- Kim, W.; Egan, J.M. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol. Rev. 2008, 60, 470–512. [Google Scholar] [CrossRef]
- Ohnuma, K.; Dang, N.H.; Morimoto, C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends. Immunol. 2008, 29, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bühling, F.; Junker, U.; Reinhold, D.; Neubert, K.; Jäger, L.; Ansorge, S. Functional role of CD26 on human B lymphocytes. Immunol. Lett. 1995, 45, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Omar, B.; Ahrén, B. Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes 2014, 63, 2196–2202. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.P.; Pratley, R.E. GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front. Endocrinol. 2020, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Xu, Q.; Yu, X.; Pan, R.; Chen, Y. Dipeptidyl peptidase 4 inhibitors and their potential immune modulatory functions. Pharmacol. Ther. 2020, 209, 107503. [Google Scholar] [CrossRef]
- Makdissi, A.; Ghanim, H.; Vora, M.; Green, K.; Abuaysheh, S.; Chaudhuri, A.; Dhindsa, S.; Dandona, P. Sitagliptin exerts an antinflammatory action. J. Clin. Endocrinol. Metab. 2012, 97, 3333–3341. [Google Scholar] [CrossRef]
- Satoh-Asahara, N.; Sasaki, Y.; Wada, H.; Tochiya, M.; Iguchi, A.; Nakagawachi, R.; Odori, S.; Kono, S.; Hasegawa, K.; Shimatsu, A. A dipeptidyl peptidase-4 inhibitor, sitagliptin, exerts anti-inflammatory effects in type 2 diabetic patients. Metabolism 2013, 62, 347–351. [Google Scholar] [CrossRef]
- Shah, P.; Ardestani, A.; Dharmadhikari, G.; Laue, S.; Schumann, D.M.; Kerr-Conte, J.; Pattou, F.; Klein, T.; Maedler, K. The DPP-4 inhibitor linagliptin restores β-cell function and survival in human isolated islets through GLP-1 stabilization. J. Clin. Endocrinol. Metab. 2013, 98, E1163–E1172. [Google Scholar] [CrossRef]
- Újhelyi, J.; Újhelyi, Z.; Szalai, A.; László, J.F.; Cayasso, M.; Vecsernyés, M.; Pórszász, R. Analgesic and anti-inflammatory effectiveness of sitagliptin and vildagliptin in mice. Regul. Pept. 2014, 194–195, 23–29. [Google Scholar] [CrossRef]
- Kagal, U.A.; Angadi, N.B.; Matule, S.M. Effect of dipeptidyl peptidase 4 inhibitors on acute and subacute models of inflammation in male Wistar rats: An experimental study. Int. J. Appl. Basic Med. Res. 2017, 7, 26–31. [Google Scholar] [CrossRef]
- Tremblay, A.J.; Lamarche, B.; Deacon, C.F.; Weisnagel, S.J.; Couture, P. Effects of sitagliptin therapy on markers of low-grade inflammation and cell adhesion molecules in patients with type 2 diabetes. Metabolism 2014, 63, 1141–1148. [Google Scholar] [CrossRef]
- Pinheiro, M.M.; Stoppa, C.L.; Valduga, C.J.; Okuyama, C.E.; Gorjão, R.; Pereira, R.M.; Diniz, S.N. Sitagliptin inhibit human lymphocytes proliferation and Th1/Th17 differentiation in vitro. Eur. J. Pharm. Sci. 2017, 100, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ohm, B.; Moneke, I.; Jungraithmayr, W. Targeting cluster of differentiation 26/dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis. Br. J. Pharmacol. 2023, 180, 2846–2861. [Google Scholar] [CrossRef]
- Zhang, K.W.; Liu, S.Y.; Jia, Y.; Zou, M.L.; Teng, Y.Y.; Chen, Z.H.; Li, Y.; Guo, D.; Wu, J.J.; Yuan, Z.D.; et al. Insight into the role of DPP-4 in fibrotic wound healing. Biomed. Pharmacother. 2022, 151, 113143. [Google Scholar] [CrossRef]
- Long, M.; Cai, L.; Li, W.; Zhang, L.; Guo, S.; Zhang, R.; Zheng, Y.; Liu, X.; Wang, M.; Zhou, X.; et al. DPP-4 Inhibitors Improve Diabetic Wound Healing via Direct and Indirect Promotion of Epithelial-Mesenchymal Transition and Reduction of Scarring. Diabetes 2018, 67, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, C.; Linke, A.; Engelmann-Pilger, K.; Steinmetz, C.; Mark, M.; Pfeilschifter, J.; Klein, T.; Frank, S. The dipeptidyl peptidase-4 inhibitor linagliptin attenuates inflammation and accelerates epithelialization in wounds of diabetic ob/ob mice. J. Pharmacol. Exp. Ther. 2012, 342, 71–80. [Google Scholar] [CrossRef]
- Lee, C.H.; Huang, C.H.; Hung, K.C.; Huang, S.C.; Kuo, C.C.; Liu, S.J. Nanofibrous Vildagliptin/PLGA Membranes Accelerate Diabetic Wound Healing by Angiogenesis. Pharmaceuticals 2022, 15, 1358. [Google Scholar] [CrossRef]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Barrera, J.A.; Hu, M.S.; Fischer, L.H.; Khong, S.; Kwon, S.H.; Wong, V.W.; Walmsley, G.G.; et al. Small molecule inhibition of dipeptidyl peptidase-4 enhances bone marrow progenitor cell function and angiogenesis in diabetic wounds. Transl. Res. 2019, 205, 51–63. [Google Scholar] [CrossRef]
- Marfella, R.; Sasso, F.C.; Rizzo, M.R.; Paolisso, P.; Barbieri, M.; Padovano, V.; Carbonara, O.; Gualdiero, P.; Petronella, P.; Ferraraccio, F.; et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp. Diabetes Res. 2012, 2012, 892706. [Google Scholar] [CrossRef]
- Vangaveti, V.N.; Jhamb, S.; Hayes, O.; Goodall, J.; Bulbrook, J.; Robertson, K.; Biros, E.; Sangla, K.S.; Malabu, U.H. Effects of vildagliptin on wound healing and markers of inflammation in patients with type 2 diabetic foot ulcer: A prospective, randomized, double-blind, placebo-controlled, single-center study. Diabetol. Metab. Syndr. 2022, 14, 183. [Google Scholar] [CrossRef]
- Samikannu, B.; Chen, C.; Lingwal, N.; Padmasekar, M.; Engel, F.B.; Linn, T. Dipeptidyl peptidase IV inhibition activates CREB and improves islet vascularization through VEGF-A/VEGFR-2 signaling pathway. PLoS ONE 2013, 8, e82639. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.; El-Hamamsy, M.H.; Ibrahim, M.Z.; Elkholy, A.A. The DPP-4 inhibitor sitagliptin improves glycaemic control and early-stage diabetic nephropathy in adolescents with type 1 diabetes using the MiniMed 780G advanced hybrid closed-loop system: A randomised controlled trial. Diabetologia 2024, 67, 2637–2649. [Google Scholar] [CrossRef]
- Hasan, A.A.; Hocher, B. Role of soluble and membrane-bound dipeptidyl peptidase-4 in diabetic nephropathy. J. Mol. Endocrinol. 2017, 59, R1–R10. [Google Scholar] [CrossRef] [PubMed]
- Elbarbary, N.S.; Ismail, E.A.R. Mitigating iftar-related glycemic excursions in adolescents and young adults with type 1 diabetes on MiniMed™ 780G advanced hybrid closed loop system: A randomized clinical trial for adjunctive oral vildagliptin therapy during Ramadan fasting. Diabetol. Metab. Syndr. 2023, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- ElKabbany, Z.A.; Ismail, E.A.R.; Hamed, E.T.; Elbarbary, N.S. The impact of vildagliptin as an add-on therapy on matrix metalloproteinase-14 levels, liver stiffness and subclinical atherosclerosis in adolescents with type 1 diabetes and non-alcoholic steatohepatitis: A randomized controlled trial. Diabetes Obes. Metab. 2024, 26, 5857–5869. [Google Scholar] [CrossRef]
- Penaforte-Saboia, J.G.; Couri, C.E.B.; Albuquerque, N.V.; Linard, L.L.P.; Araújo, D.A.C.; de Oliveira, S.K.P.; Gomes, T.F.P.; Pinheiro, M.M.; Castelo, M.H.C.G.; Fernandes, V.O.; et al. PRE1BRAZIL Protocol: A Randomized Controlled Trial to Evaluate the Effectiveness and Safety of the DPP-4 Inhibitor Alogliptin in Delaying the Progression of Stage 2 Type 1 Diabetes. Diabetes Metab. Syndr. Obes. 2024, 17, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Ergin, A.B.; Poggio, E.; Krishnamurthi, V.; Jaber, T.; Hatipoglu, B.A. DPP-4 Inhibitor Therapy in Patients After Pancreatic Transplant. Endocr. Pract. 2015, 21, 567–573. [Google Scholar] [CrossRef]
- Jang, H.W.; Jung, C.H.; Ko, Y.; Lim, S.J.; Kwon, H.E.; Jung, J.H.; Kwon, H.; Kim, Y.H.; Shin, S. Beneficial effects of posttransplant dipeptidyl peptidase-4 inhibitor administration after pancreas transplantation to improve β cell function. Ann. Surg. Treat. Res. 2021, 101, 187–196. [Google Scholar] [CrossRef]
- Kim, S.J.; Nian, C.; Doudet, D.J.; McIntosh, C.H. Dipeptidyl peptidase IV inhibition with MK0431 improves islet graft survival in diabetic NOD mice partially via T-cell modulation. Diabetes 2009, 58, 641–651. [Google Scholar] [CrossRef]
- Senior, P.A.; Koh, A.; Yau, J.; Imes, S.; Dinyari, P.; Malcolm, A.J.; Light, P.; Shapiro, A.M. Sitagliptin plus pantoprazole can restore but not maintain insulin independence after clinical islet transplantation: Results of a pilot study. Diabet. Med. 2017, 34, 204–212. [Google Scholar] [CrossRef]
- Jin, L.; Lim, S.W.; Doh, K.C.; Piao, S.G.; Jin, J.; Heo, S.B.; Chung, B.H.; Yang, C.W. Dipeptidyl peptidase IV inhibitor MK-0626 attenuates pancreatic islet injury in tacrolimus-induced diabetic rats. PLoS ONE 2014, 9, e100798. [Google Scholar] [CrossRef] [PubMed]
- Penfornis, A.; Kury-Paulin, S. Immunosuppressive drug-induced diabetes. Diabetes Metab. 2006, 32, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.T.; Odegaard, D.E.; Haire, C.E.; Collier, D.S.; Wrenshall, L.E.; Stevens, R.B. Sitagliptin therapy in kidney transplant recipients with new-onset diabetes after transplantation. Transplantation 2011, 92, e56–e57. [Google Scholar] [CrossRef]
- Yamada, Y.; Sato, T.; Oda, H.; Harada, N.; Yoshizawa, A.; Nishikawa, S.; Kayawake, H.; Tanaka, S.; Yutaka, Y.; Hamaji, M.; et al. Favorable effect of CD26/DPP-4 inhibitors on postoperative outcomes after lung transplantation: A propensity-weighted analysis. J. Heart Lung Transplant. 2024, 43, 66–76. [Google Scholar] [CrossRef]
- Moneke, I.; Ögütür, E.; Chatterjee, S.; Haberecker, M.; Jang, J.H.; Fähndrich, S.; Senbaklavaci, Ö.; Faccioli, E.; Opitz, I.; Passlick, B.; et al. CD26-inhibition correlates with the absence of chronic lung allograft dysfunction and decreases fibroblast activity in vitro. Br. J. Surg. 2022, 9 (Suppl. S3), znac176.007. [Google Scholar] [CrossRef]
- Farag, S.S.; Abu Zaid, M.; Schwartz, J.E.; Thakrar, T.C.; Blakley, A.J.; Abonour, R.; Robertson, M.J.; Broxmeyer, H.E.; Zhang, S. Dipeptidyl Peptidase 4 Inhibition for Prophylaxis of Acute Graft-versus-Host Disease. N. Engl. J. Med. 2021, 384, 11–19. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Angelucci, E.; Raiola, A.M.; Varaldo, R.; Di Grazia, C.; Gualandi, F.; Benedetti, E.; Risitano, A.; Musso, M.; Zallio, F.; et al. Treatment of steroid resistant acute graft versus host disease with an anti-CD26 monoclonal antibody-Begelomab. Bone Marrow Transplant. 2020, 55, 1580–1587. [Google Scholar] [CrossRef]
- Qiao, M.; Yang, X.; Bao, X.; Zhou, J.; Zhu, H.; Zhang, Y.; You, T.; Qiu, H.; Wang, Y.; Xue, S.; et al. Sitagliptin for Prevention of aGVHD in Patients Received Alternative Donor Transplantations: A Prospective, Multicenter, Open-Label, Randomized Controlled Trial. Blood 2023, 142 (Suppl. S1), 4922. [Google Scholar] [CrossRef]
- Telikani, Z.; Sheikh, V.; Zamani, A.; Borzouei, S.; Salehi, I.; Amirzargar, M.A.; Alahgholi-Hajibehzad, M. Effects of sitagliptin and vitamin D3 on T helper cell transcription factors and cytokine production in clinical subgroups of type 2 diabetes mellitus: Highlights upregulation of FOXP3 and IL-37. Immunopharmacol. Immunotoxicol. 2019, 41, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Wahba, N.S.; Abdel-Ghany, R.H.; Ghareib, S.A.; Abdel-Aal, M.; Alsemeh, A.E. Vitamin D3 potentiates the renoprotective effects of vildagliptin in a rat model of fructose/salt-induced insulin resistance. Eur. J. Pharm. Sci. 2020, 144, 105196. [Google Scholar] [CrossRef] [PubMed]
- Elrashidy, R.A.; Zakaria, E.M.; Elmaghraby, A.M.; Abd El Aziz, R.E.M.; Abdelgalil, R.M.; Megahed, R.M.; Elshiech, A.A.; Salama, D.E.A.; Ibrahim, S.E. Linagliptin and Vitamin D3 Synergistically Rescue Testicular Steroidogenesis and Spermatogenesis in Cisplatin-Exposed Rats: The Crosstalk of Endoplasmic Reticulum Stress with NF-κB/iNOS Activation. Molecules 2022, 27, 7299. [Google Scholar] [CrossRef]
- Shoier, N.O.; Ghareib, S.A.; Kothayer, H.; Alsemeh, A.E.; El-Sayed, S.S. Vitamin D3 mitigates myopathy and metabolic dysfunction in rats with metabolic syndrome: The potential role of dipeptidyl peptidase-4. Naunyn-Schmiedebergs Arch. Pharmacol. 2025, 398, 3697–3715. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Ramezani, M.; Katsiki, N.; Mirmohammadkhani, M.; Tabaei, N.S. Efficacy of Adding Sitagliptin to Ongoing Metformin on Metabolic Profile, Triglyceride-Glucose Index, Vitamin D3, and Liver Tests in Patients Type 2 Diabetes Mellitus and Nonalcoholic Fatty Liver Disease: A Double-Blind Randomized Clinical Trial. Curr. Ther. Res. Clin. Exp. 2024, 101, 100764. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Cimini, F.A.; Bloise, D.; Cavallo, M.G. Dipeptidyl peptidase-4 inhibitors and bone metabolism: Is vitamin D the link? Acta Diabetol. 2016, 53, 839–844. [Google Scholar] [CrossRef]
- Yan, X.; Li, X.; Liu, B.; Huang, J.; Xiang, Y.; Hu, Y.; Tang, X.; Zhang, Z.; Huang, G.; Xie, Z.; et al. Combination therapy with saxagliptin and vitamin D for the preservation of β-cell function in adult-onset type 1 diabetes: A multi-center, randomized, controlled trial. Signal Transduct. Target. Ther. 2023, 8, 158. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, X.; Wu, C.; Pei, X.; Li, X.; Wang, X.; Niu, X.; Jiang, H.; Zeng, X.; Zhou, Z. Adding vitamin D3 to the dipeptidyl peptidase-4 inhibitor saxagliptin has the potential to protect β-cell function in LADA patients: A 1-year pilot study. Diabetes Metab. Res. Rev. 2020, 36, e3298. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.; Pinheiro, F.M.; Torres, M.A. Four-year clinical remission of type 1 diabetes mellitus in two patients treated with sitagliptin and vitamin D3. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016, 16-0099. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.M.; Pinheiro, F.M.M.; de Arruda, M.M.; Beato, G.M.; Verde, G.A.C.L.; Bianchini, G.; Casalenuovo, P.R.M.; Argolo, A.A.A.; de Souza, L.T.; Pessoa, F.G.; et al. Association between sitagliptin plus vitamin D3 (VIDPP-4i) use and clinical remission in patients with new-onset type 1 diabetes: A retrospective case-control study. Arch. Endocrinol. Metab. 2023, 67, e000652. [Google Scholar] [CrossRef]
- Rapti, E.; Karras, S.; Grammatiki, M.; Mousiolis, A.; Tsekmekidou, X.; Potolidis, E.; Zebekakis, P.; Daniilidis, M.; Kotsa, K. Combined treatment with sitagliptin and vitamin D in a patient with latent autoimmune diabetes in adults. Endocrinol. Diabetes Metab. Case Rep. 2016, 2016, 150136. [Google Scholar] [CrossRef]
- Kasina, S.V.S.K.; Baradhi, K.M. Dipeptidyl Peptidase IV (DPP IV) Inhibitors. [Updated 22 May 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542331/ (accessed on 8 July 2025).
- Marcinowska-Suchowierska, E.; Kupisz-Urbańska, M.; Łukaszkiewicz, J.; Płudowski, P.; Jones, G. Vitamin D Toxicity—A Clinical Perspective. Front. Endocrinol. 2018, 9, 550. [Google Scholar] [CrossRef]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Herman, G.A.; Stevens, C.; Van Dyck, K.; Bergman, A.; Yi, B.; De Smet, M.; Snyder, K.; Hilliard, D.; Tanen, M.; Tanaka, W.; et al. Pharmacokinetics and pharmacodynamics of sitagliptin, an inhibitor of dipeptidyl peptidase IV, in healthy subjects: Results from two randomized, double-blind, placebo-controlled studies with single oral doses. Clin. Pharmacol. Ther. 2005, 78, 675–688. [Google Scholar] [CrossRef]
- Mita, T.; Katakami, N.; Shiraiwa, T.; Yoshii, H.; Gosho, M.; Shimomura, I.; Watada, H. Dose-Dependent Effect of Sitagliptin on Carotid Atherosclerosis in Patients with Type 2 Diabetes Mellitus Receiving Insulin Treatment: A Post Hoc Analysis. Diabetes Ther. 2017, 8, 1135–1146. [Google Scholar] [CrossRef]
- Ben Nasr, M.; Usuelli, V.; Dellepiane, S.; Seelam, A.J.; Fiorentino, T.V.; D’Addio, F.; Fiorina, E.; Xu, C.; Xie, Y.; Balasubramanian, H.B.; et al. Glucagon-like peptide 1 receptor is a T cell-negative costimulatory molecule. Cell Metab. 2024, 36, 1302–1319.e12. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Jeon, S.J.; Cho, K.S.; Moon, E.; Sapkota, A.; Jun, H.S.; Ryu, J.H.; Choi, J.W. Activation of Glucagon-Like Peptide-1 Receptor Promotes Neuroprotection in Experimental Autoimmune Encephalomyelitis by Reducing Neuroinflammatory Responses. Mol. Neurobiol. 2018, 55, 3007–3020. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; McLean, B.A.; Baggio, L.L.; Koehler, J.A.; Hammoud, R.; Rittig, N.; Yabut, J.M.; Seeley, R.J.; Brown, T.J.; Drucker, D.J. Central glucagon-like peptide 1 receptor activation inhibits Toll-like receptor agonist-induced inflammation. Cell Metab. 2024, 36, 130–143.e5. [Google Scholar] [CrossRef]
- Wong, C.K.; Yusta, B.; Koehler, J.A.; Baggio, L.L.; McLean, B.A.; Matthews, D.; Seeley, R.J.; Drucker, D.J. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T cell-induced inflammation. Cell Metab. 2022, 34, 1514–1531.e1517. [Google Scholar] [CrossRef]
- Hammoud, R.; Kaur, K.D.; Koehler, J.A.; Baggio, L.L.; Wong, C.K.; Advani, K.E.; Yusta, B.; Efimova, I.; Gribble, F.M.; Reimann, F.; et al. Glucose-dependent insulinotropic polypeptide receptor signaling alleviates gut inflammation in mice. JCI Insight 2024, 10, e174825. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Salem, A.; Hoole, A.L.; Obaid, D.R.; Halcox, J.P.J.; Stephens, J.W. Glucagon-like peptide-1 receptor agonists improve biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomised controlled trials. Diabetes Obes. Metab. 2021, 23, 1806–1822. [Google Scholar] [CrossRef]
- Infante, M.; Silvestri, F.; Padilla, N.; Pacifici, F.; Pastore, D.; Pinheiro, M.M.; Caprio, M.; Tesauro, M.; Fabbri, A.; Novelli, G.; et al. Unveiling the Therapeutic Potential of the Second-Generation Incretin Analogs Semaglutide and Tirzepatide in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. J. Clin. Med. 2025, 14, 1303. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.M.; Nunes, R.; Carvalho, A.M.; Florindo, H.; Ferreira, D.; Sarmento, B. GLP-1 Analogue-Loaded Glucose-Responsive Nanoparticles as Allies of Stem Cell Therapies for the Treatment of Type I Diabetes. ACS Pharmacol. Transl. Sci. 2024, 7, 1650–1663. [Google Scholar] [CrossRef]
- Aggeletopoulou, I.; Kalafateli, M.; Geramoutsos, G.; Triantos, C. Recent Advances in the Use of Vitamin D Organic Nanocarriers for Drug Delivery. Biomolecules 2024, 14, 1090. [Google Scholar] [CrossRef]
- Thondawada, M.; Wadhwani, A.D.; S Palanisamy, D.; Rathore, H.S.; Gupta, R.C.; Chintamaneni, P.K.; Samanta, M.K.; Dubala, A.; Varma, S.; Krishnamurthy, P.T.; et al. An effective treatment approach of DPP-IV inhibitor encapsulated polymeric nanoparticles conjugated with anti-CD-4 mAb for type 1 diabetes. Drug Dev. Ind. Pharm. 2018, 44, 1120–1129. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).