Optimizing Recovery in Cardiac Surgery: A Narrative Review of Enhanced Recovery After Surgery Protocols and Clinical Outcomes
Abstract
1. Introduction
2. Methods
3. ERAS in Non-Cardiac Surgery
3.1. History and Evolution
3.2. Structure of ERAS in Non-Cardiac Surgery
3.2.1. Preoperative Phase
3.2.2. Intraoperative Phase
3.2.3. Postoperative Phase
3.3. Variations of ERAS Among Different Non-Cardiac Surgeries
3.4. Financial Outcomes of ERAS in Non-Cardiac Surgeries for Healthcare Systems
3.5. Pitfalls and Challenges
4. ERAS in Cardiac Surgery and Cardiac Interventions
4.1. History of ERAS in Cardiac Surgery
4.2. Elements of ERAS for Cardiac Surgery
4.2.1. Preoperative Phase
4.2.2. Intraoperative Phase
4.2.3. Postoperative Phase
4.2.4. 2024 Guideline Updates
4.3. Successful Implementation of ERAS for Cardiac Surgery in an Adult Population
4.4. Challenges of ERACS
5. Perspective
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Taurchini, M.; Del Naja, C.; Tancredi, A. Enhanced Recovery After Surgery: A Patient Centered Process. J. Vis. Surg. 2018, 4, 40. [Google Scholar] [CrossRef]
- Agüero-Martínez, M.O.; Tapia-Figueroa, V.M.; Hidalgo-Costa, T. Improved Recovery Protocols in Cardiac Surgery: A Systematic Review and Meta-Analysis of Observational and Quasi-Experimental Studies. MEDICC Rev. 2021, 23, 46–53. [Google Scholar] [CrossRef]
- Shaw, A.D.; Guinn, N.R.; Brown, J.K.; Arora, R.C.; Lobdell, K.W.; Grant, M.C.; Gan, T.J.; Engelman, D.T. Controversies in enhanced recovery after cardiac surgery. Perioper. Med. 2022, 11, 19. [Google Scholar] [CrossRef]
- Engelman, D.T.; Ali, W.B.; Williams, J.B.; Perrault, L.P.; Reddy, V.S.; Arora, R.C.; Roselli, E.E.; Khoynezhad, A.; Gerdisch, M.; Levy, J.H.; et al. Guidelines for Perioperative Care in Cardiac Surgery: Enhanced Recovery After Surgery Society Recommendations. JAMA Surg. 2019, 154, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, M.; Lamarche, Y. Enhanced Recovery After Cardiac Surgery (ERACS) Pilot Program Saves Hospital Days, Readmissions and Costs. In Proceedings of the 102nd Annual Meeting, Boston, MA, USA, 16 May 2022. [Google Scholar]
- Golder, H.J.; Papalois, V. Enhanced Recovery After Surgery: History, Key Advancements and Developments in Transplant Surgery. J. Clin. Med. 2021, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Turaga, A.H. Enhanced Recovery After Surgery (ERAS) Protocols for Improving Outcomes for Patients Undergoing Major Colorectal Surgery. Cureus 2023, 15, e41755. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.-L.; Ye, X.-Z.; Zhang, X.-D.; Chen, B.-C.; Yu, Z. Enhanced Recovery After Surgery Programs Versus Traditional Care for Colorectal Surgery: A Meta-Analysis of Randomized Controlled Trials. Dis. Colon Rectum 2013, 56, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.-B.; Li, Y.-Y.; Hung, K.-C.; Illias, A.M.; Tsai, Y.-F.; Yang, Y.-L.; Chin, J.-C.; Wu, S.-C. The Impact of Rocuronium and Sugammadex on Length of Stay in Patients Undergoing Open Spine Surgery: A Propensity Score-Matched Analysis. Bioengineering 2023, 10, 959. [Google Scholar] [CrossRef]
- Zhang, B.; Ochuba, A.J.; Mullen, G.R.; Rai, A.; Aro, T.; Hoenig, D.M.; Okeke, Z.; Winoker, J.S. How I Do It: ERAS protocol featuring erector spinae plane block for percutaneous nephrolithotomy. Can. J. Urol. 2023, 30, 11639–11643. [Google Scholar]
- Mitric, C.; Kosa, S.D.; Kim, S.R.; Nelson, G.; Laframboise, S.; Bouchard-Fortier, G. Cost impact analysis of enhanced recovery after minimally invasive gynecologic oncology surgery. Int. J. Gynecol. Cancer 2023, 33, 1786–1793. [Google Scholar] [CrossRef]
- Götz, J.; Maderbacher, G.; Leiss, F.; Zeman, F.; Meyer, M.; Reinhard, J.; Grifka, J.; Greimel, F. Better early outcome with enhanced recovery total hip arthroplasty (ERAS-THA) versus conventional setup in randomized clinical trial (RCT). Arch. Orthop. Trauma Surg. 2023, 144, 439–450. [Google Scholar] [CrossRef]
- Bisch, S.P.; Nelson, G. Outcomes of Enhanced Recovery After Surgery (ERAS) in Gynecologic Oncology: A Review. Curr. Oncol. 2022, 29, 631–640. [Google Scholar] [CrossRef]
- Azhar, R.A.; Bochner, B.; Catto, J.; Goh, A.C.; Kelly, J.; Patel, H.D.; Pruthi, R.S.; Thalmann, G.N.; Desai, M. Enhanced Recovery After Urological Surgery: A Contemporary Systematic Review of Outcomes, Key Elements, and Research Needs. Eur. Urol. 2016, 70, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Awad, S.; Varadhan, K.K.; Ljungqvist, O.; Lobo, D.N. A meta-analysis of randomised controlled trials on preoperative oral carbohydrate treatment in elective surgery. Clin. Nutr. 2013, 32, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.A.; Alton, S.; Seung, H.; Pahlavan, A.; Trilling, A.R.; Coghlan, M.; Goetzinger, K.R.; Cojocaru, L. Enhanced recovery after cesarean from the patient perspective: A prospective study of the ERAC Questionnaire (ERAC-Q). J. Perinat. Med. 2023, 52, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Olsén, M.F.; Andersson, T.; Al Nouh, M.; Johnson, E.; Block, L.; Vakk, M.; Wennerblom, J. Development of and adherence to an ERAS® and prehabilitation protocol for patients undergoing pancreatic surgery: An observational study. Scand. J. Surg. 2023, 112, 235–245. [Google Scholar] [CrossRef]
- Xia, Z.; Chen, Y.; Xie, J.; Zhang, W.; Tan, L.; Shi, Y.; Liu, J.; Wang, X.; Tan, G.; Zeng, A. Faster Return to Daily Activities and Better Pain Control: A Prospective Study of Enhanced Recovery After Surgery Protocol in Breast Augmentation. Aesthetic Plast. Surg. 2023, 47, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Probst, S.; Calandra, C.; Davis, R.; Sugimoto, K.; Nie, L.; Gan, T.J.; Bennett-Guerrero, E. Enhanced recovery after surgery (ERAS) program for lumbar spine fusion. Perioper. Med. 2019, 8, 4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelson, G.; Bakkum-Gamez, J.; Kalogera, E.; Glaser, G.; Altman, A.; A Meyer, L.; Taylor, J.S.; Iniesta, M.; Lasala, J.; Mena, G.; et al. Guidelines for perioperative care in gynecologic/oncology: Enhanced Recovery After Surgery (ERAS) Society Recommendations—2019 Update. Int. J. Gynecol. Cancer 2019, 29, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Zaouter, C.; Imbault, J.; Labrousse, L.; Abdelmoumen, Y.; Coiffic, A.; Colonna, G.; Jansens, J.-L.; Ouattara, A. Association of Robotic Totally Endoscopic Coronary Artery Bypass Graft Surgery Associated with a Preliminary Cardiac Enhanced Recovery After Surgery Program: A Retrospective Analysis. J. Cardiothorac. Vasc. Anesth. 2015, 29, 1489–1497. [Google Scholar] [CrossRef] [PubMed]
- Sola, M.; Ramm, C.J.; Kolarczyk, L.M.; Teeter, E.G.; Yeung, M.; Caranasos, T.G.; Vavalle, J.P. Application of a Multidisciplinary Enhanced Recovery After Surgery Pathway to Improve Patient Outcomes After Transcatheter Aortic Valve Implantation. Am. J. Cardiol. 2016, 118, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Noss, C.; Prusinkiewicz, C.; Nelson, G.; Patel, P.A.; Augoustides, J.G.; Gregory, A.J. Enhanced Recovery for Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2018, 32, 2760–2770. [Google Scholar] [CrossRef] [PubMed]
- Takata, E.T.; Eschert, J.; Mather, J.; McLaughlin, T.; Hammond, J.; Hashim, S.W.; McKay, R.G.; Sutton, T.S. Enhanced Recovery After Surgery Is Associated with Reduced Hospital Length of Stay After Urgent or Emergency Isolated Coronary Artery Bypass Surgery at an Urban, Tertiary Care Teaching Hospital: An Interrupted Time Series Analysis with Propensity Score Matching. J. Cardiothorac. Vasc. Anesth. 2023, 37, 31–41. [Google Scholar]
- Kubitz, J.C.; Schulte-Uentrop, L.; Zoellner, C.; Lemke, M.; Messner-Schmitt, A.; Kalbacher, D.; Sill, B.; Reichenspurner, H.; Koell, B.; Girdauskas, E.; et al. Establishment of an enhanced recovery after surgery protocol in minimally invasive heart valve surgery. PLoS ONE 2020, 15, e0231378. [Google Scholar] [CrossRef]
- Smith, T.W., Jr.; Wang, X.; Singer, M.A.; Godellas, C.V.; Vaince, F.T. Enhanced recovery after surgery: A clinical review of implementation across multiple surgical subspecialties. Am. J. Surg. 2020, 219, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.M.; Liverpool, A.; Romeiser, J.L.; Thacker, J.; Gan, T.J.; Bennett-Guerrero, E. Types of surgical patients enrolled in enhanced recovery after surgery (ERAS) programs in the USA. Perioper. Med. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grant, M.C.; Crisafi, C.; Alvarez, A.; Arora, R.C.; Brindle, M.E.; Chatterjee, S.; Ender, J.; Fletcher, N.; Gregory, A.J.; Gunaydin, S.; et al. Perioperative Care in Cardiac Surgery: A Joint Consensus Statement by the Enhanced Recovery After Surgery (ERAS) Cardiac Society, ERAS International Society, and The Society of Thoracic Surgeons (STS). Ann. Thorac. Surg. 2024, 117, 669–689. [Google Scholar] [CrossRef] [PubMed]
- Ching, P.R. Care Bundles in Surgical Site Infection Prevention: A Narrative Review. Curr. Infect. Dis. Rep. 2024, 26, 163–172. [Google Scholar] [CrossRef]
- Loria, C.M.; Zborek, K.; Millward, J.B.; Anderson, M.P.; Richardson, C.M.; Namburi, N. Enhanced recovery after cardiac surgery protocol reduces perioperative opioid use. JTCVS Open 2022, 12, 280–296. [Google Scholar] [CrossRef]
- Schneider, C.; Marguerite, S.; Ramlugun, D.; Saadé, S.; Maechel, A.-L.; Oulehri, W.; Collange, O.; Mertes, P.-M.; Mazzucotelli, J.-P.; Kindo, M. Enhanced recovery after surgery program for patients undergoing isolated elective coronary artery bypass surgery improves postoperative outcomes. J. Thorac. Cardiovasc. Surg. 2023, 168, 597–607. [Google Scholar] [CrossRef]
- Sutton, T.S.; McKay, R.G.; Mather, J.; Takata, E.; Eschert, J.; Cox, M.; Douglas, A.; McLaughlin, T.; Loya, D.; Mennett, R.; et al. Enhanced Recovery After Surgery Is Associated with Improved Outcomes and Reduced Racial and Ethnic Disparities After Isolated Coronary Artery Bypass Surgery: A Retrospective Analysis with Propensity-Score Matching. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2418–2431. [Google Scholar] [CrossRef]
- Brown, A.; Beaudry, R.I.; Moen, J.; Kang, S.; Hassanabad, A.F.; Vasanthan, V.; Gregory, A.J.; Kent, W.D.; Adams, C. Patient-reported outcome measures after minimally invasive mitral valve surgery: The benefit may be early. JTCVS Open 2024, 20, 26–28. [Google Scholar] [CrossRef] [PubMed]
- Maj, G.; Regesta, T.; Campanella, A.; Cavozza, C.; Parodi, G.; Audo, A. Optimal Management of Patients Treated with Minimally Invasive Cardiac Surgery in the Era of Enhanced Recovery After Surgery and Fast-Track Protocols: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.J.; Kent, W.D.; Adams, C.; Arora, R.C. Closing the care gap: Combining enhanced recovery with minimally invasive valve surgery. Curr. Opin. Cardiol. 2024, 39, 380–387. [Google Scholar] [CrossRef]
- Zaouter, C.; Oses, P.; Assatourian, S.; Labrousse, L.; Rémy, A.; Ouattara, A. Reduced Length of Hospital Stay for Cardiac Surgery—Implementing an Optimized Perioperative Pathway: Prospective Evaluation of an Enhanced Recovery After Surgery Program Designed for Mini-Invasive Aortic Valve Replacement. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3010–3019. [Google Scholar] [CrossRef]
- Petersen, J.; Kloth, B.; Konertz, J.; Kubitz, J.; Schulte-Uentrop, L.; Ketels, G.; Reichenspurner, H.; Girdauskas, E. Economic impact of enhanced recovery after surgery protocol in minimally invasive cardiac surgery. BMC Health Serv. Res. 2021, 21, 254. [Google Scholar] [CrossRef]
- Brown, A.; Hassanabad, A.F.; Moen, J.; Wiens, K.; Gregory, A.J.; Parhar, K.K.S.; Adams, C.; Kent, W.D. Rapid-recovery protocol for minimally invasive mitral valve repair. JTCVS Open 2024, 22, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Demir, Z.A.; Marczin, N. ERAS in Cardiac Surgery: Wishful Thinking or Reality. Turk. J. Anaesthesiol. Reanim. 2023, 51, 370–373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stone, A.B.; Yuan, C.T.; Rosen, M.A.; Grant, M.C.; Benishek, L.E.; Hanahan, E.; Lubomski, L.H.; Ko, C.; Wick, E.C. Barriers to and Facilitators of Implementing Enhanced Recovery Pathways Using an Implementation Framework: A Systematic Review. JAMA Surg. 2018, 153, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.J.; Arora, R.C.; Chatterjee, S.; Crisafi, C.; Morton-Bailey, V.; Rea, A.; Salenger, R.; Engelman, D.T.; Grant, M.C.; Cangut, B.; et al. Enhanced Recovery After Surgery (ERAS) cardiac turnkey order set for perioperative pain management in cardiac surgery: Proceedings from the American Association for Thoracic Surgery (AATS) ERAS Conclave 2023. JTCVS Open 2024, 22, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Cangut, B.; Rea, A.; Salenger, R.; Arora, R.C.; Grant, M.C.; Morton-Bailey, V.; Hirji, S.; Engelman, D.T.; Gregory, A.J.; et al. Enhanced Recovery After Surgery Cardiac Society turnkey order set for prevention and management of postoperative atrial fibrillation after cardiac surgery: Proceedings from the American Association for Thoracic Surgery ERAS Conclave 2023. JTCVS Open 2024, 18, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Crisafi, C.; Grant, M.C.; Rea, A.; Morton-Bailey, V.; Gregory, A.J.; Arora, R.C.; Chatterjee, S.; Lother, S.A.; Cangut, B.; Engelman, D.T.; et al. Enhanced Recovery After Surgery Cardiac Society turnkey order set for surgical-site infection prevention: Proceedings from the American Association for Thoracic Surgery ERAS Conclave 2023. J. Thorac. Cardiovasc. Surg. 2024, 168, 1500–1509. [Google Scholar] [CrossRef] [PubMed]
- Salenger, R.; Hirji, S.; Rea, A.; Cangut, B.; Morton-Bailey, V.; Gregory, A.J.; Arora, R.C.; Grant, M.C.; Raphael, J.; Engelman, D.T.; et al. ERAS Cardiac Society turnkey order set for patient blood management: Proceedings from the AATS ERAS Conclave 2023. J. Thorac. Cardiovasc. Surg. 2024, 168, 890–897. [Google Scholar] [CrossRef]
- Rea, A.; Salenger, R.; Grant, M.C.; Yeh, J.; Damas, B.; Crisalfi, C.; Arora, R.; Gregory, A.J.; Morton-Bailey, V.; Engelman, D.T.; et al. Preoperative medication management turnkey order set for nonemergent adult cardiac surgery. JTCVS Open 2024, 22, 1–13. [Google Scholar] [CrossRef]
- Zurek, S.; Kurowicki, A.; Borys, M.; Iwasieczko, A.; Woloszczuk-Gebicka, B.; Czuczwar, M.; Widenka, K. Early removal of chest drains in patients following off-pump coronary artery bypass graft (OPCAB) is not inferior to standard care—Study in the Enhanced Recovery After Surgery (ERAS) group. Kardiochirurgia Torakochirurgia Pol. Pol. J. Thorac. Cardiovasc. Surg. 2021, 18, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.; Chen, X.; Song, Z.; Wang, Y.; Zhou, Y.; Zhang, D. A Scientometric Analysis and Visualization Discovery of Enhanced Recovery After Surgery. Front. Surgery. 2022, 9, 894083. [Google Scholar] [CrossRef] [PubMed]
- Yazdchi, F.; Hirji, S.; Harloff, M.; McGurk, S.; Morth, K.; Zammert, M.; Shook, D.; Varelmann, D.; Shekar, P.; Kaneko, T.; et al. Enhanced Recovery After Cardiac Surgery: A Propensity-Matched Analysis. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.G.; Ebrahimian, S.; Sakowitz, S.; Tran, Z.; Kim, S.T.; Benharash, P. Outcomes of Expedited Discharge After Isolated Coronary Artery Bypass Grafting. J. Cardiothorac. Vasc. Anesth. 2022, 36, 3766–3772. [Google Scholar] [CrossRef]
- Williams, J.B.; McConnell, G.; Allender, J.E.; Woltz, P.; Kane, K.; Smith, P.K.; Engelman, D.T.; Bradford, W.T. One-year results from the first US-based enhanced recovery after cardiac surgery (ERAS Cardiac) program. J. Thorac. Cardiovasc. Surg. 2019, 157, 1881–1888. [Google Scholar] [CrossRef]
- Greco, M.; Capretti, G.; Beretta, L.; Gemma, M.; Pecorelli, N.; Braga, M. Enhanced recovery program in colorectal surgery: A meta-analysis of randomized controlled trials. World J. Surg. 2014, 38, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Hirji, S.A.; Salenger, R.; Boyle, E.M.; Williams, J.; Reddy, V.S.; Grant, M.C.; Chatterjee, S.; Gregory, A.J.; Arora, R.; Engelman, D.T. Expert Consensus of Data Elements for Collection for Enhanced Recovery After Cardiac Surgery. World J. Surg. 2021, 45, 917–925. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, J.; Obanor, O.; Clarizia, N.; Singh, G.; Lobdell, K.; Bailey, D.; Barnett, C.F.; Shaw, A.D.; Engelman, D.T.; Arora, R.C.; et al. Best Management Practices on Temporary Mechanical Circulatory Support: Joint Consensus Report of the PeriOperative Quality Initiative and the Enhanced Recovery After Surgery Cardiac Society. Ann. Thorac. Surg. 2025, 120, 225–243. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, D.; van Genderen, M.E.; Verhoef, C.; Huiskens, J.; Gommers, D.; van Unen, E.; Schasfoort, R.A.; Schepers, J.; van Bommel, J.; Grünhagen, D.J. Optimizing discharge after major surgery using an artificial intelligence-based decision support tool (DESIRE): An external validation study. Surgery 2022, 172, 663–669. [Google Scholar] [CrossRef] [PubMed]
| Care Element | JAMA 2019 | ATS/STS 2024 |
|---|---|---|
| Auditing of process measures | Not Included | Moderate |
| Avoidance of hypothermia <36 °C after CPB | I; COR B-NR (Moderate) | Covered intraop (Moderate) |
| Avoiding PA catheters in low-risk patients | Not Included | Moderate |
| Carbohydrate loading | IIb; COR C-LD (Low) | Covered under NPO/Prehab (Low) |
| Chemical/mechanical thromboprophylaxis | IIa; COR C-LD (Low) | Covered under screening (Moderate) |
| Chest tube patency | I; COR B-NR (Moderate) | Not Included |
| Chest wall regional analgesia | Not Included | Moderate |
| Clear liquids up to 2–4 h preop | IIb; COR C-LD (Low) | Low |
| Comprehensive patient blood management | Not Included | Moderate |
| Continuing ventilation on CPB | Not Included | Moderate |
| Correction of nutritional deficiency | IIa; COR C-LD (Low) | Not Included |
| Early ambulation and upper extremity exercise | Not Included | Moderate |
| Establishment of multidisciplinary team | Not Included | Moderate |
| Facilitate extubation within 6 hrs | IIa; COR B-R (Moderate) | Moderate |
| Goal-directed fluid and hemodynamic therapy | I; COR B-R (High) | Moderate |
| Goal-directed perfusion while on CPB | Not Included | Low |
| Hyperthermia >37.9 °C rewarming CPB | III Harm | N/A |
| Insulin infusion for hyperglycemia | IIa; COR B-NR (Moderate) | Covered under screening (Moderate) |
| Limiting NPO status for clear fluids >2 h | IIb; COR C-LD (Low) | Low |
| Mechanical ventilation with lung protective strategies | Not Included | High |
| Multicomponent prehabilitation | IIa; COR B-R (Low) | Low |
| Multifaceted screening and risk assessment | Not Included | Moderate |
| Multimodal analgesia with opioid stewardship | I; COR B-NR (Moderate) | Moderate |
| Multimodal pain plan | I; COR B-NR (Moderate) | Moderate |
| Patient engagement through shared decision-making | IIa; COR C-LD (Low) | Low |
| Patient engagement tools/education | IIa; COR C-LD (Low) | Low |
| Perioperative glycemic control | I; COR B-R (High) | Covered under screening (Moderate) |
| Preop HbA1c measurement | IIa; COR C-LD (Low) | Covered under screening (Moderate) |
| Prevention of POAF | Not Included | Moderate |
| Rigid sternal fixation for high-risk | IIa; COR B-R (Moderate) | Not Included |
| Risk assessment/prophylaxis for PONV | Not Included | Moderate |
| Routine delirium screening and nonpharm tx | I; COR B-NR (Moderate) | High |
| Screening and AKI care model | IIa; COR B-R (Moderate) | Moderate |
| Selective intra/post-op extubation in low-risk | Not Included | Low |
| Smoking and alcohol cessation ≥4 wks preop | I; COR C-LD (Low) | Covered under screening (Low) |
| Standard use of CNS monitoring | Not Included | Moderate |
| Stripping/breaking sterile chest tubes | III No Benefit | N/A |
| Surgical site infection reduction bundle | I; COR B-R (High) | High |
| TEE in moderate-/high-risk patients | Not Included | Moderate |
| Tranexamic acid for on-pump surgery | I; COR A (High) | Not Included |
| Reference Number | Study Title | Year | Number of ERACS Patients | Number of Control Patients | Intervention | Results of ERACS Implementation |
|---|---|---|---|---|---|---|
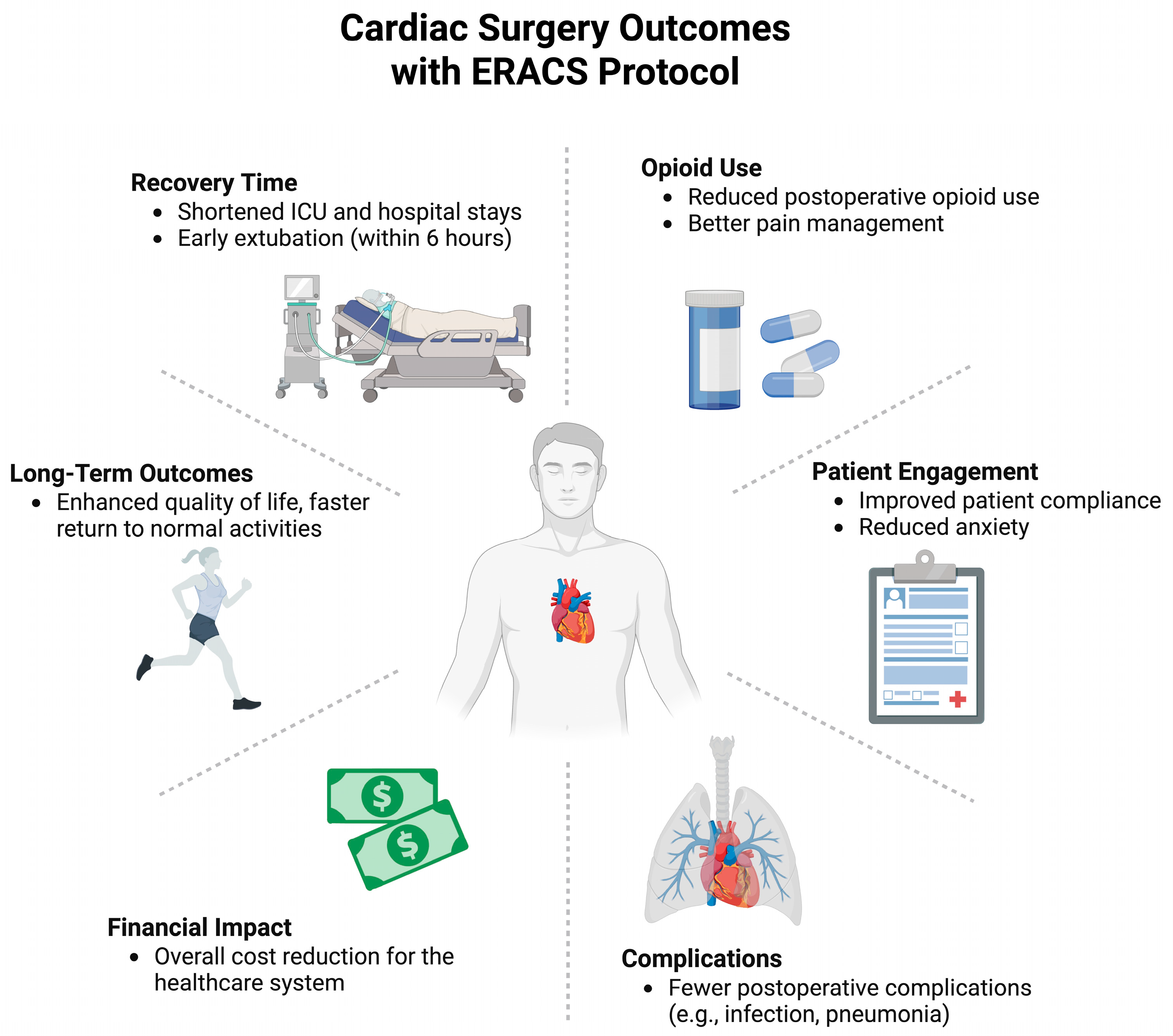
| [26] | Enhanced Recovery After Surgery Is Associated with Reduced Hospital Length of Stay after Urgent or Emergency Isolated Coronary Artery Bypass Surgery at an Urban, Tertiary Care Teaching Hospital: An Interrupted Time Series Analysis with Propensity Score Matching | 2023 | 565 | 330 | Isolated Coronary Artery Bypass Surgery | Higher likelihood of early extubation (46.0% vs. 35.8%) No significant differences in 30-day morbidity or ICU stay in urgent and emergent CABG |
| [30] | Enhanced recovery after cardiac surgery protocol reduces perioperative opioid use | 2022 | 250 | 216 | Non-emergent cardiac surgery with median sternotomy (including ascending aorta repair, CABG, valve repair, and a combination of CABG and valve repair) | 57% reduction in opioid use from postoperative days 0–5 Earlier chest tube removal ICU and hospital length of stay and 30-day morbidity and mortality showed no significant differences |
| [31] | Enhanced recovery after surgery program for patients undergoing isolated elective coronary artery bypass surgery improves postoperative outcomes | 2023 | 362 | 362 | Isolated, elective CABG surgery | 53.1% reduction in mechanical ventilation time 28.0% shorter ICU stay 10.5% shorter hospital stay Significant reductions in complications like bronchopneumonia, acute respiratory distress syndrome, and severe acute kidney injury |
| [32] | Enhanced Recovery After Surgery Is Associated With Improved Outcomes and Reduced Racial and Ethnic Disparities After Isolated Coronary Artery Bypass Surgery: A Retrospective Analysis With Propensity-Score Matching. | 2022 | 1079 | 656 | Isolated Coronary Artery Bypass Surgery | Eliminated racial disparities in postoperative ICU readmission and length of stay |
| [36] | Reduced Length of Hospital Stay for Cardiac Surgery-Implementing an Optimized Perioperative Pathway: Prospective Evaluation of an Enhanced Recovery After Surgery Program Designed for Mini-Invasive Aortic Valve Replacement. | 2019 | 23 | 23 | Mini-Invasive Aortic Valve Replacement | Decreased hospital length of stay by 3 days Lower pain scores, Trends toward global complication reduction |
| [37] | Economic impact of enhanced recovery after surgery protocol in minimally invasive cardiac surgery. | 2021 | 61 | 69 | Elective minimally invasive aortic or mitral valve surgery | Average reduction in hospital stay by 2 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaffer, A.; Yang, K.; Ebrahim, A.; Brown, A.N.; EL-Andari, R.; Dokollari, A.; Gregory, A.J.; Adams, C.; Kent, W.D.T.; Fatehi Hassanabad, A. Optimizing Recovery in Cardiac Surgery: A Narrative Review of Enhanced Recovery After Surgery Protocols and Clinical Outcomes. Med. Sci. 2025, 13, 128. https://doi.org/10.3390/medsci13030128
Jaffer A, Yang K, Ebrahim A, Brown AN, EL-Andari R, Dokollari A, Gregory AJ, Adams C, Kent WDT, Fatehi Hassanabad A. Optimizing Recovery in Cardiac Surgery: A Narrative Review of Enhanced Recovery After Surgery Protocols and Clinical Outcomes. Medical Sciences. 2025; 13(3):128. https://doi.org/10.3390/medsci13030128
Chicago/Turabian StyleJaffer, Arzina, Kayleigh Yang, Alisha Ebrahim, Amy N. Brown, Ryaan EL-Andari, Aleksander Dokollari, Alex J. Gregory, Corey Adams, William D. T. Kent, and Ali Fatehi Hassanabad. 2025. "Optimizing Recovery in Cardiac Surgery: A Narrative Review of Enhanced Recovery After Surgery Protocols and Clinical Outcomes" Medical Sciences 13, no. 3: 128. https://doi.org/10.3390/medsci13030128
APA StyleJaffer, A., Yang, K., Ebrahim, A., Brown, A. N., EL-Andari, R., Dokollari, A., Gregory, A. J., Adams, C., Kent, W. D. T., & Fatehi Hassanabad, A. (2025). Optimizing Recovery in Cardiac Surgery: A Narrative Review of Enhanced Recovery After Surgery Protocols and Clinical Outcomes. Medical Sciences, 13(3), 128. https://doi.org/10.3390/medsci13030128






