Intraosseous Versus Intravenous Vascular Access in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
2. Methodology
2.1. Protocol Registration
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria
Outcomes
2.4. Study Selection
2.5. Data Extraction
2.6. Risk of Bias and Certainty of Evidence
2.7. Statistical Analysis
3. Results
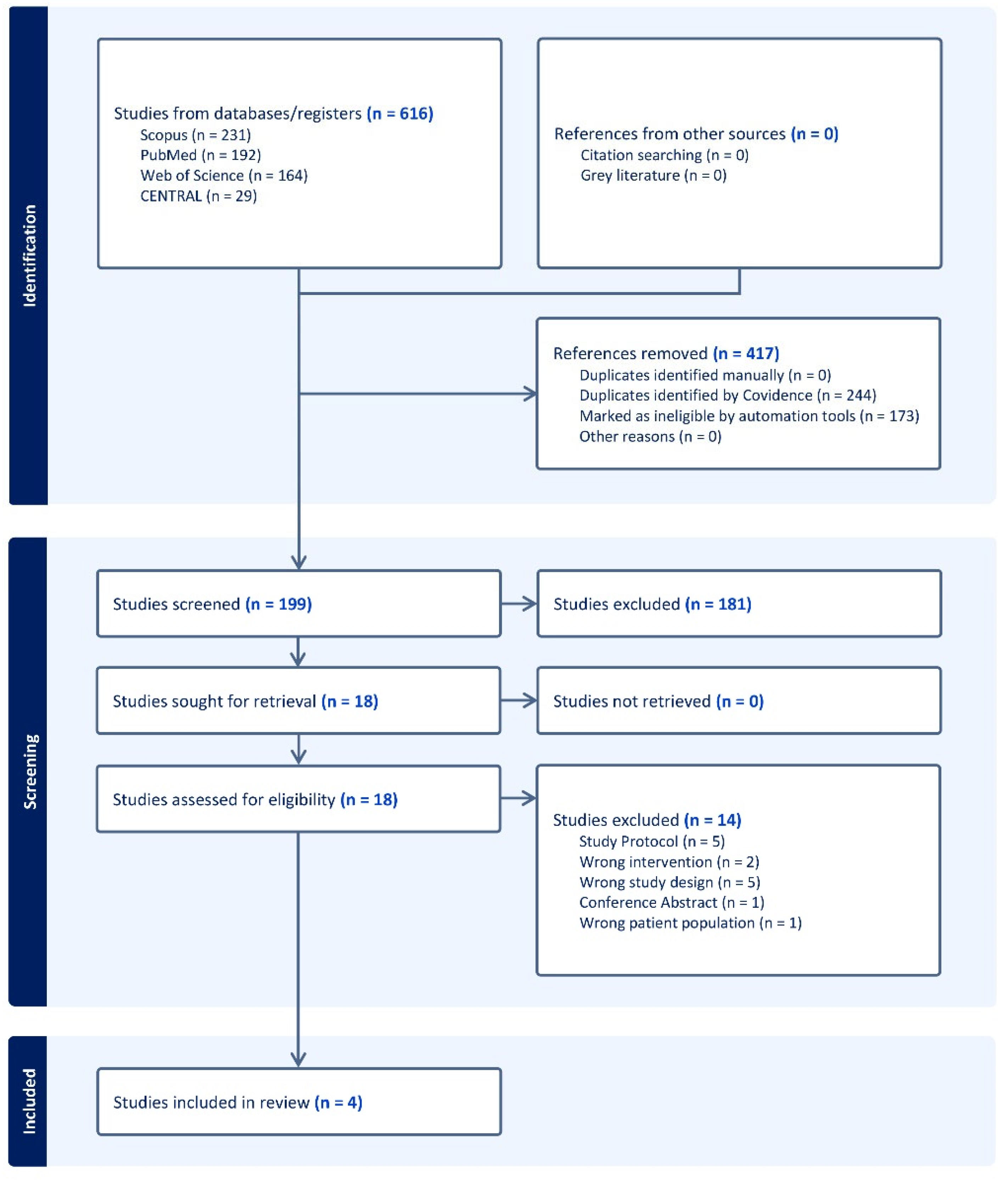
3.1. Search Results and Study Selection
3.2. Characteristics of Included Studies
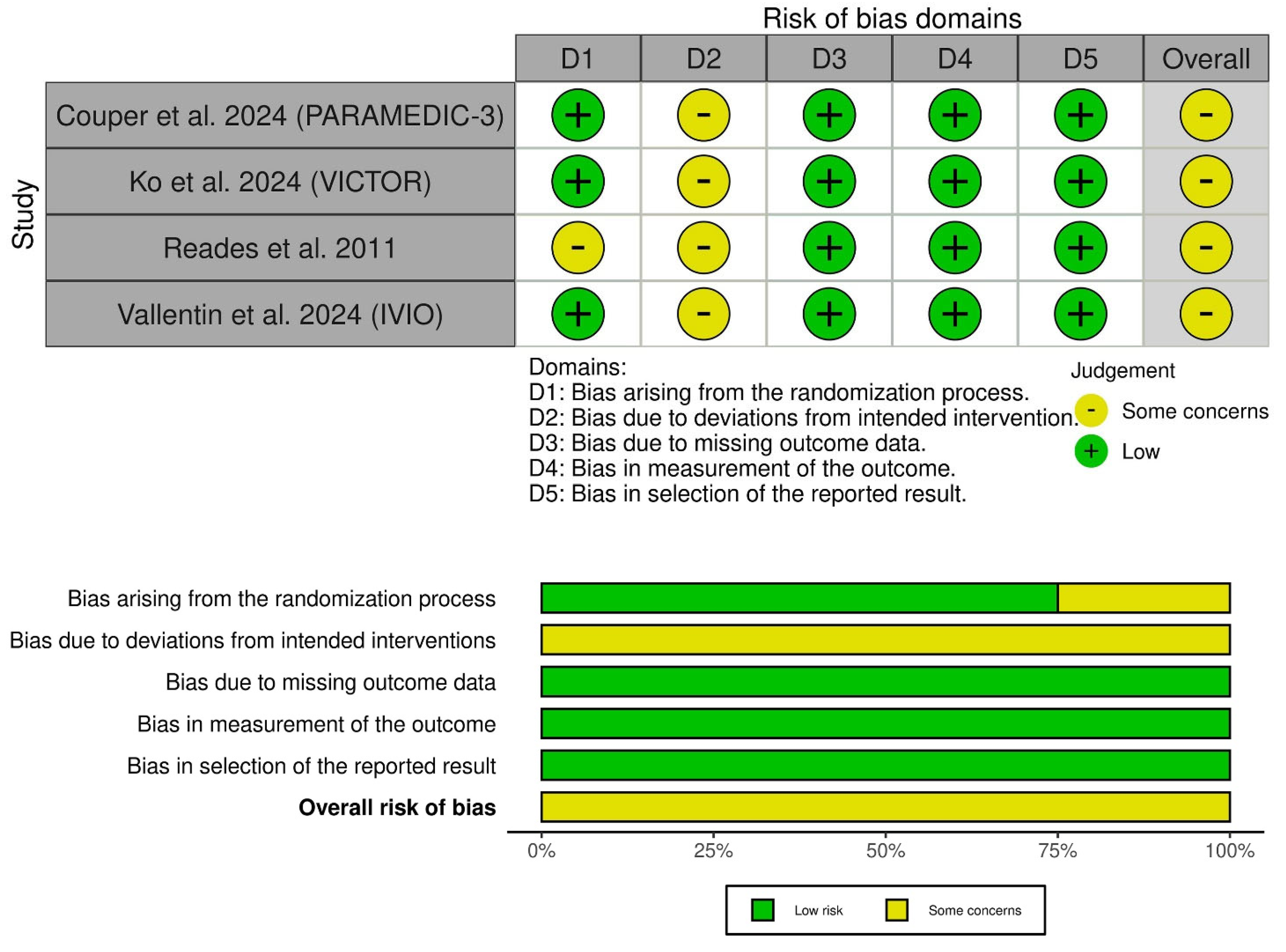
3.3. Risk of Bias and Certainty of Evidence
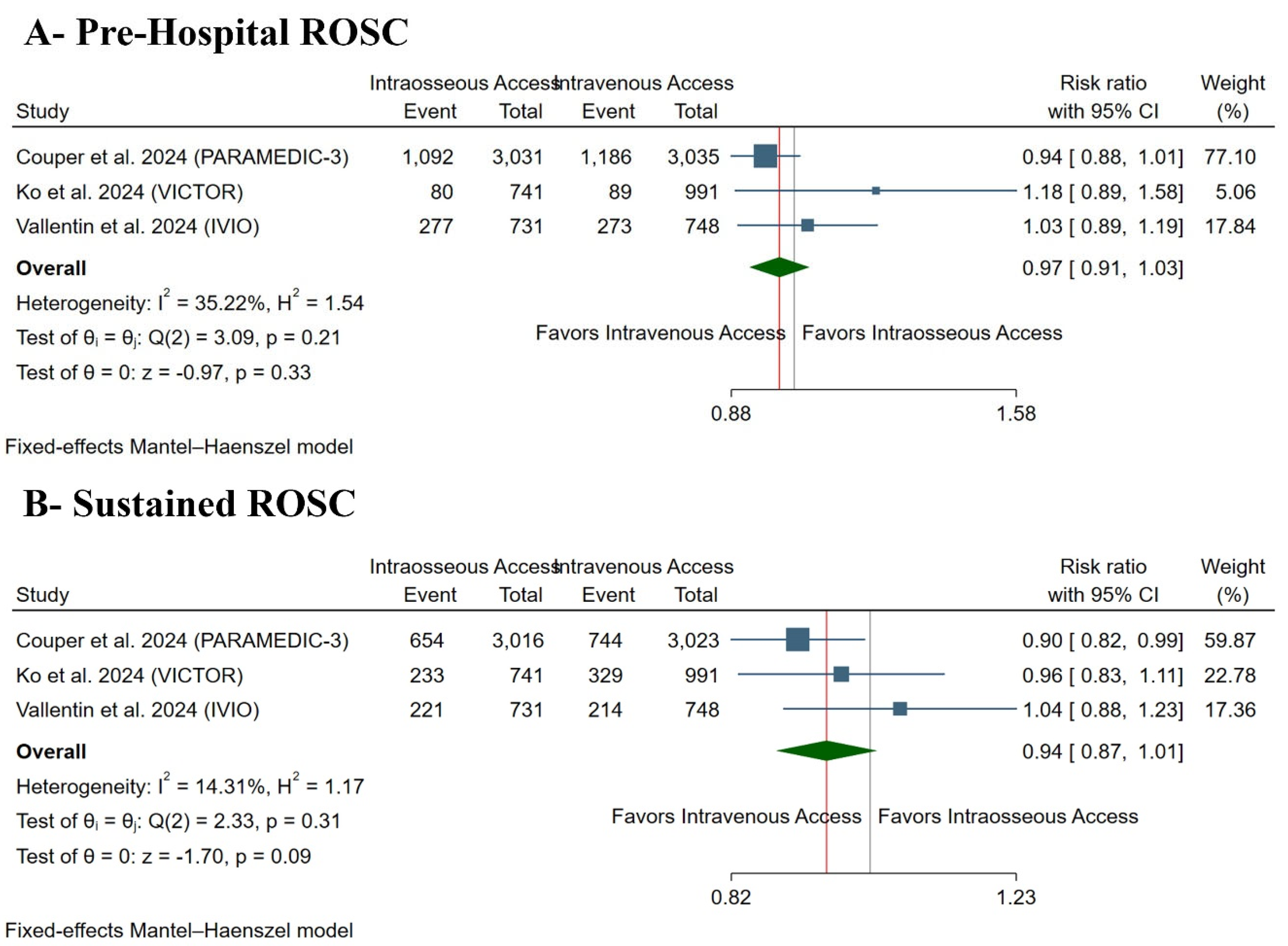
3.4. Primary Outcome: ROSC
3.5. Secondary Outcomes
3.5.1. Secondary Clinical Outcomes
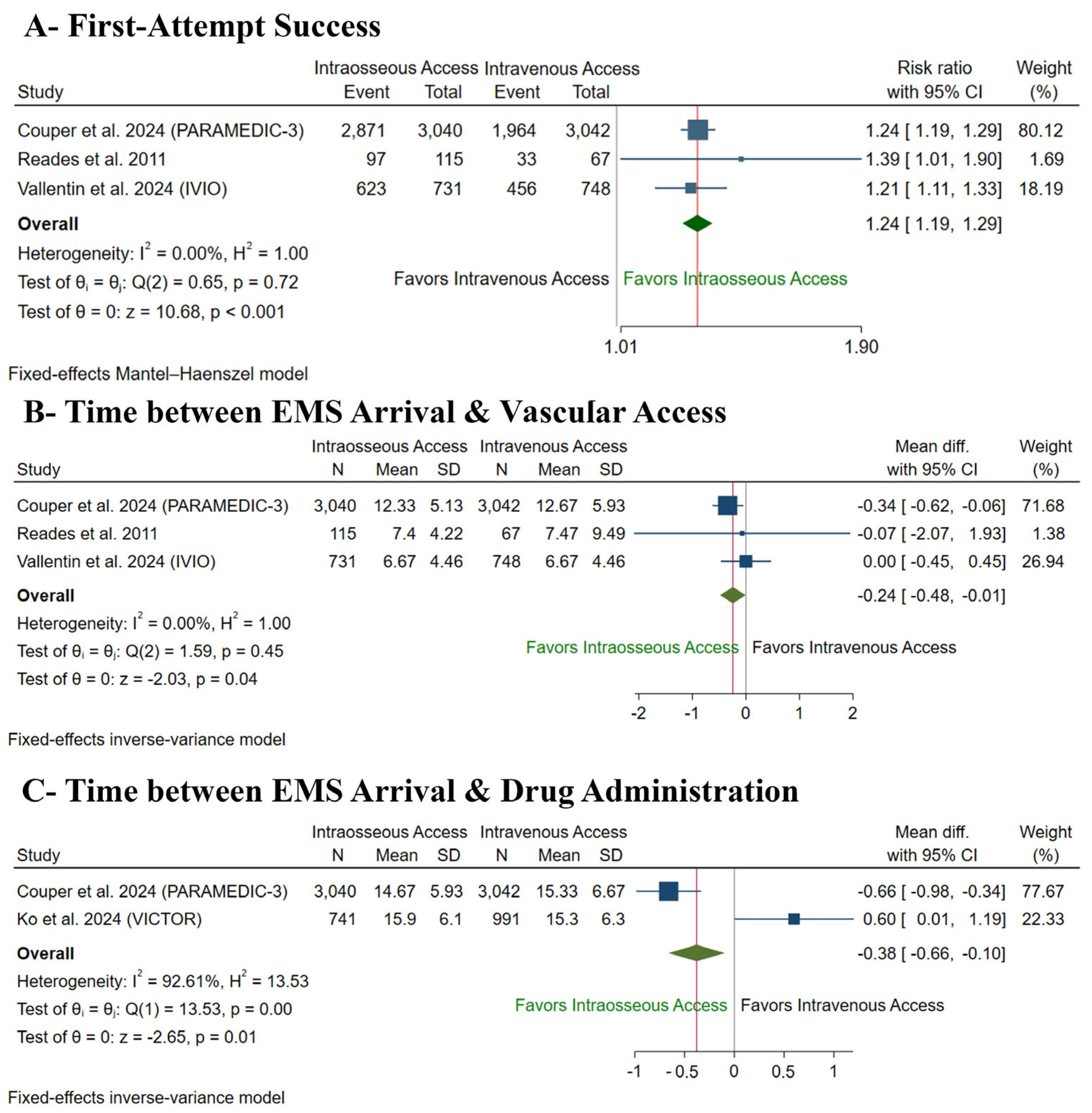
3.5.2. Secondary Procedural Outcomes
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abuelazm, M.; Ali, S.; Mahmoud, A.; Mechi, A.; Kadhim, H.; Katamesh, B.E.; Elzeftawy, M.A.; Ibrahim, A.A.; Abdelazeem, B. High versus low mean arterial pressure targets after out-of-hospital cardiac arrest: A systematic review and meta-analysis of randomized controlled trials. J. Crit. Care 2023, 78, 154365. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.; Nolan, J.P.; Rees, N.; Walker, A.; Perkins, G.D.; Couper, K. Drug routes in out-of-hospital cardiac arrest: A summary of current evidence. Resuscitation 2022, 181, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kiguchi, T.; Okubo, M.; Nishiyama, C.; Maconochie, I.; Ong ME, H.; Kern, K.B.; Wyckoff, M.H.; McNally, B.; Christensen, E.F.; Tjelmeland, I.; et al. Out-of-hospital cardiac arrest across the World: First report from the International Liaison Committee on Resuscitation (ILCOR). Resuscitation 2020, 152, 39–49. [Google Scholar] [CrossRef]
- Gräsner, J.-T.; Wnent, J.; Herlitz, J.; Perkins, G.D.; Lefering, R.; Tjelmeland, I.; Koster, R.W.; Masterson, S.; Rossell-Ortiz, F.; Maurer, H.; et al. Survival after out-of-hospital cardiac arrest in Europe—Results of the EuReCa TWO study. Resuscitation 2020, 148, 218–226. [Google Scholar] [CrossRef]
- Semeraro, F.; Greif, R.; Böttiger, B.W.; Burkart, R.; Cimpoesu, D.; Georgiou, M.; Yeung, J.; Lippert, F.; Lockey, A.S.; Olasveengen, T.M.; et al. European Resuscitation Council Guidelines 2021: Systems saving lives. Resuscitation 2021, 161, 80–97. [Google Scholar] [CrossRef]
- Hawkes, C.; Booth, S.; Ji, C.; Brace-McDonnell, S.J.; Whittington, A.; Mapstone, J.; Cooke, M.W.; Deakin, C.D.; Gale, C.P.; Fothergill, R.; et al. Epidemiology and outcomes from out-of-hospital cardiac arrests in England. Resuscitation 2017, 110, 133–140. [Google Scholar] [CrossRef]
- Abuelazm, M.T.; Ghanem, A.; Katamesh, B.E.; Hassan, A.R.; Abdalshafy, H.; Seri, A.R.; Awad, A.K.; Abdelnabi, M.; Abdelazeem, B. Defibrillation strategies for refractory ventricular fibrillation out-of-hospital cardiac arrest: A systematic review and network meta-analysis. Ann. Noninvasive Electrocardiol. 2023, 28, e13075. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Böttiger, B.W.; Carli, P.; Couper, K.; Deakin, C.D.; Djärv, T.; Lott, C.; Olasveengen, T.; Paal, P.; Pellis, T.; et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation 2021, 161, 115–151. [Google Scholar] [CrossRef]
- Perman, S.M.; Elmer, J.; Maciel, C.B.; Uzendu, A.; May, T.; Mumma, B.E.; Bartos, J.A.; Rodriguez, A.J.; Kurz, M.C.; Panchal, A.R.; et al. 2023 American Heart Association Focused Update on Adult Advanced Cardiovascular Life Support: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2024, 149, e254–e273. [Google Scholar] [CrossRef]
- Couper, K.; Ji, C.; Deakin, C.D.; Fothergill, R.T.; Nolan, J.P.; Long, J.B.; Mason, J.M.; Michelet, F.; Norman, C.; Nwankwo, H.; et al. A Randomized Trial of Drug Route in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2024, 392, 336–348. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Lin, H.-Y.; Huang, E.P.-C.; Lee, A.-F.; Hsieh, M.-J.; Yang, C.-W.; Lee, B.-C.; Wang, Y.-C.; Yang, W.-S.; Chien, Y.-C.; et al. Intraosseous versus intravenous vascular access in upper extremity among adults with out-of-hospital cardiac arrest: Cluster randomised clinical trial (VICTOR trial). BMJ 2024, 386, e079878. [Google Scholar] [CrossRef] [PubMed]
- Reades, R.; Studnek, J.R.; Vandeventer, S.; Garrett, J. Intraosseous versus intravenous vascular access during out-of-hospital cardiac arrest: A randomized controlled trial. Ann. Emerg. Med. 2011, 58, 509–516. [Google Scholar] [CrossRef]
- Vallentin, M.F.; Granfeldt, A.; Klitgaard, T.L.; Mikkelsen, S.; Folke, F.; Christensen, H.C.; Povlsen, A.L.; Petersen, A.H.; Winther, S.; Frilund, L.W.; et al. Intraosseous or Intravenous Vascular Access for Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2025, 392, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Feinstein, B.A.; Stubbs, B.A.; Rea, T.; Kudenchuk, P.J. Intraosseous compared to intravenous drug resuscitation in out-of-hospital cardiac arrest. Resuscitation 2017, 117, 91–96. [Google Scholar] [CrossRef]
- Nolan, J.P.; Deakin, C.D.; Ji, C.; Gates, S.; Rosser, A.; Lall, R.; Perkins, G.D. Intraosseous versus intravenous administration of adrenaline in patients with out-of-hospital cardiac arrest: A secondary analysis of the PARAMEDIC2 placebo-controlled trial. Intensive Care Med. 2020, 46, 954–962. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; Emberson, J.R.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Vist, G.E.; Falck-Ytter, Y.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: What is “quality of evidence” and why is it important to clinicians? BMJ Br. Med. J. 2008, 336, 995. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. Rating Quality of Evidence and Strength of Recommendations: GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ Br. Med. J. 2008, 336, 924. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Granfeldt, A.; Avis, S.R.; Lind, P.C.; Holmberg, M.J.; Kleinman, M.; Maconochie, I.; Hsu, C.H.; de Almeida, M.F.; Wang, T.-L.; Neumar, R.W.; et al. Intravenous vs. intraosseous administration of drugs during cardiac arrest: A systematic review. Resuscitation 2020, 149, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Tabowei, G.; Dadzie, S.K.; Khoso, A.A.; Riyalat, A.A.; Ali, M.; Atta, M.I.M.S.I.; Wei, C.R.; Ali, N. Efficacy of Intraosseous Versus Intravenous Drug Administration in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e72276. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.W.; Grossestreuer, A.V.; Donnino, M.W. “Resuscitation time bias”—A unique challenge for observational cardiac arrest research. Resuscitation 2018, 125, 79–82. [Google Scholar] [CrossRef]
- Perkins, G.D.; Neumar, R.; Hsu, C.H.; Hirsch, K.G.; Aneman, A.; Becker, L.B.; Couper, K.; Callaway, C.W.; Hoedemaekers, C.W.E.; Lim, S.L.; et al. Improving Outcomes after Post-Cardiac Arrest Brain Injury: A Scientific Statement from the International Liaison Committee on Resuscitation. Circulation 2024, 150, e158-80. [Google Scholar] [CrossRef]
- Ross, E.M.; Mapp, J.; Kharod, C.; Wampler, D.A.; Velasquez, C.; Miramontes, D.A. Time to epinephrine in out-of-hospital cardiac arrest: A retrospective analysis of intraosseous versus intravenous access. Am. J. Disaster Med. 2016, 11, 119–123. [Google Scholar] [CrossRef]
- Berger, D.; Petrie, A.; Lubin, J.S. The Ability of Paramedics to Accurately Locate Correct Anatomical Sites for Intraosseous Needle Insertion. Cureus 2023, 15, e33355. [Google Scholar] [CrossRef]
- Daya, M.R.; Leroux, B.G.; Dorian, P.; Rea, T.D.; Newgard, C.D.; Morrison, L.J.; Lupton, J.R.; Menegazzi, J.J.; Ornato, J.P.; Sopko, G.; et al. Survival After Intravenous Versus Intraosseous Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Shock-Refractory Cardiac Arrest. Circulation 2020, 141, 188–198. [Google Scholar] [CrossRef]
- Greenstein, Y.Y.; Koenig, S.J.; Mayo, P.H.; Narasimhan, M. A Serious Adult Intraosseous Catheter Complication and Review of the Literature. Crit. Care Med. 2016, 44, e904–e909. [Google Scholar] [CrossRef]
- Kristiansen, S.; Storm, B.; Dahle, D.; Domaas Josefsen, T.; Dybwik, K.; Nilsen, B.A.; Waage-Nielsen, E. Intraosseous fluid resuscitation causes systemic fat emboli in a porcine hemorrhagic shock model. Scand. J. Trauma Resusc. Emerg. Med. 2021, 29, 172. [Google Scholar] [CrossRef]
- Maxien, D.; Wirth, S.; Peschel, O.; Sterzik, A.; Kirchhoff, S.; Kreimeier, U.; Reiser, M.F.; Mück, F.G. Intraosseous needles in pediatric cadavers: Rate of malposition. Resuscitation 2019, 145, 1–7. [Google Scholar] [CrossRef]
- Hallas, P.; Brabrand, M.; Folkestad, L. Complication with intraosseous access: Scandinavian users’ experience. West. J. Emerg. Med. 2013, 14, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Alilou, S.; Moskowitz, A.; Rashedi, S. Intraosseous versus intravenous vascular access in out-of-hospital cardiac arrest: A systematic review and meta-analysis of randomized controlled trials. Crit. Care 2025, 29, 124. [Google Scholar] [CrossRef] [PubMed]
- Rath, S.; Abdelraouf, M.R.; Hassan, W.; Mehmood, Q.; Ansab, M.; Salamah, H.M.; Singh, P.K.; Punukollu, A.; Jain, H.; Ahmed, R. The impact of intraosseous vs. intravenous vascular access during resuscitation in out-of-hospital cardiac arrest: A comprehensive systematic review and meta-analysis. Heart Lung 2025, 72, 20–31. [Google Scholar] [CrossRef] [PubMed]
| Study ID | Study Design | Country | Total Participants | Intraosseous Access Route | Main Inclusion Criteria | Sustained ROSC Definition | Primary Outcome | Follow-Up Duration |
|---|---|---|---|---|---|---|---|---|
| Couper et al. 2024 (PARAMEDIC-3) [10] | Multicenter, open-label RCT | UK | 6082 | Humeral or tibial | Adults (≥18 years) with OHCA (of any cause) and requiring vascular access (during ongoing CPR) | NA | Survival at 30 days | 6 months |
| Ko et al. 2024 (VICTOR) [11] | Multicenter, open-label, cluster RCT | Taiwan | 1732 | Humeral | Adults (20–80 years) with atraumatic OHCA | ROSC for at least two hours and survival with a favorable neurological outcome | Survival to hospital discharge | Hospital discharge |
| Reades et al. 2011 [12] | Single-center, open-label RCT | USA | 182 | Humeral or tibial | Adults (>18 years) with atraumatic OHCA | NA | First-attempt success | NA |
| Vallentin et al. 2024 (IVIO) [13] | Multicenter, single-blinded RCT | Denmark | 1479 | Humeral or tibial | Adults (>18 years) with atraumatic OHCA | ROSC with no further use of chest compressions for at least 20 min | Sustained ROSC | 6 months |
| Study ID | Number of Patients in Each Group | Age (Years), Mean (SD) | Gender (Male), N. (%) | Bystander CPR, N. (%) | OHCA Cause, N. (%) | OHCA Location, N. (%) | Witness, N. (%) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IO | IV | IO | IV | IO | IV | IO | IV | Medical | Traumatic | Drug Overdose | Drowning | Asphyxia | Unknown or Not Recorded | Home | Public | Bystander | EMS | Unwitnessed | |||||||||||||
| IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | IO | IV | ||||||||||
| Couper et al. 2024 (PARAMEDIC-3) [10] | 3040 | 3042 | 67.8 (16.3) | 68.3 (15.9) | 1941 (63.8) | 1951 (64.1) | 2089 (68.7) | 2145 (70.5) | 2484 (81.7) | 2480 (81.5) | 48 (1.6) | 38 (1.2) | 57 (1.9) | 67 (2.2) | 8 (0.3) | 7 (0.2) | 86 (2.8) | 87 (2.9) | 355 (11.7) | 362 (11.9) | 2392 (78.7) | 2422 (79.6) | 99 (3.3) | 96 (3.2) | 1645 (54.1) | 1703 (56.0) | 194 (6.4) | 183 (6.0) | 1164 (38.3) | 1109 (36.5) | |
| Ko et al. 2024 (VICTOR) [11] | 741 | 991 | 64.0 (54.0–72.0) | 66.0 (56.0–74.0) | 521 (70.3) | 713 (71.9) | 531 (71.7) | 701 (70.7) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | 536 (72.3) | 702 (70.8) | 187 (25.2) | 274 (27.6) | NA | NA | NA | NA | NA | NA | |
| Reades et al. 2011 [12] | 115 | 67 | 65.7 (2.6) | 64.7 (2.2) | 77 (65) | 42 (63) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Vallentin et al. 2024 (IVIO) [13] | 731 | 748 | 69 (15) | 70 (14) | 517 (71) | 516 (69) | 585 (84) | 594 (83) | 216 (29) | 213 (29) | NA | NA | 13 (2) | 12 (2) | 4 (1) | 4 (1) | 22 (3) | 20 (3) | 474 (65) | 494 (66) | NA | NA | NA | NA | 383 (52) | 391 (52) | 38 (5) | 29 (4) | 310 (42) | 328 (44) | |
| Certainty Assessment | Summary of Findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (Studies) Follow-Up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates (%) | Relative Effect (95% CI) | Anticipated Absolute Effects | ||
| With [IV] | With [IO] | Risk with [IV] | Risk Difference with [IO] | ||||||||
| Prehospital ROSC | |||||||||||
| 9277 (3 RCTs) | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ Moderate a | 1548/4774 (32.4%) | 1449/4503 (32.2%) | RR 0.97 (0.91 to 1.03) | 1548/4774 (32.4%) | 10 fewer per 1000 (from 29 fewer to 10 more) |
| Maintained ROSC | |||||||||||
| 9250 (3 RCTs) | serious a | not serious | serious b | not serious | none | ⨁⨁◯◯ Low a,b | 1287/4762 (27.0%) | 1108/4488 (24.7%) | RR 0.94 (0.84 to 1.01) | 1287/4762 (27.0%) | 16 fewer per 1000 (from 43 fewer to 3 more) |
| Survival to Discharge | |||||||||||
| 9185 (3 RCTs) | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ Moderate a | 296/4723 (6.3%) | 278/4462 (6.2%) | RR 1.00 (0.97 to 1.03) | 296/4723 (6.3%) | 0 fewer per 1000 (from 2 fewer to 2 more) |
| 30-Day Discharge | |||||||||||
| 7543 (3 RCTs) | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ Moderate a | 113/3782 (3.0%) | 219/3761 (5.8%) | RR 0.98 (0.82 to 1.17) | 113/3782 (3.0%) | 1 fewer per 1000 (from 5 fewer to 5 more) |
| Favorable Neurological Recovery | |||||||||||
| 9185 (3 RCTs) | serious a | not serious | not serious | seriousc | none | ⨁⨁◯◯ Low a,c | 222/4723 (4.7%) | 218/4462 (4.9%) | RR 1.07 (0.90 to 1.29) | 222/4723 (4.7%) | 3 more per 1000 (from 5 fewer to 14 more) |
| Unfavorable Neurological Recovery | |||||||||||
| 9185 (3 RCTs) | serious a | not serious | not serious | not serious | none | ⨁⨁⨁◯ Moderate a | 4501/4723 (95.3%) | 4244/4462 (95.1%) | RR 1.03 (0.88 to 1.21) | 4501/4723 (95.3%) | 29 more per 1000 (from 114 fewer to 200 more) |
| First-Attempt Success | |||||||||||
| 7743 (3 RCTs) | serious a | not serious | not serious | seriousc | none | ⨁⨁◯◯ Low a,c | 2453/3857 (63.6%) | 1197/3886 (30.8%) | RR 1.24 (1.19 to 1.29) | 2453/3857 (63.6%) | 153 more per 1000 (from 121 more to 184 more) |
| Time Between EMS Arrival and Vascular Access | |||||||||||
| 7743 (3 RCTs) | serious a | not serious | not serious | serious c | none | ⨁⨁◯◯ Low a,c | 3857 | 3886 | - | 3857 | MD 0.24 min lower (0.48 lower to 0.01 lower) |
| Time Between EMS Arrival and Drug Administration | |||||||||||
| 7814 (2 RCTs) | serious a | very serious d | not serious | serious c | none | ⨁◯◯◯ Very low a,c,d | 4033 | 3781 | - | 4033 | MD 0.38 min lower (0.66 lower to 0.1 lower) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsagban, A.; Saab, O.; Al-Obaidi, H.; Algodi, M.; Yu, A.; Abuelazm, M.; Hochberg, C. Intraosseous Versus Intravenous Vascular Access in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2025, 13, 78. https://doi.org/10.3390/medsci13020078
Alsagban A, Saab O, Al-Obaidi H, Algodi M, Yu A, Abuelazm M, Hochberg C. Intraosseous Versus Intravenous Vascular Access in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medical Sciences. 2025; 13(2):78. https://doi.org/10.3390/medsci13020078
Chicago/Turabian StyleAlsagban, Alhareth, Omar Saab, Hasan Al-Obaidi, Marwah Algodi, Amy Yu, Mohamed Abuelazm, and Chad Hochberg. 2025. "Intraosseous Versus Intravenous Vascular Access in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Medical Sciences 13, no. 2: 78. https://doi.org/10.3390/medsci13020078
APA StyleAlsagban, A., Saab, O., Al-Obaidi, H., Algodi, M., Yu, A., Abuelazm, M., & Hochberg, C. (2025). Intraosseous Versus Intravenous Vascular Access in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Medical Sciences, 13(2), 78. https://doi.org/10.3390/medsci13020078