Adherence to Mediterranean Diet and Ocular Dryness Severity in Sjögren’s Syndrome: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
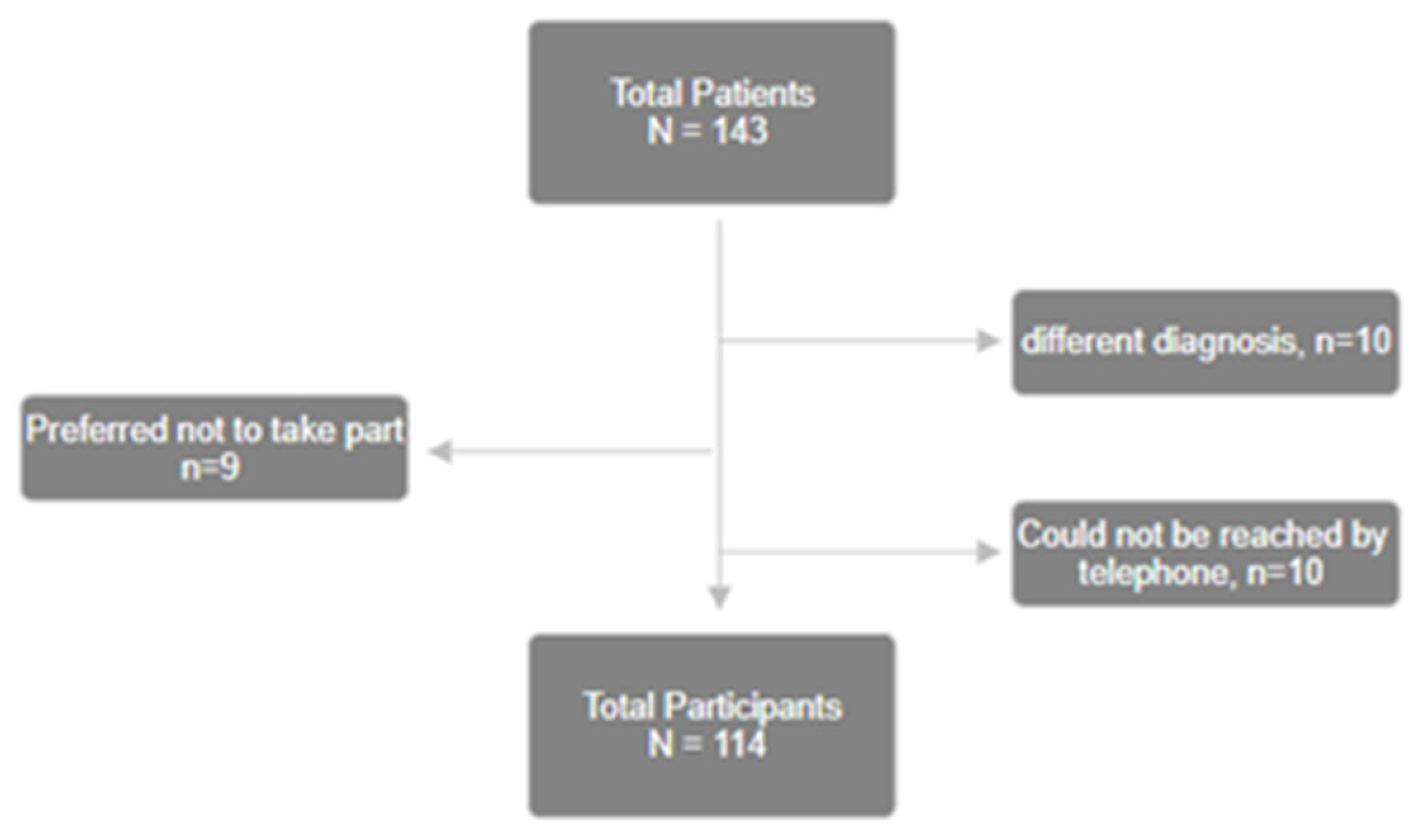
2.1. Study Population
2.2. Questionnaires
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Demographics
3.2. Population
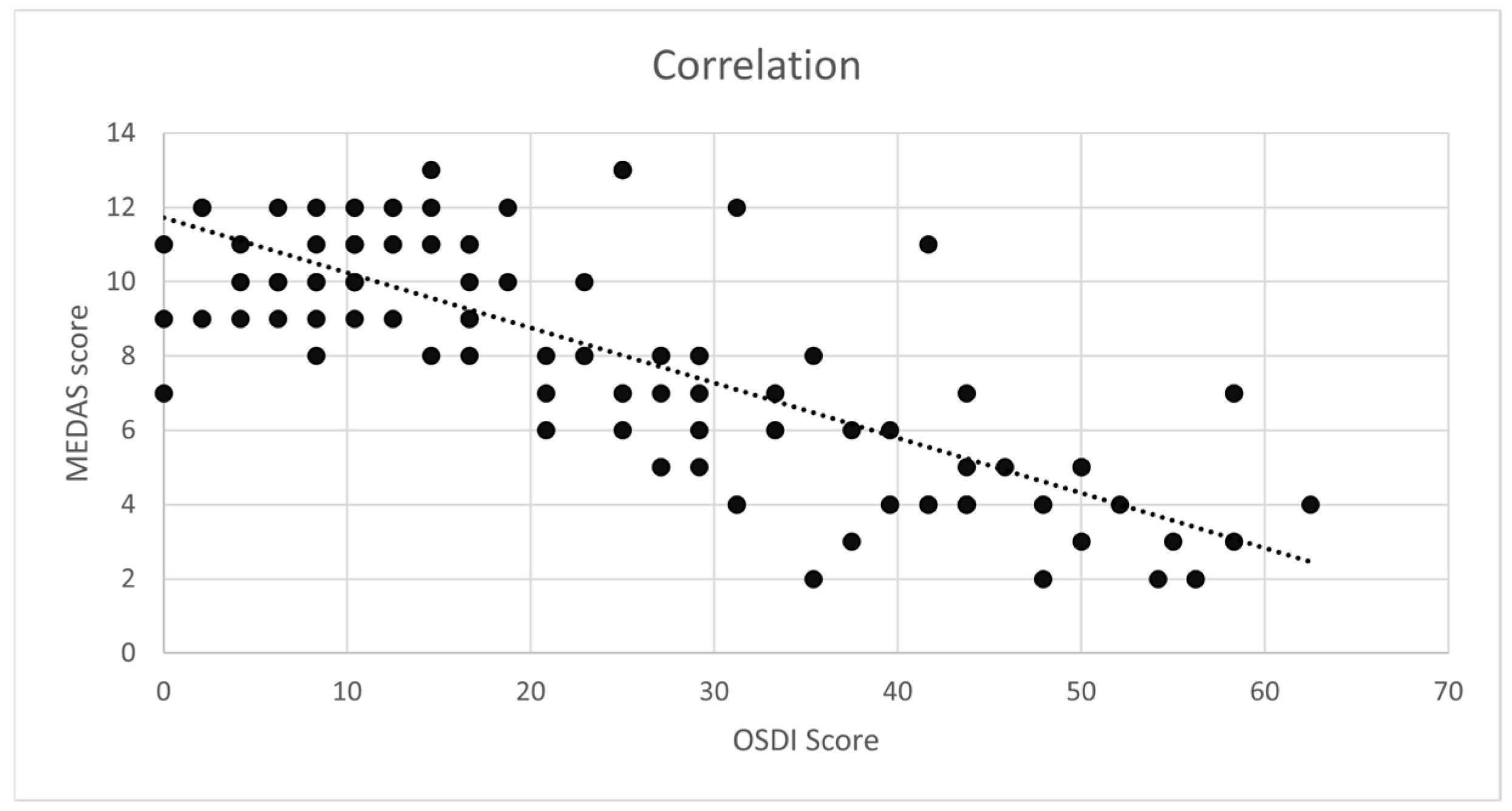
3.3. Correlation Between OD and the MD
3.4. Comparison of OD Based on Various Risk Factors
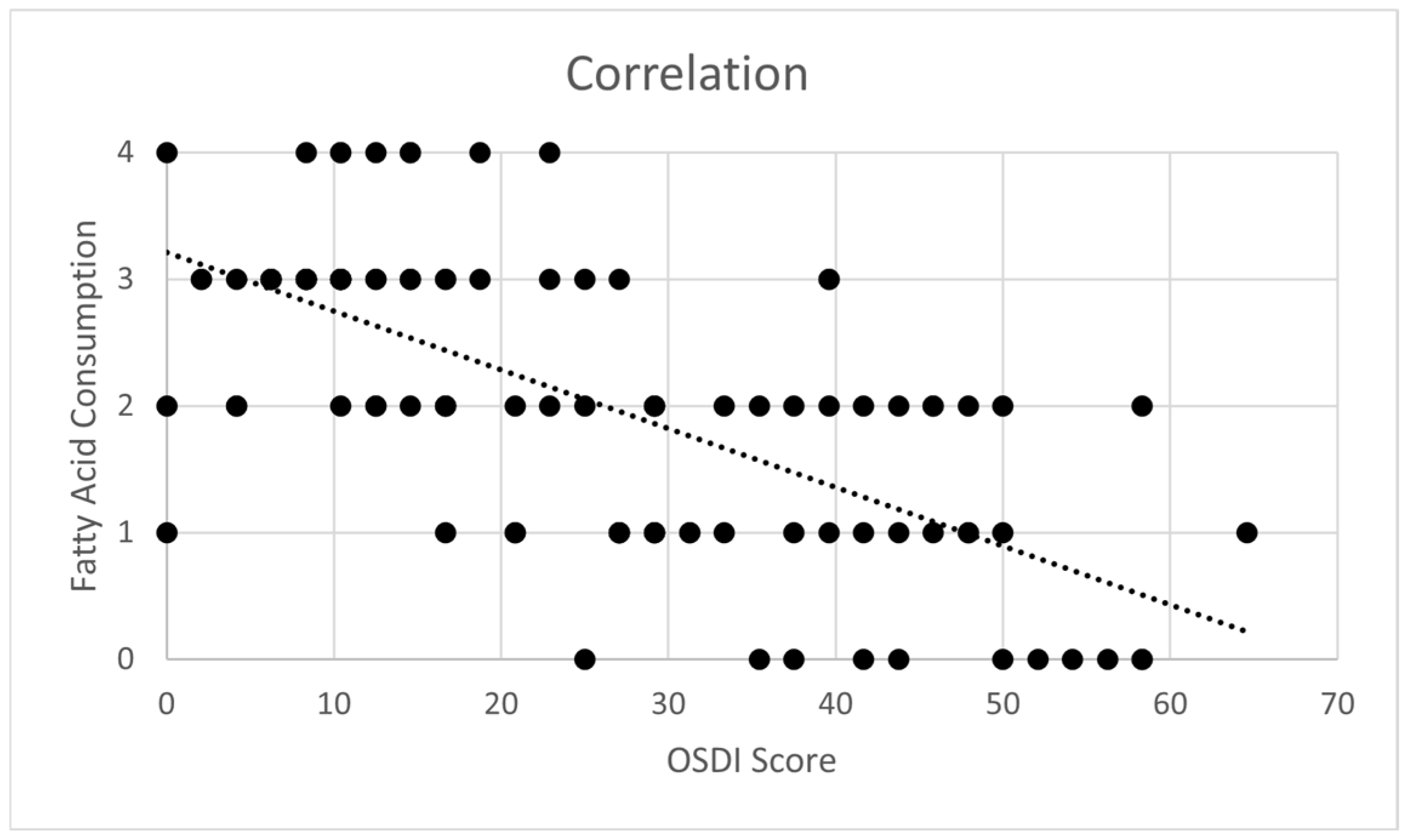
3.5. Impact of Polyunsaturated Fatty Acid Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MD | Mediterranean Diet |
| MEDAS | Mediterranean Diet Adherence Screener |
| OSDI | Ocular Surface Disease Index |
| OD | Ocular Dryness |
| SS | Sjögren Syndrome |
References
- Sjögren, H. Zur Kenntnis der Keratoconjunctivitis Sicca II: Allgemeine Symptomatologie und Ätiologies. Acta Ophthalmol. 1935, 13, 1–39. [Google Scholar] [CrossRef]
- Lessard, C.J.; Li, H.; Adrianto, I.; Ice, J.A.; Rasmussen, A.; Grundahl, K.M.; Kelly, J.A.; Dozmorov, M.G.; Miceli-Richard, C.; Bowman, S.; et al. Variants at multiple loci implicated in both innate and adaptive immune responses are associated with Sjögren’s syndrome. Nat. Genet. 2013, 45, 11. [Google Scholar] [CrossRef]
- Qin, B.; Wang, J.; Yang, Z.; Yang, M.; Ma, N.; Huang, F.; Zhong, R. Epidemiology of primary Sjögren’s syndrome: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 1983–1989. [Google Scholar] [CrossRef]
- Narváez, J.; Sánchez-Fernández, S.Á.; Seoane-Mato, D.; Díaz-González, F.; Bustabad, S. Prevalence of Sjögren’s syndrome in the general adult population in Spain: Estimating the proportion of undiagnosed cases. Sci. Rep. 2020, 10, 10627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vehof, J.; Utheim, T.P.; Bootsma, H.; Hammond, C.J. Advances, limitations and future perspectives in the diagnosis and management of dry eye in Sjögren’s syndrome. Clin. Exp. Rheumatol. 2020, 38 (Suppl. 126), 301–309. [Google Scholar] [PubMed]
- Sutcliffe, N.; Recchioni, A.; Hilmi, S.; Rauz, S.; Tappuni, A.R. What’s new in ocular and oral aspects of Sjögren’s syndrome and do new treatments work? Rheumatology 2021, 60, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Methodologies to diagnose and monitor dry eye disease: Report of the Diagnostic Methodology Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 108–152. [CrossRef]
- Ahn, H.; Ji, Y.W.; Jun, I.; Kim, T.-I.; Lee, H.K.; Seo, K.Y. Comparison of Treatment Modalities for Dry Eye in Primary Sjögren’s Syndrome. J. Clin. Med. 2022, 11, 463. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sumida, T.; Azuma, N.; Moriyama, M.; Takahashi, H.; Asashima, H.; Honda, F.; Abe, S.; Ono, Y.; Hirota, T.; Hirata, S.; et al. Clinical practice guideline for Sjögren’s syndrome 2017. Mod. Rheumatol. 2018, 28, 383–408. [Google Scholar] [CrossRef] [PubMed]
- Both, T.; Dalm, V.A.S.H.; van Hagen, P.M.; van Daele, P.L.A. Reviewing primary Sjögren’s syndrome: Beyond the dryness—From pathophysiology to diagnosis and treatment. Int. J. Med. Sci. 2017, 14, 191–200. [Google Scholar] [CrossRef]
- Cohn, G.S.; Corbett, D.; Tenen, A.; Coroneo, M.; McAlister, J.; Craig, J.P.; Gray, T.; Kent, D.; Murray, N.; Petsoglou, C.; et al. Randomized, Controlled, Double-Masked, Multicenter, Pilot Study Evaluating Safety and Efficacy of Intranasal Neurostimulation for Dry Eye Disease. Investig. Ophthalmol. Vis. Sci. 2019, 60, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Fulvio, G.; La Rocca, G.; Ferro, F. Update on the pathophysiology and treatment of primary Sjögren syndrome. Nat. Rev. Rheumatol. 2024, 20, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, G.K.; Patel, J.; Malvankar-Mehta, M.S.; Mather, R. Work productivity among Sjögren’s Syndrome and non-Sjögren’s dry eye patients: A systematic review and meta-analysis. Eye 2021, 35, 12. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Garcia, E.; Rodriguez-Artalejo, F.; Li, T.Y.; Fung, T.T.; Li, S.; Willett, W.C.; Rimm, E.B.; Hu, F.B. The Mediterranean-style dietary pattern and mortality among men and women with cardiovascular disease. Am. J. Clin. Nutr. 2014, 99, 172–180. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Mai, F.; Mercuri, A.; Centorame, D.; Cipollone, J.; Mariani, F.M.; Rossi, M.; Bartoloni, E.; Grassi, D.; et al. Adherence to the Mediterranean diet and the impact on clinical features in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 133), 190–196. [Google Scholar] [CrossRef]
- Molina-Leyva, I.; Molina-Leyva, A.; Riquelme-Gallego, B.; Cano-Ibáñez, N.; García-Molina, L.; Bueno-Cavanillas, A. Effectiveness of Mediterranean Diet Implementation in Dry Eye Parameters: A Study of PREDIMED-PLUS Trial. Nutrients 2020, 12, 1289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1402S–1406S. [Google Scholar] [CrossRef]
- Martínez-González, M.Á.; Corella, D.; Salas-Salvadó, J.; Ros, E.; Covas, M.I.; Fiol, M.; Wärnberg, J.; Arós, F.; Ruíz-Gutiérrez, V.; Lamuela-Raventós, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef]
- Gregório, M.J.; Rodrigues, A.M.; Salvador, C.; Dias, S.S.; de Sousa, R.D.; Mendes, J.M.; Coelho, P.S.; Branco, J.C.; Lopes, C.; Martínez-González, M.A.; et al. Validation of the Telephone-Administered Version of the Mediterranean Diet Adherence Screener (MEDAS) Questionnaire. Nutrients 2020, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Moutsopoulos, H.M. The geoepidemiology of Sjögren’s syndrome. Autoimmun. Rev. 2010, 9, A305–A310. [Google Scholar] [CrossRef]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjögren’s syndrome: A consensus and data-driven methodology involving three international patient cohorts. Ann. Rheum. Dis. 2017, 76, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.M.; Powell-Griner, E.; McFann, K.; Nahin, R.L. Complementary and alternative medicine use among adults: United States, 2002. Adv. Data 2004, 1–19. [Google Scholar] [CrossRef]
- Miljanović, B.; Trivedi, K.A.; Dana, M.R.; Gilbard, J.P.; Buring, J.E.; Schaumberg, D.A. Relation between dietary n-3 and n-6 fatty acids and clinically diagnosed dry eye syndrome in women. Am. J. Clin. Nutr. 2005, 82, 887–893. [Google Scholar] [CrossRef]
- Hong, S.; Woo, M.; Eom, Y.; Kim, H.K.; Yoon, K.C.; Na, K.S.; Cho, K.J.; Lee, H.K.; Song, J.S. A Multicenter, Randomized, Clinical Trial Assessing the Effect of rTG-Omega 3 Supplementation on Meibomian Gland Dysfunction Patients after Cataract Surgery rTG-Omega 3 for Meibomian Gland Dysfunction. J. Ocul. Pharmacol. Ther. 2025, 41, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Yoo, Y.S.; Shin, E.; Han, G.; Shin, K.; Lim, D.H.; Chung, T.Y. Effects of the re-esterified triglyceride (rTG) form of omega-3 supplements on dry eye following cataract surgery. Br. J. Ophthalmol. 2021, 105, 1504–1509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-González, M.Á.; Martín-Calvo, N.; Bretos-Azcona, T.; Carlos, S.; Delgado-Rodríguez, M. Mediterranean Diet and Cardiovascular Prevention: Why Analytical Observational Designs Do Support Causality and Not Only Associations. Int. J. Environ. Res. Public Health 2022, 19, 13653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| All | Ocular Surface Disease Index | p-Value | ||
|---|---|---|---|---|
| Minimal (<12) | Considerable or High (>13) | |||
| N | 114 | 31 | 83 | |
| Age, mean (SD) | 51 ± 13.4 | 49.0 | 52.2 | 0.293 |
| Sex, N (%) | 0.042 | |||
| Female | 98 (86) | 30 (26) | 68 (60) | |
| Male | 16 (14) | 1 (1) | 15 (13) | |
| Marital status, N (%) | 0.484 | |||
| Married or in a couple | 88 (77) | 22 (19) | 66 (58) | |
| Single | 26 (23) | 10 (9) | 16 (14) | |
| Smokers, N (%) | 16 (14) | 5 (4) | 11 ((1) | 0.646 |
| Disease duration of less than 5 years, N (%) | 82 (72) | 47 (41) | 35 (31) | 0.323 |
| Diagnosis, N (%) | 0.360 | |||
| Histology (Chisholm and Mason score of ≥3) | 33 (29) | 7 (6) | 26 (23) | |
| Anti-SSA or SSB antibody | 81 (71) | 24 (21) | 57 (50) | |
| Treatment with Hydroxychloroquine, N (%) | 61 (54) | 17 (15) | 44 (39) | 0.862 |
| Ocular treatment with artificial tears, N (%) | 75 (65) | 17 (15) | 58 (50) | 0.134 |
| Associated diseases, N (%) | 0.429 | |||
| No associated diseases | 86 (75) | 25 (22) | 61 (53) | |
| SLE | 13 (11) | 4 (3) | 9 (8) | |
| Thyroid | 15 (14) | 2 (2) | 13 (12) | |
| Presence of subjective OD, N (%) | 92 (81) | 16 (14) | 76 (67) | <0.001 |
| MEDAS, median (IQR) | 8 (5–11) | 69 | 45 | <0.001 |
| MEDAS score categories, N (%) | <0.001 | |||
| Minimal | 31 (27) | 0 (0) | 31 (27) | |
| Moderate | 38 (33) | 8 (7) | 30 (26) | |
| Good | 45 (40) | 23 (20) | 22 (20) | |
| MEDAS | Frequency | Mean ± SD | p-Value | |
|---|---|---|---|---|
| OSDI | Poor or moderate adherence | 69 | 33 ± 16.0 | <0.01 |
| High adherence | 45 | 13 ± 8.1 |
| Bivariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Correlation with OSDI | p-Value | Coefficient | 95% CI | |
| Age | 0.037 | 0.694 | −0.003 | −0.336; 0.329 |
| Sex | 0.108 | 0.249 | 0.772 | 0.270; 1.173 |
| Marital status | 0.123 | 0.156 | −0.087 | −0.472; 0.298 |
| Smoking | −0.063 | 0.504 | −0.037 | −0.469; 0.384 |
| Diagnostic modality | −0.192 | 0.056 | 0.138 | −0.392; 0.667 |
| Treatment with Hydroxychloroquine | 0.026 | 0.783 | 0.025 | −0.280; 0.331 |
| Use of artificial tears | 0.205 | 0.183 | 0.128 | −0.176; 0.431 |
| MEDAS score | −0.73 | <0.01 | −0.293 | −0.344; −0.241 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaaya, C.; Raad, E.; Kahale, F.; Chelala, E.; Ziade, N.; Maalouly, G. Adherence to Mediterranean Diet and Ocular Dryness Severity in Sjögren’s Syndrome: A Cross-Sectional Study. Med. Sci. 2025, 13, 64. https://doi.org/10.3390/medsci13020064
Chaaya C, Raad E, Kahale F, Chelala E, Ziade N, Maalouly G. Adherence to Mediterranean Diet and Ocular Dryness Severity in Sjögren’s Syndrome: A Cross-Sectional Study. Medical Sciences. 2025; 13(2):64. https://doi.org/10.3390/medsci13020064
Chicago/Turabian StyleChaaya, Celine, Elie Raad, Francesca Kahale, Elias Chelala, Nelly Ziade, and Georges Maalouly. 2025. "Adherence to Mediterranean Diet and Ocular Dryness Severity in Sjögren’s Syndrome: A Cross-Sectional Study" Medical Sciences 13, no. 2: 64. https://doi.org/10.3390/medsci13020064
APA StyleChaaya, C., Raad, E., Kahale, F., Chelala, E., Ziade, N., & Maalouly, G. (2025). Adherence to Mediterranean Diet and Ocular Dryness Severity in Sjögren’s Syndrome: A Cross-Sectional Study. Medical Sciences, 13(2), 64. https://doi.org/10.3390/medsci13020064






