A Potential Pitfall in the Interpretation of Microscope-Integrated Fluorescence Angiography: The Center–Periphery Effect
Abstract
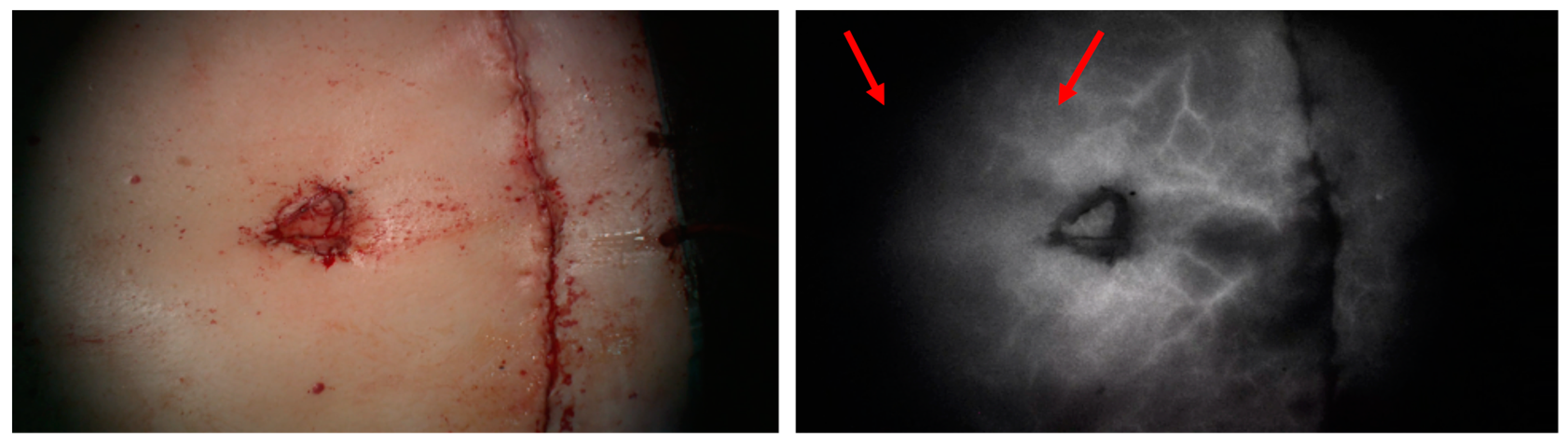
1. Introduction
2. Materials and Methods
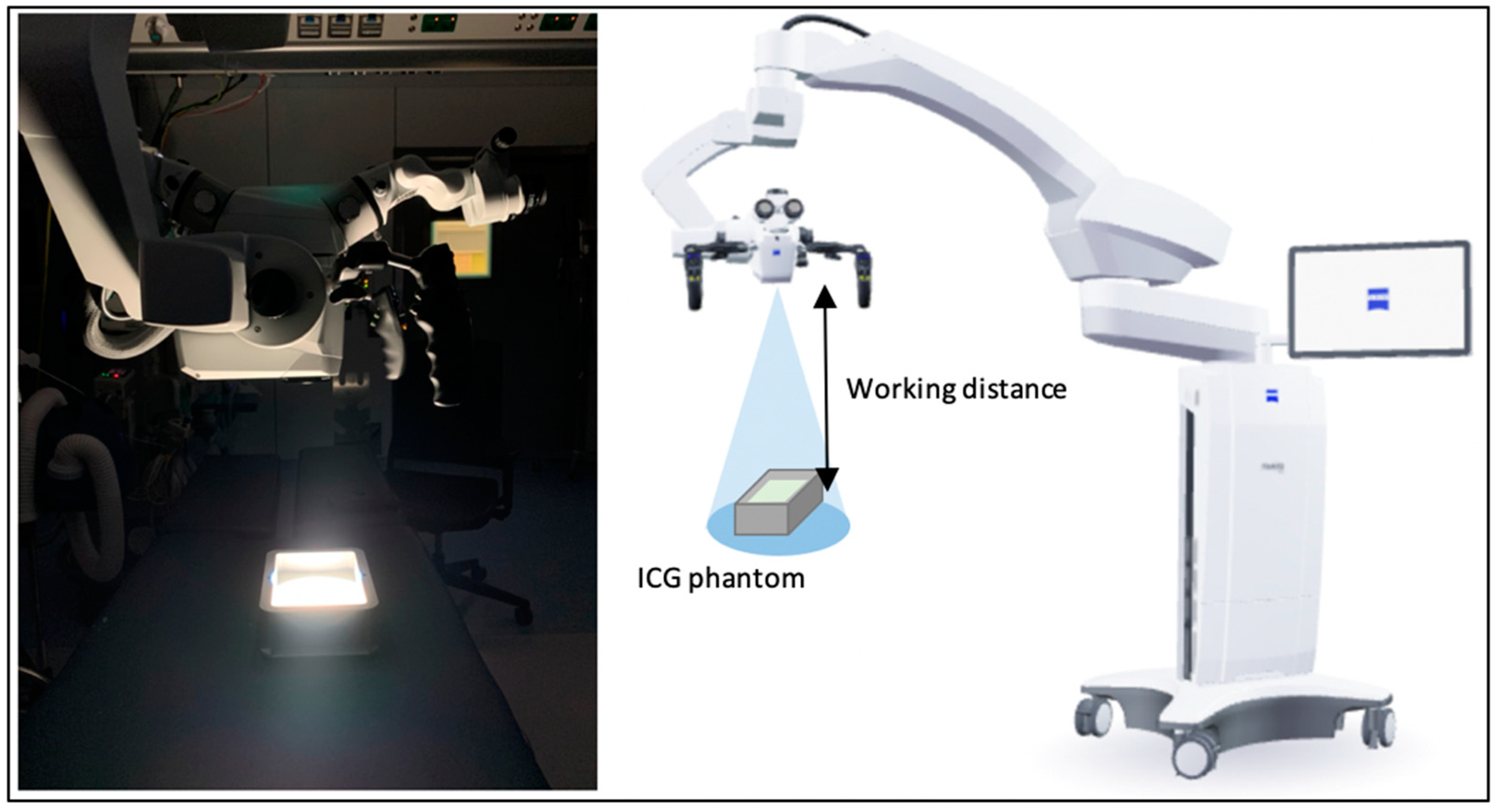
2.1. Phantom
2.2. Fluorescent Assessment
2.3. Analysis
2.4. Photobleaching
3. Results
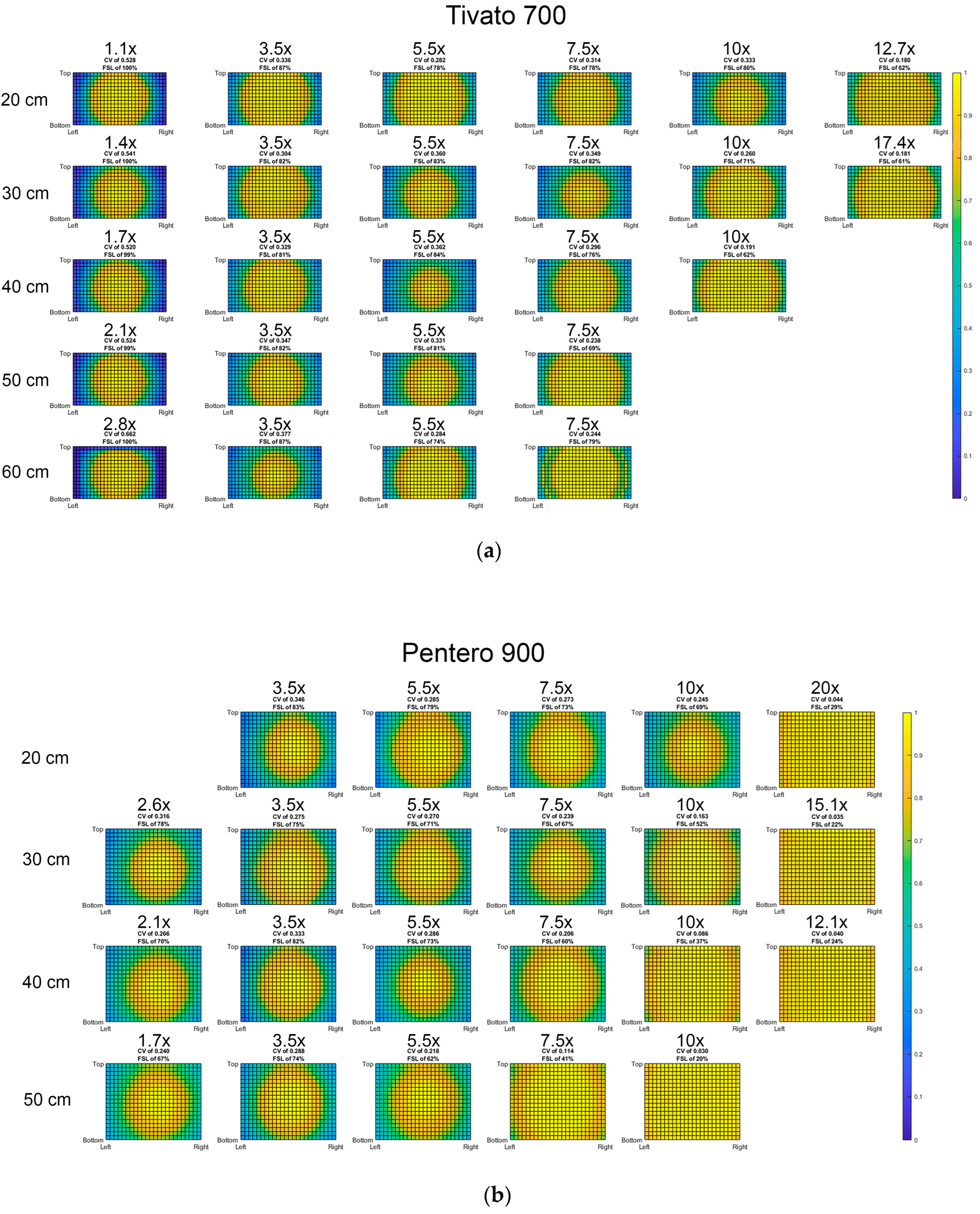
3.1. Light Distribution
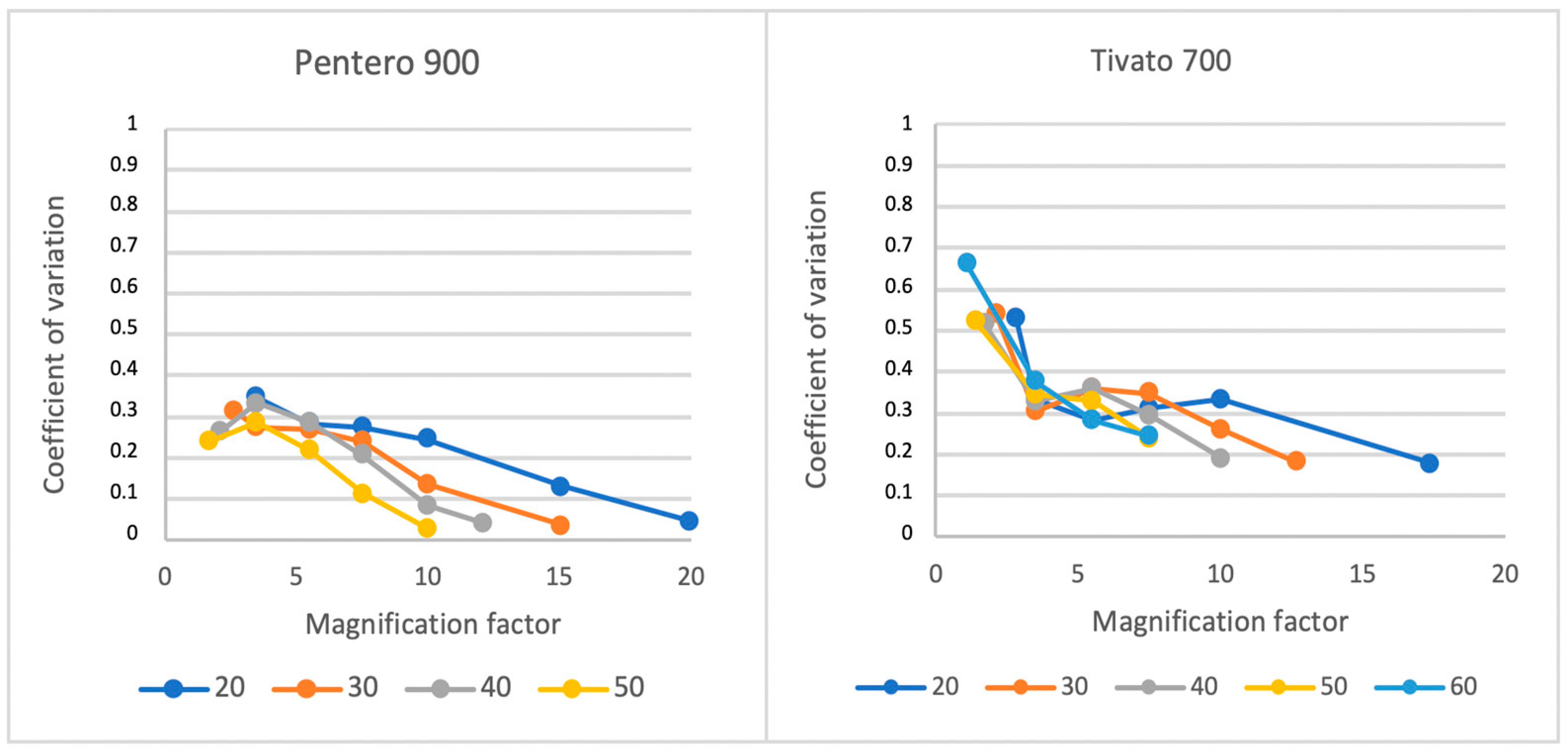
3.2. Influence of Light Beam Diameter
3.3. Absolute Maximum Light Intensity
3.4. Reliability Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIRF | Near-infrared fluorescence |
| ICG | Indocyanine green |
| ICG-FA | Indocyanine green fluorescence angiography |
| WD | Working distance |
| FoV | Field of view |
| MF | Magnification factor |
| PBS | Phosphate-buffered saline |
| FSL | Fluorescence signal loss |
| Flow | Lowest fluorescent intensity |
| Fhigh | Highest fluorescent intensity |
| AMI | Maximum light intensity |
| CV | Coefficient of variation |
Appendix A
| Pentero | Tivato | ||
|---|---|---|---|
| Magnification | FoV (mm) Horizontal × Vertical | Magnification | FoV (mm) Horizontal × Vertical |
| 20 | 13 × 10 | 17.4 | 19 × 10 |
| 15.1 | 17 × 14 | 12.7 | 14 × 8 |
| 12.1 | 22 × 18 | 10 | 23 × 13 |
| 10 | 26 × 21 | 7.5 | 31 × 18 |
| 7.5 | 35 × 28 | 5.5 | 43 × 24 |
| 5.5 | 47 × 39 | 3.5 | 67 × 37 |
| 3.5 | 75 × 61 | 2.8 | 206 × 116 |
| 2.6 | 98 × 80 | 2.1 | 167 × 94 |
| 2.1 | 131 × 107 | 1.7 | 139 × 78 |
| 1.7 | 148 × 121 | 1.4 | 112 × 63 |
| 1.1 | 82 × 46 | ||
References
- Stewart, H.L.; Birch, D.J.S. Fluorescence Guided Surgery. Methods Appl. Fluoresc. 2021, 9, 042002. [Google Scholar] [CrossRef]
- Blanco-Colino, R.; Espin-Basany, E. Intraoperative use of ICG fluorescence imaging to reduce the risk of anastomotic leakage in colorectal surgery: A systematic review and meta-analysis. Tech. Coloproctol. 2018, 22, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Raimondo, D.; Oliviero, A.; Raspollini, A.; Travaglino, A.; Torella, M.; Riemma, G.; La Verde, M.; De Franciscis, P.; Casadio, P.; et al. The Use of near Infra-Red Radiation Imaging after Injection of Indocyanine Green (NIR-ICG) during Laparoscopic Treatment of Benign Gynecologic Conditions: Towards Minimalized Surgery. A Systematic Review of Literature. Medicina 2022, 58, 792. [Google Scholar] [CrossRef] [PubMed]
- Raabe, A.; Beck, J.; Seifert, V. Technique and image quality of intraoperative indocyanine green angiography during aneurysm surgery using surgical microscope integrated near-infrared video technology. Zentralbl. Neurochir. 2005, 66, 1–6, discussion 7-8. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Sheckter, C. Intraoperative Laser-Assisted Indocyanine Green Imaging Can Reduce the Rate of Fat Necrosis in Microsurgical Breast Reconstruction. Plast. Reconstr. Surg. 2020, 145, 507e–513e. [Google Scholar] [CrossRef]
- Komorowska-Timek, E.; Gurtner, G.C. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast. Reconstr. Surg. 2010, 125, 1065–1073. [Google Scholar] [CrossRef]
- Holm, C.; Tegeler, J.; Mayr, M.; Becker, A.; Pfeiffer, U.; Mühlbauer, W. Monitoring free flaps using laser-induced fluorescence of indocyanine green: A preliminary experience. Microsurgery 2002, 22, 278–287. [Google Scholar] [CrossRef]
- Parmeshwar, N.; Sultan, S.M.; Kim, E.A.; Piper, M.L. A Systematic Review of the Utility of Indocyanine Angiography in Autologous Breast Reconstruction. Ann. Plast. Surg. 2021, 86, 601–606. [Google Scholar] [CrossRef]
- Kuriyama, M.; Yano, A.; Yoshida, Y.; Kubo, M.; Akita, S.; Mitsukawa, N.; Satoh, K.; Yamamoto, S.; Sasaguri, S.; Orihashi, K. Reconstruction using a divided latissimus dorsi muscle flap after conventional posterolateral thoracotomy and the effectiveness of indocyanine green-fluorescence angiography to assess intraoperative blood flow. Surg. Today 2016, 46, 326–334. [Google Scholar] [CrossRef]
- Holm, C.; Mayr, M.; Höfter, E.; Becker, A.; Pfeiffer, U.; Mühlbauer, W. Intraoperative evaluation of skin-flap viability using laser-induced fluorescence of indocyanine green. Br. J. Plast. Surg. 2002, 55, 635–644. [Google Scholar] [CrossRef]
- Marshall, J.; Johnsen, S. Fluorescence as a means of colour signal enhancement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160335. [Google Scholar] [CrossRef] [PubMed]
- Yoneya, S.; Saito, T.; Komatsu, Y.; Koyama, I.; Takahashi, K.; Duvoll-Young, J. Binding properties of indocyanine green in human blood. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1286–1290. [Google Scholar]
- Morales-Conde, S.; Licardie, E.; Alarcón, I.; Balla, A. Indocyanine green (ICG) fluorescence guide for the use and indications in general surgery: Recommendations based on the descriptive review of the literature and the analysis of experience. Cirugia Espanola 2022, 100, 534–554. [Google Scholar] [CrossRef] [PubMed]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef]
- Nerup, N.; Andersen, H.S.; Ambrus, R.; Strandby, R.B.; Svendsen, M.B.S.; Madsen, M.H.; Svendsen, L.B.; Achiam, M.P. Quantification of fluorescence angiography in a porcine model. Langenbecks Arch. Surg. 2017, 402, 655–662. [Google Scholar] [CrossRef]
- Lütken, C.D.; Achiam, M.P.; Osterkamp, J.; Svendsen, M.B.; Nerup, N. Quantification of fluorescence angiography: Toward a reliable intraoperative assessment of tissue perfusion—A narrative review. Langenbecks Arch. Surg. 2021, 406, 251–259. [Google Scholar] [CrossRef]
- Hoven, P.V.D.; Verduijn, P.; Van Capelle, L.; Tange, F.; Michi, M.; Corion, L.; Mulder, B.S.; Mureau, M.; Vahrmeijer, A.; Van Der Vorst, J. Quantification of near-infrared fluorescence imaging with indocyanine green in free flap breast reconstruction. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 1820–1825. [Google Scholar] [CrossRef]
- Ruiz, A.J.; Wu, M.; LaRochelle, E.P.M.; Gorpas, D.; Ntziachristos, V.; Pfefer, T.J.; Pogue, B.W. Indocyanine green matching phantom for fluorescence-guided surgery imaging system characterization and performance assessment. J. Biomed. Opt. 2020, 25, 056003. [Google Scholar] [CrossRef]
- Gorpas, D.; Koch, M.; Anastasopoulou, M.; Klemm, U.; Ntziachristos, V. Benchmarking of fluorescence cameras through the use of a composite phantom. J. Biomed. Opt. 2017, 22, 016009. [Google Scholar] [CrossRef]
- Dalli, J.; Gallagher, G.; Jindal, A.; Epperlein, J.P.; Hardy, N.P.; Malallah, R.; O’Donoghue, K.; Cantillon-Murphy, P.; Mac Aonghusa, P.G.; Cahill, R.A. Objective interrogation of signal presentation from surgical near-infrared fluorescence systems for user and computerised interpretation. In Proceedings of the SPIE Photonics Europe, Strasbourg, France, 3 April 2022; SPIE: Bellingham, WA, USA, 2022; Volume 12146. [Google Scholar]
- Joosten, J.J.; Bloemen, P.R.; Elzen, R.M.v.D.; Dalli, J.; Cahill, R.A.; Henegouwen, M.I.v.B.; Hompes, R.; de Bruin, D.M. Investigating and Compensating for Periphery-Center Effect among Commercial Near Infrared Imaging Systems Using an Indocyanine Green Phantom. Appl. Sci. 2023, 13, 2042. [Google Scholar] [CrossRef]
- Lai, L.T.; Morgan, M.K. Use of indocyanine green videoangiography during intracranial aneurysm surgery reduces the incidence of postoperative ischaemic complications. J. Clin. Neurosci. 2014, 21, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Mayr, M.; Höfter, E.; Dornseifer, U.; Ninkovic, M. Assessment of the patency of microvascular anastomoses using microscope-integrated near-infrared angiography: A preliminary study. Microsurgery 2009, 29, 509–514. [Google Scholar] [CrossRef]
- Ma, L.; Fei, B. Comprehensive review of surgical microscopes: Technology development and medical applications. J. Biomed. Opt. 2021, 26, 010901. [Google Scholar] [CrossRef]
- Nadler, S.B.; Hidalgo, J.H.; Bloch, T. Prediction of blood volume in normal human adults. Surgery 1962, 51, 224–232. [Google Scholar] [PubMed]
- Everitt, B. ; Skrondal, The Cambridge Dictionary of Statistics, 4th ed.; Cambridge University Press: Cambridge, UK, 2010; 468p. [Google Scholar]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Aernouts, B.; Van Beers, R.; Watté, R.; Lammertyn, J.; Saeys, W. Dependent scattering in Intralipid(R) phantoms in the 600-1850 nm range. Opt. Express 2014, 22, 6086–6098. [Google Scholar] [CrossRef]
- Di Ninni, P.; Martelli, F.; Zaccanti, G. Intralipid: Towards a diffusive reference standard for optical tissue phantoms. Phys. Med. Biol. 2011, 56, N21–N28. [Google Scholar] [CrossRef]

| Pentero 900 | ||||||
|---|---|---|---|---|---|---|
| Working Distance | Magnification Factor | |||||
| Min | 3.5 | 5.5 | 7.5 | 10 | Max | |
| 20 cm | 144 | 144 | 135 | 123 | 61 | |
| 30 cm | 130 | 130 | 132 | 117 | 65 | 32 |
| 40 cm | 71 | 151 | 153 | 100 | 58 | 40 |
| 50 cm | 53 | 101 | 78 | 42 | ||
| Tivato 700 | ||||||
|---|---|---|---|---|---|---|
| Working Distance | Magnification Factor | |||||
| Min | 3.5 | 5.5 | 7.5 | 10 | Max | |
| 20 cm | 215 | 214 | 214 | 214 | 200 | 79 |
| 30 cm | 135 | 135 | 134 | 110 | 61 | 40 |
| 40 cm | 84 | 84 | 81 | 44 | 25 | |
| 50 cm | 56 | 56 | 39 | 81 | ||
| 60 cm | 34 | 33 | 14 | 6 | ||
| WD; MF | 50% Light Intensity | 100% Light Intensity | |
|---|---|---|---|
| 30 cm; 12.7× (phantom 2) | CV | 0.181 | 0.177 |
| AMI | 39.7 | 113.7 | |
| 60 cm; 2.8× (phantom 2) | CV | 0.64 | 0.64 |
| AMI | 25.3 | 66.9 | |
| 60 cm; 3.5× (phantom 2) | CV | 0.398 | 0.367 |
| AMI | 24.8 | 67.5 | |
| 60 cm; 5.5× (phantom 1) | CV | 0.284 | 0.254 |
| AMI | 14.5 | 42 | |
| 60 cm; 7.5× (phantom 1) | CV | 0.244 | 0.177 |
| AMI | 6.4 | 19.1 | |
| 60 cm; 5.5× (phantom 2) | CV | 0.303 | 0.263 |
| AMI | 10.2 | 30.9 | |
| 60 cm; 7.5× (phantom 2) | CV | 0.2 | 0.172 |
| AMI | 8.68 | 27.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolk, D.; Bloemen, P.; van den Elzen, R.M.; de Bruin, M.; Driessen, C. A Potential Pitfall in the Interpretation of Microscope-Integrated Fluorescence Angiography: The Center–Periphery Effect. Med. Sci. 2025, 13, 54. https://doi.org/10.3390/medsci13020054
Stolk D, Bloemen P, van den Elzen RM, de Bruin M, Driessen C. A Potential Pitfall in the Interpretation of Microscope-Integrated Fluorescence Angiography: The Center–Periphery Effect. Medical Sciences. 2025; 13(2):54. https://doi.org/10.3390/medsci13020054
Chicago/Turabian StyleStolk, Dieder, Paul Bloemen, Richard Martin van den Elzen, Martijn de Bruin, and Caroline Driessen. 2025. "A Potential Pitfall in the Interpretation of Microscope-Integrated Fluorescence Angiography: The Center–Periphery Effect" Medical Sciences 13, no. 2: 54. https://doi.org/10.3390/medsci13020054
APA StyleStolk, D., Bloemen, P., van den Elzen, R. M., de Bruin, M., & Driessen, C. (2025). A Potential Pitfall in the Interpretation of Microscope-Integrated Fluorescence Angiography: The Center–Periphery Effect. Medical Sciences, 13(2), 54. https://doi.org/10.3390/medsci13020054






