State-of-the-Art Review on the Treatment of Axial Spondyloarthritis
Abstract
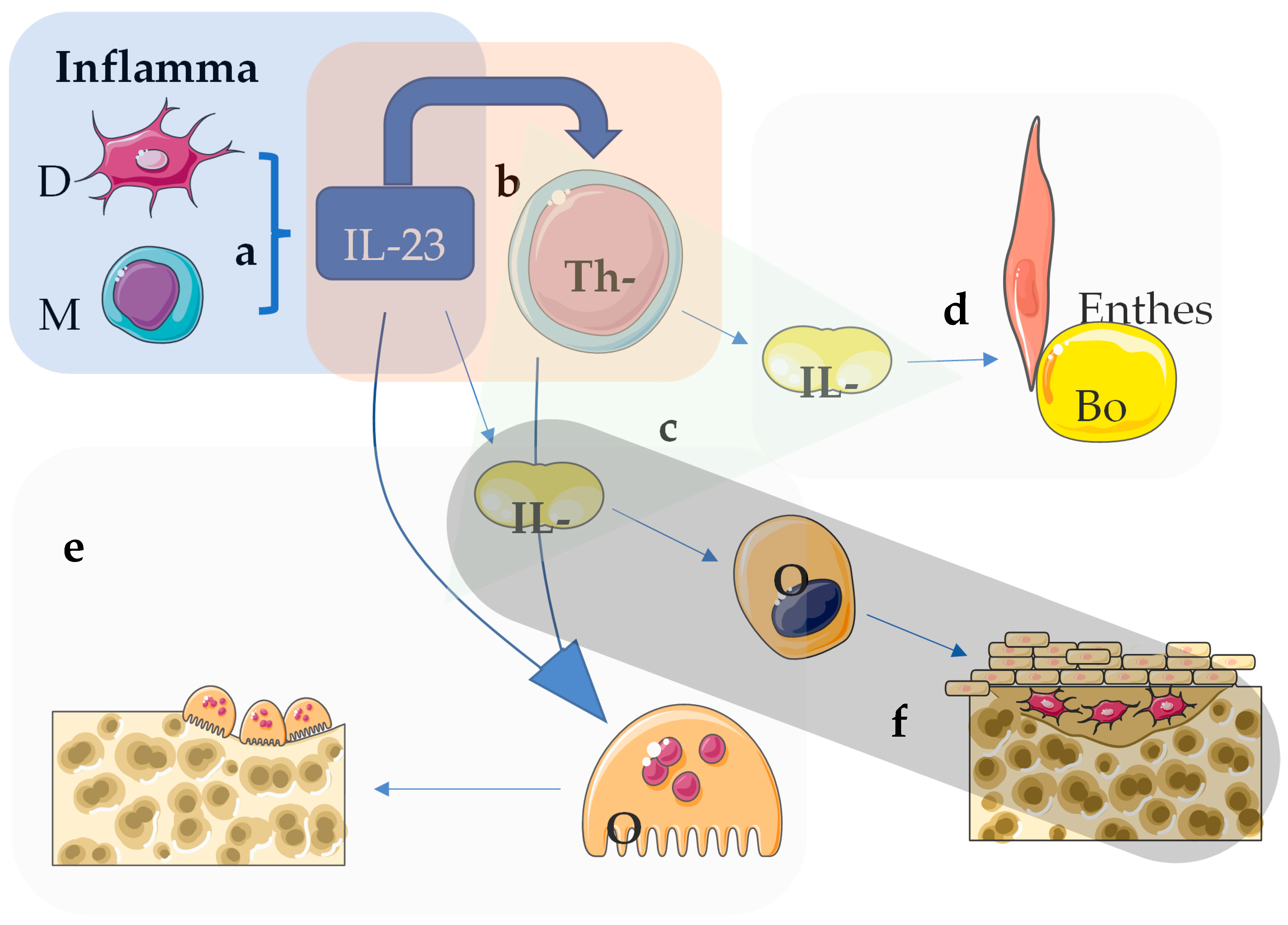
1. Introduction
2. Methods
2.1. Physical Therapy and Exercise in axSPA
2.2. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
2.3. Glucocorticoids and Conventional Synthetic DMARDs (csDMARDs)
2.4. When to Initiate Biological Therapy in axSpA
2.5. TNF-α Inhibitors
2.6. Interleukin-17 Inhibitors (IL-17i)
2.7. Janus Kinase Inhibitors (JAKi)
2.8. Interleukin-12/23 and IL-23 Inhibitors
2.9. Unmet Needs
- Suboptimal Treatment Response: A considerable number of patients do not achieve ideal therapeutic responses.
- Limited Drug Options: Available medications are fewer compared to other conditions like rheumatoid arthritis.
- Lack of Biomarkers: There is an absence of biomarkers to identify early which patients with chronic back pain are at increased risk of progressing to axial spondyloarthritis.
- Predictive Indicators: Predictors for treatment response, indicating the most suitable drug, are lacking.
- Extra-Skeletal Manifestations: Determining which patients will develop extra-skeletal manifestations remains challenging.
- Non-Inflammatory Pain Mechanisms: Understanding and addressing mechanisms underlying non-inflammatory pain (e.g., depression, anxiety) are needed.
- Delayed Diagnosis: Strategies to improve delayed diagnosis are still required.
3. Discussion
4. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deodhar, A.; Strand, V.; Kay, J.; Braun, J. The term “non-radiographic axial spondyloarthritis” is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann. Rheum. Dis. 2016, 75, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Ramiro, S.; Nikiphorou, E.; Spriano, A.; Ortolan, A.; Webers, C.; Baraliakos, X.; Landewé, R.B.; van der Heijde, D. ASAS-EULAR recommendations for the management of axial spondyloarthritis: 2022 update. Ann. Rheum. Dis. 2023, 82, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Nikiphorou, E.; Baraliakos, X. Treat-to-target in axial spondyloarthritis. Rheum. Dis. Clin. N. Am. 2019, 45, 519–535. [Google Scholar] [CrossRef]
- Dougados, M. Treat-to-target in axial spondyloarthritis: From its concept to its implementation. J. Autoimmun. 2020, 110, 102398. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Martey, C.; Sengupta, R. Physical therapy in axial spondyloarthritis: Guidelines, evidence and clinical practice. Curr. Opin. Rheumatol. 2020, 32, 365–370. [Google Scholar] [CrossRef]
- Milner, J.R.; Barron, J.S.; Beinke, K.M.; Butterworth, R.H.; Chasle, B.E.; Dutton, L.J.; Lewington, M.A.; Lim, E.G.S.; Morley, T.B.; O’Reilly, J.E.; et al. Exercise for ankylosing spondylitis: An evidence-based consensus statement. Semin. Arthritis Rheum. 2016, 45, 411–427. [Google Scholar] [CrossRef]
- Gwinnutt, J.M.; Wieczorek, M.; Cavalli, G.; Balanescu, A.; Bischoff-Ferrari, H.A.; Boonen, A.; de Souza, S.; de Thurah, A.; Dorner, T.E.; Moe, R.H.; et al. Effects of physical exercise and body weight on disease-specific outcomes of people with rheumatic and musculoskeletal diseases (RMDs): Systematic reviews and meta-analyses informing the 2021 EULAR recommendations for lifestyle improvements in people with RMDs. RMD Open 2022, 8, e002168. [Google Scholar] [CrossRef]
- Danve, A.; Deodhar, A. Treatment of axial spondyloarthritis: An update. Nat. Rev. Rheumatol. 2022, 18, 205–216. [Google Scholar] [CrossRef]
- Navarro-Compán, V.; Sepriano, A.; El-Zorkany, B.; van der Heijde, D. Axial spondyloarthritis. Ann. Rheum. Dis. 2021, 80, 1511–1521. [Google Scholar] [CrossRef]
- Ward, M.M.; Deodhar, A.; Gensler, L.S.; Dubreuil, M.; Yu, D.; Khan, M.A.; Haroon, N.; Borenstein, D.; Wang, R.; Biehl, A.; et al. 2019 update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network recommendations for the treatment of Ankylosing spondylitis and Nonradiographic axial spondyloarthritis. Arthritis Care Res. 2019, 71, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.L.; Otón, T.; Sanz, J. Anti-TNF alpha therapy in ankylosing spondylitis: Symptom control and structural damage modification. Reumatol. Clin. 2011, 7, 51–55. [Google Scholar] [CrossRef]
- Neerinckx, B.; Lories, R.J. Structural disease progression in axial spondyloarthritis: Still a cause for concern. Curr. Rheumatol. Rep. 2017, 19, 14. [Google Scholar] [CrossRef]
- Kocijan, R.; Muschitz, C.; Rech, J. Anti-TNFs in axial spondyloarthritis. Wien. Med. Wochenschr. 2015, 165, 10–13. [Google Scholar] [CrossRef]
- Molto, A.; López-Medina, C.; van den Bosch, F.E.; Booren, A.; Webers, C.; Dernis, E.; van Gaalen, F.A.; Soubrier, M.; Claudepierre, P.; Baillet, A.; et al. Efficacy of a tight-control and treat-to-target strategy in axial spondyloarthritis: Results of the open-label, pragmatic, cluster-randomised TICOSPA trial. Ann. Rheum. Dis. 2021, 80, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Danve, A.; Deodhar, A. Treat-to-target in Axial Spondyloarthritis. What are the issues? Curr. Rheumatol. Rep. 2017, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.; Adamopoulos, I.E. Axial spondyloarthritis: New advances in diagnosis and management. BMJ 2021, 372, m4447. [Google Scholar] [CrossRef] [PubMed]
- Pécourneau, V.; Degboé, Y.; Barnetche, T.; Cantagrel, A.; Constantin, A.; Ruyssen-Witrand, A. Effectiveness of exercise programs in ankylosing Spondylitis: A meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2018, 99, 383–389.e1. [Google Scholar] [CrossRef]
- Sharan, D.; Rajkumar, J.S. Physiotherapy for ankylosing spondylitis: Systematic review and a proposed rehabilitation protocol. Curr. Rheumatol. Rev. 2017, 13, 121–125. [Google Scholar] [CrossRef]
- Dagfinrud, H.; Kvien, T.K.; Hagen, K.B. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst. Rev. 2008, 2008, CD002822. [Google Scholar] [CrossRef]
- Haroon, N.; Inman, R.D.; Learch, T.J.; Weisman, M.H.; Lee, M.; Rahbar, M.H.; Ward, M.M.; Reveille, J.D.; Gensler, L.S. The impact of tumor necrosis factor α inhibitors on radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2013, 65, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Regel, A.; Sepriano, A.; Baraliakos, X.; van der Heijde, D.; Braun, J.; Landewé, R.; van den Bosch, F.; Falzon, L.; Ramiro, S. Efficacy and safety of non-pharmacological and non-biological pharmacological treatment: A systematic literature review informing the 2016 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. RMD Open 2017, 3, e000397. [Google Scholar] [CrossRef] [PubMed]
- Baraliakos, X.; Kiltz, U.; Peters, S.; Appel, H.; Dybowski, F.; Igelmann, M.; Kalthoff, L.; Krause, D.; Menne, H.J.; Saracbasi-Zender, E.; et al. Efficiency of treatment with non-steroidal anti-inflammatory drugs according to current recommendations in patients with radiogtraphic and non-radiographic axial spondyloarthritis. Rheumatology 2017, 56, 95–102. [Google Scholar] [CrossRef]
- Kroon, F.P.; van der Burg, L.R.; Ramiro, S.; Landewé, R.B.M.; Buchbinder, R.; Falzon, L.; van der Jeijde, D. Non0steroidal anti-inflammatory drugs (NSAIDs) for axial spondyloarthritis (ankylosing spondylitis and non-radiographic axial spondyloarthritis). Cochrane Database Syst. Rev. 2015, 2015, CD010952. [Google Scholar] [CrossRef]
- Baraliakos, X.; van der Heijde, D.; Sieper, J.; Inman, R.D.; Kameda, H.; Li, Y.; Bu, X.; Shmagel, A.; Wung, P.; Song, I.H.; et al. Efficacy and safety of upadacitinib in patients with ankylosing spondylitis refractory to biologic therapy: 1-year results from the open-label extension of a phase III study. Arthritis Res. Ther. 2023, 25, 172. [Google Scholar] [CrossRef]
- Sieper, J.; Poddubnyy, D. Axial spondyloarthritis. Lancet 2017, 390, 73–81. [Google Scholar] [CrossRef]
- Haibel, H.; Fendler, C.; Listing, J.; Callhoff, J.; Braun, J.; Sieper, J. Efficacy of oral prednisolone in active ankylosing spondylitis: Results of a double-blind, randomised, placebo-controlled short term trial. Ann. Rheum. Dis. 2014, 73, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.M.; Kim, M.; Kim, Y.J.; Lee, Y.; Kim, Y. Risk of acute anterior uveitis in Ankylosing Spondylitis according to the type of tumor necrosis factor-alpha inhibitor and history of uveitis: A nationwide population-based study. J Clin Med. 2022, 11, 631. [Google Scholar] [CrossRef]
- Lian, F.; Zhou, J.; Wei, C.; Wang, Y.; Xu, H.; Liang, L.; Yang, X. Anti-TNF alpha agents and methotrexate in spondyloarthritis related uveitis in a Chinese population. Clin. Rheumatol. 2015, 34, 1913–1920. [Google Scholar] [CrossRef]
- Dougados, M.; Lardy-Cléaud, A.; Desfleurs, E.; Claudepierre, P.; Goupille, P.; Ryussen-Witrand, A.; Saraux, A.; Tournadre, A.; Wendling, D.; Lukas, C. Impact of the time of initiation and line of biologic therapy on the retention rate of secukinumab in axial spondyloarthritis (axSpA): Data from the French multicentre retrospective FORSYA study. RMD Open 2024, 10, e003942. [Google Scholar] [CrossRef]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Singh, J.A.; Veras, M.M.S.; Ghogomu, E.T.; Jandu, M.B.; Tugwell, P.; Wells, G.A. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. 2015, 2015, CD005468. [Google Scholar] [CrossRef] [PubMed]
- Baraliakos, X.; Haibel, H.; Listing, J.; Sieper, J.; Braun, J. Continuous long-term anti-TNF therapy does not lead to an increase in the rate of new bone formation over 8 years in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2014, 73, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Kampman, A.; Wink, F.; Paap, D.; Carbo, M.; Siderius, M.; Kieskamp, S.; Maas, F.; Spoorenberg, A.; Arends, S. Patients’ perspectives on axial pain in relation to inflammation and structural damage in a large cohort of axial spondyloarthritis patients. Arthritis Care Res. 2024, 76, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Karmacharya, P.; Duarte-Garcia, A.; Dubreuil, M.; Murad, M.H.; Shahukhal, R.; Shrestha, P.; Myasoedova, E.; Crowson, C.S.; Wright, K.; Davis, J.M., 3rd. Effect of Therapy on radiographic progression in axial spondyloarthritis: A systematic review and meta-analysis. Arthritis Rheumatol. 2020, 72, 733–749. [Google Scholar] [CrossRef]
- Goupille, P.; Wendling, D. Are the recommendations for the use of anti-TNF drugs during axial spondyloarthritis relevant for nonradiographic forms? Joint Bone Spine 2020, 87, 381–383. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Wu, J.; Xing, Q.; Shi, G.; Li, J.; Liu, X.; Wu, L.; Li, X.; Tan, W.; et al. IBI303, a biosimilar to adalimumab, for the treatment of patients with ankylosing spondylitis in China: A randomised, double-blind, phase 3 equivalence trial. Lancet Rheumatol. 2019, 1, e35–e343. [Google Scholar] [CrossRef]
- Su, J.; Li, M.; He, L.; Zhao, D.; Wan, W.; Liu, Y.; Xu, J.; Xu, J.; Liu, H.; Jiang, L.; et al. Comparison of the efficacy and safety of adalimumab (Humira) and the adalimumab biosimilar candidate (HS016) in Chinese patients with active ankylosing spondylitis: A multicenter, randomized, double-blind, parallel, phase III clinical trial. BioDrugs 2020, 34, 381–393. [Google Scholar] [CrossRef]
- Zhao, D.; He, D.; Bi, L.; Wu, H.; Liu, Y.; Wu, Z.; Li, Y.; Wang, G.; Li, X.; Bao, C.; et al. Safety and efficacy of prefilled liquid etanercept-biosimilar Yisaipu for active ankylosing spondylitis: A multi-center phase III trial. Rheumatol. Ther. 2021, 8, 361–374. [Google Scholar] [CrossRef]
- Jørgensen, K.K.; Olsen, I.C.; Goll, G.L.; Lorentzen, M.; Bolstad, N.; Haavardsholm, E.A.; Lundin, K.E.A.; Mørk, C.; Jahnsen, J.; Kvien, T.K. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 2017, 389, 2304–2316. [Google Scholar] [CrossRef]
- Kaltsonoudis, E.; Pelechas, E.; Voulgari, P.V.; Drosos, A.A. Maintained clinical remission in Ankylosing spondylitis patients switched from reference infliximab to its biosimilar: An 18-month comparative open-label study. J. Clin. Med. 2019, 8, 956. [Google Scholar] [CrossRef]
- Saoussen, M.; Yasmine, M.; Lilia, N.; Alia, F.; Hiba, B.; Kawther, B.A.; Ahmed, L. Tapering biologics in axial spondyloarthritis: A systematic literature review. Int. Immunopharmacol. 2022, 112, 109256. [Google Scholar] [CrossRef] [PubMed]
- Kaltsonoudis, E.; Pelechas, E.; Voulgari, O.V.; Drosos, A.A. Prolongation or discontinuation of TNF inhibitors in the treatment of rheumatoid arthritis. Could this be a realistic scenario? Scand. J. Rheumatol. 2018, 47, 63–64. [Google Scholar] [CrossRef]
- Corthay, A.; Hansson, A.S.; Homdahl, R. T lymphocytes are not required for the spontaneous development of entheseal ossification leading to marginal ankylosis in the DBA/1 mouse. Arthritis Rheum. 2000, 43, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Webers, C.; Ortolan, A.; Sepriano, A.; Falzon, L.; Baraliakos, X.; Landewé, R.B.M.; Ramiro, S.; van der Heijde, D.; Nikiphorou, E. Efficacy and safety of biological DMARDs: A systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann. Rheum. Dis. 2023, 82, 130–141. [Google Scholar] [CrossRef]
- Sieper, J.; Deodhar, A.; Marzo-Ortega, H.; Aelion, J.A.; Blanco, R.; Jui-Cheng, T.; Andersson, M.; Porter, B.; Richards, H.B. Secukinumab efficacy in anti-TNF-naïve and anti-TNF-experienced subjects with active ankylosing spondylitis: Results from the MEASURE 2 study. Ann. Rheum. Dis. 2017, 76, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, A.; Poddubnyy, D.; Pacheco-Tena, C.; Salvarani, C.; Lespessailles, E.; Rahman, P.; Järvinen, P.; Sanchez-Burson, J.; Gaffney, K.; Lee, E.B.; et al. Efficacy and safety of ixekizumab in the treatment of radiographic axial spondyloarthritis: Sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheumatol. 2019, 71, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Deodhar, A.; Baeten, D.; Sieper, J.; Emery, P.; Readie, A.; Martin, R.; Mpofu, S.; Richards, H.B. Effect of secukinumab on clinical and radiographic outcomes in ankylosing spondylitis: 2-year results from the randomised phase III MEASURE 1 study. Ann. Rheum. Dis. 2017, 76, 1070–1077. [Google Scholar] [CrossRef]
- Baraliakos, X.; Deodhar, A.; van der Heijde, D.; Magrey, M.; Maksymowych, W.P.; Tomita, T.; Xu, H.; Massow, U.; Fleurinck, C.; Ellis, A.M.; et al. Bimekizumab treatment in patients with active axial spondyloarthritis: 52-week efficacy and safety from the randomised parallel phase 3 BE MOBILE 1 and BE MOBILE 2 studies. Ann. Rheum. Dis. 2024, 83, 199–213. [Google Scholar] [CrossRef]
- Van der Haijde, D.; Deodhar, A.; Baraliakos, X.; Brown, M.A.; Dobashi, H.; Dougados, M.; Elewaut, D.; Ellis, A.M.; Fleurinck, C.; Gaffney, K.; et al. Efficacy and safety of Bimekizumab in axial spondyloarthritis: Results of two parallel phase 3 randomised controlled trials. Ann. Rheum. Dis. 2023, 82, 515–526. [Google Scholar] [CrossRef]
- Veale, D.J.; McGonagle, D.; McInnes, I.B.; Krueger, J.G.; Ritchlin, C.T.; Elewaut, D.; Kanik, K.S.; Hendrikx, T.; Berstein, G.; Hodge, J.; et al. The rationale for janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology 2019, 58, 197–205. [Google Scholar] [CrossRef]
- Ciccia, F.; Guggino, G.; Rizzo, A.; Saieva, L.; Peralta, S.; Giardina, A.; Cannizaro, A.; Sireci, G.; De Leo, G.; Alessandro, R.; et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 2015, 74, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Thong, D.; Miller, N.; Duffield, S.J.; Hughes, D.M.; Chadwick, L.; Goodson, N.J. The prevalence of depression in axial spondyloarthritis and its association with disease activity: A systematic review and meta-analysis. Arthritis Res. Ther. 2018, 20, 140. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.S.; Duffield, S.J.; Goodson, N.J. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best. Pract. Res. Clin. Rheumatol. 2019, 33, 101423. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Webers, C.; Sepriano, A. Efficacy and safety of non-pharmacological and pharmacological non-biological interventions: A systematic literature review informing the 2022 update of the ASAS-EULAR recommendations for the management of axial spondyloarthritis. Ann. Rheum. Dis. 2023, 82, 142–152. [Google Scholar] [CrossRef]
- Sutton, C.E.; Lalor, S.J.; Sweeney, C.M.; Brereton, C.F.; Lavelle, E.C.; Mills, K.H. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 2009, 31, 331–341. [Google Scholar] [CrossRef]
- Poddubnyy, D.; Hermann, K.G.; Callhoff, J.; Listing, J.; Sieper, J. Ustekinumab for the treatment of patients with active ankylosing spondylitis: Results of a 28-week, prospective, open-label, proof-of-concept study (TOPAS). Ann. Rheum. Dis. 2014, 73, 817–823. [Google Scholar] [CrossRef]
- Deodhar, A.; Gensler, L.S.; Sieper, J.; Clark, M.; Calderon, C.; Wand, Y.; Zhou, Y.; Leu, J.H.; Campbell, K.; Sweet, K.; et al. Three multicenter, randomized, double-blind, placebo-controlled studies evaluating the efficacy and safety of Ustekinumab in axial spondyloarhtiritis. Arthritis Rheumatol. 2019, 71, 258–270. [Google Scholar] [CrossRef]
- Pfeifle, R.; Rothe, T.; Ipseiz, N.; Scherer, H.U.; Culemann, S.; Harre, U.; Ackermann, J.A.; Seefried, M.; Kleyer, A.; Uderhardt, S.; et al. Regulation of autoantibody activity by the IL-23-Th17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017, 18, 104–113. [Google Scholar] [CrossRef]
- Baeten, D.; Østergaard, M.; Wei, J.C.C.; Sieper, J.; Järvinen, P.; Tam, L.S.; Salvarani, C.; Kim, T.H.; Solinger, A.; Datsenko, Y.; et al. Risankizumab, an IL-23 inhibitor, for ankylosing spondylitis: Results of a randomised, double-blind, placebo-controlled, proof-of-concept, dose-finding phase 2 study. Ann. Rheum. Dis. 2018, 77, 1295–1302. [Google Scholar] [CrossRef]
- Pelechas, E.; Kaltsonoudis, E.; Voulgari, P.V.; Drosos, A.A. Unmet needs in the treatment of ankylosing spondylitis: A long-term observational study from a single university center. Rheumatol. Int. 2019, 39, 663–668. [Google Scholar] [CrossRef]
- Gossec, L.; Baraliakos, X.; Kerschbaumer, A.; de WIT, M.; McInnes, I.; Dougados, M. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann. Rheum. Dis. 2020, 79, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Landewé, R.B.M. The unsustainable bubble of disease-modifying antirheumatic drugs in rheumatology. Lancet Rheumatol. 2021, 3, e306–e312. [Google Scholar] [CrossRef] [PubMed]
- Gialouri, C.G.; Pappa, M.; Evangelatos, G.; Nikiphorou, E.; Fragoulis, G.E. Effect of body mass index on treatment response of biologic/targeted-synthetic DMARDs in patients with rheumatoid arthritis, psoriatic arthritis or axial spondyloarthritis. A systematic review. Autoimmun. Rev. 2023, 22, 103357. [Google Scholar] [CrossRef] [PubMed]
- Duffield, S.J.; Miller, N.; Zhao, S.; Goodson, N.J. Concomitant fibromyalgia complicating chronic inflammatory arthritis: A systematic review and meta-analysis. Rheumatology 2018, 57, 1453–1460. [Google Scholar] [CrossRef]
| Category of Treatment | Drug Class | Example Medications | Mechanism of Action | Approved for r-axSpA | Approved for nr-axSpA |
|---|---|---|---|---|---|
| NSAIDs | NSAIDs | Ibuprofen, Naproxen, Diclofenac, Etoricoxib | Inhibition of COX-1 and COX-2 enzymes, reducing inflammation and pain | YES | YES |
| Glucocorticoids | Steroids | Prednisone, Methylprednisolone | Reduction in inflammation and suppression of the immune system | Conditionally, depending on disease’s phenotype; not for axial disease | Conditionally, depending on disease’s phenotype; not for axial disease |
| DMARDs | Conventional synthetic DMARDs | Methotrexate, Sulfasalazine, Leflunomide | Reduction in inflammation and modulation of the immune response | Conditionally, depending on disease’s phenotype; not for axial disease | Conditionally, depending on disease’s phenotype; not for axial disease |
| Biologic DMARDs | TNF inhibitors | Adalimumab | Inhibition of TNF, a cytokine involved in inflammation | YES | YES |
| Etanercept | YES | YES | |||
| Infliximab | YES | NO | |||
| Certolizumab | YES | YES | |||
| Golimumab | YES | YES | |||
| IL-17 inhibitors | Secukinumab | Inhibition of IL-17, a cytokine involved in inflammationBimekizumab inhibits IL-17A and F | YES | YES | |
| Ixekizumab | YES | YES | |||
| Bimekizumab | YES | YES | |||
| Targeted synthetic DMARDs | JAK inhibitors | Tofacitinib | YES | NO | |
| Upadacitinib | YES | YES | |||
| Physical Therapy and Exercise | Physical Therapy Exercises | Stretching, Strengthening exercises | Improvement in mobility and reduction in pain through increased flexibility and muscle strengthening |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaltsonoudis, E.; Karagianni, P.; Memi, T.; Pelechas, E. State-of-the-Art Review on the Treatment of Axial Spondyloarthritis. Med. Sci. 2025, 13, 32. https://doi.org/10.3390/medsci13010032
Kaltsonoudis E, Karagianni P, Memi T, Pelechas E. State-of-the-Art Review on the Treatment of Axial Spondyloarthritis. Medical Sciences. 2025; 13(1):32. https://doi.org/10.3390/medsci13010032
Chicago/Turabian StyleKaltsonoudis, Evripidis, Panagiota Karagianni, Tereza Memi, and Eleftherios Pelechas. 2025. "State-of-the-Art Review on the Treatment of Axial Spondyloarthritis" Medical Sciences 13, no. 1: 32. https://doi.org/10.3390/medsci13010032
APA StyleKaltsonoudis, E., Karagianni, P., Memi, T., & Pelechas, E. (2025). State-of-the-Art Review on the Treatment of Axial Spondyloarthritis. Medical Sciences, 13(1), 32. https://doi.org/10.3390/medsci13010032





