FGF-23 as a Biomarker for Carotid Plaque Vulnerability: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
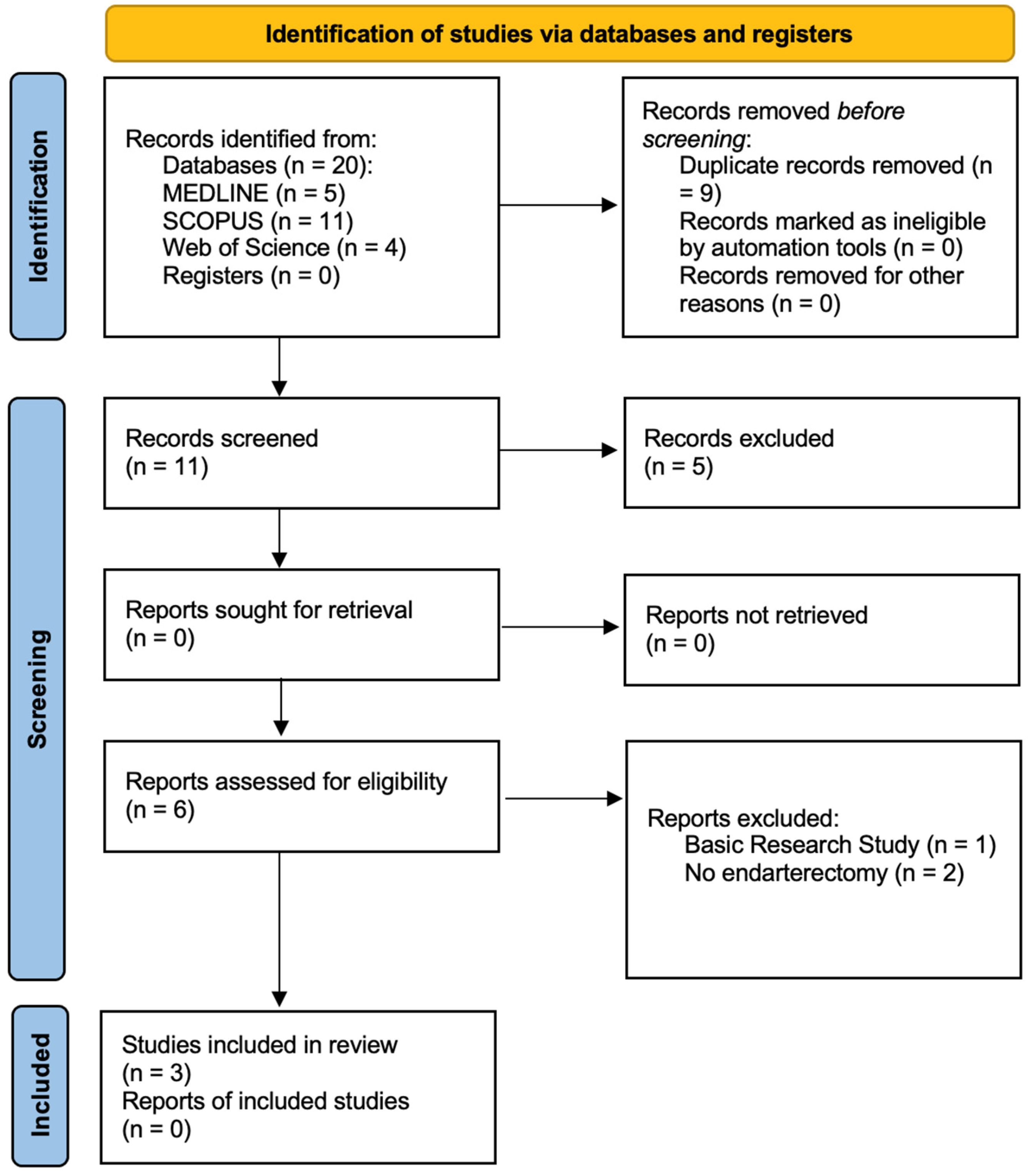
3.1. Search Results
3.2. Description of Studies
3.3. Main Findings
3.3.1. Primary Outcomes
3.3.2. Secondary Outcomes
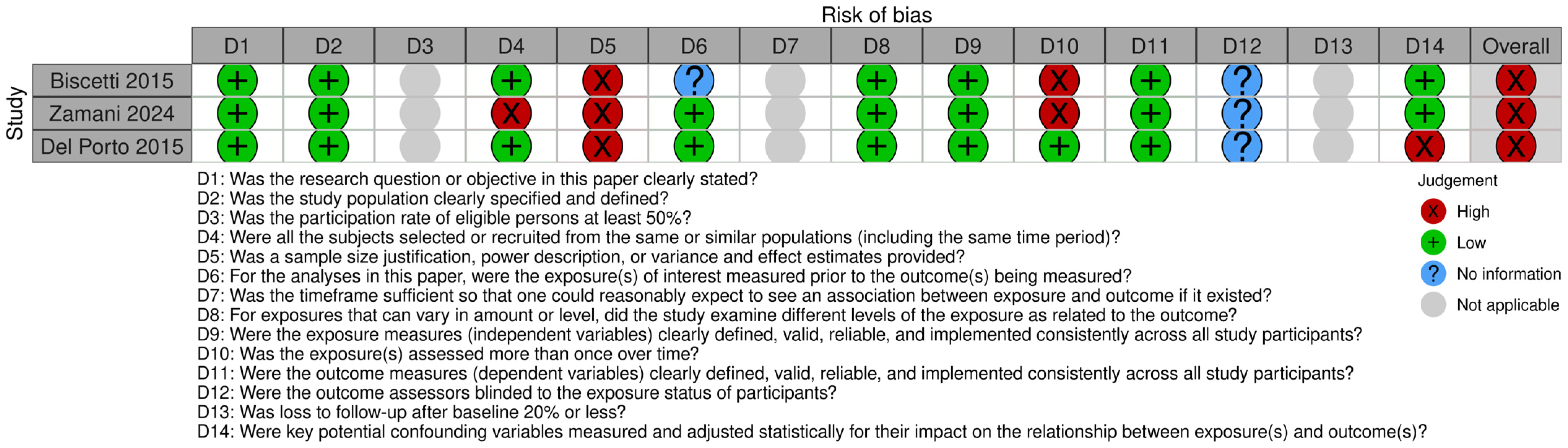
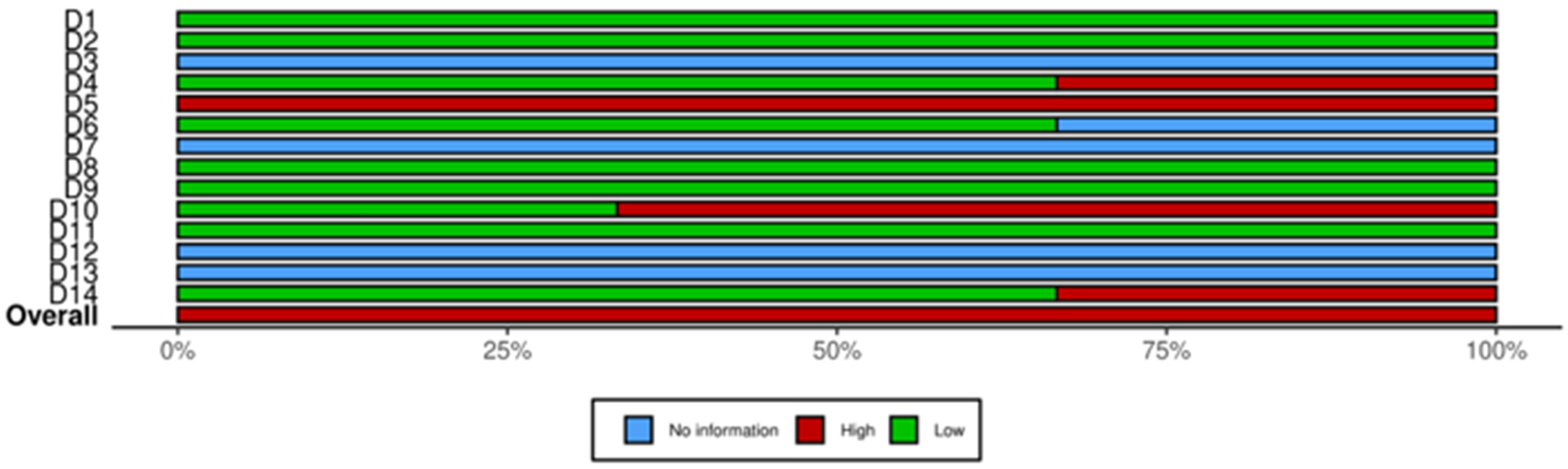
3.4. Quality of Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SMI | Superb Microvascular Imaging |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| OPG | Osteoprotegerin |
| CKD | Chronic Kidney Disease |
| CVD | Cardiovascular Disease |
| PRISMA | Preferred Reporting Items for a Systematic Review and Meta-analysis |
| CEA | Carotid Endarterectomy |
| JOS | Joana Oliveira-Sousa |
| JRN | João Rocha Neves |
| MFM | Mariana Fragão-Marques |
| NHLBI | National Heart, Lung and Blood Institute |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluation |
| IMVF | Intraplaque Microvascular Flow |
| IPN | Intraplaque Neovascularization |
| AHA | American Heart Association |
| HsCRP | High Sensitivity C Reactive Protein |
| VEGF | Vascular Endothelial Growth Factor |
| IL-8 | Interleukin-8 |
| b-FGF | basic Fibroblast Growth Factor |
| TNF-α | Tumor Necrosis Factor–α |
| CRP | C Reactive Protein |
| ICAS | Internal Carotid Artery Stenosis |
| IL-1B | Interleukin-1B |
| HIF1-α | Hypoxia-Induced Transcription Factor 1-α |
| ACS | Asymptomatic Carotid Stenosis |
| ESVS | European Society of Vascular Surgery |
References
- Dossabhoy, S.; Arya, S. Epidemiology of atherosclerotic carotid artery disease. Semin. Vasc. Surg. 2021, 34, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Méd. 2016, 45 Pt 2, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef]
- Shinohara, Y.; Nagayama, M.; Origasa, H. Postpublication external review of the Japanese guidelines for the management of stroke 2004. Stroke 2009, 40, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef]
- Verhoeven, B.A.; de Vries, J.P.; Pasterkamp, G.; Ackerstaff, R.G.; Schoneveld, A.H.; Velema, E.; de Kleijn, D.P.; Moll, F.L. Carotid atherosclerotic plaque characteristics are associated with microembolization during carotid endarterectomy and procedural outcome. Stroke 2005, 36, 1735–1740. [Google Scholar] [CrossRef]
- Niculescu, R.; Russu, E.; Arbanasi, E.M.; Kaller, R.; Arbanasi, E.M.; Melinte, R.M.; Cosarca, C.M.; Cocuz, I.G.; Sabau, A.H.; Tinca, A.C.; et al. Carotid Plaque Features and Inflammatory Biomarkers as Predictors of Restenosis and Mortality Following Carotid Endarterectomy. Int. J. Environ. Res. Public Health 2022, 19, 13934. [Google Scholar] [CrossRef]
- Miceli, G.; Basso, M.G.; Pintus, C.; Pennacchio, A.R.; Cocciola, E.; Cuffaro, M.; Profita, M.; Rizzo, G.; Tuttolomondo, A. Molecular Pathways of Vulnerable Carotid Plaques at Risk of Ischemic Stroke: A Narrative Review. Int. J. Mol. Sci. 2024, 25, 4351. [Google Scholar] [CrossRef]
- Seiler, S.; Reichart, B.; Roth, D.; Seibert, E.; Fliser, D.; Heine, G.H. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrol. Dial. Transplant. 2010, 25, 3983–3989. [Google Scholar] [CrossRef]
- Kumar, T.; Mohanty, S.; Rani, A.; Malik, A.; Kumar, R.; Bhashker, G. Fibroblast Growth Factor-23 in Pre-Dialysis Chronic Kidney Disease Patients and its Correlation with Carotid Artery Calcification. Indian J. Nephrol. 2022, 32, 560–566. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ustundag, S.; Temizoz, O.; Sut, N.; Demir, M.; Ermis, V.; Sevinc, C.; Ustundag, A. Fibroblast Growth Factor-23 and Carotid Artery Intima Media Thickness in Chronic Kidney Disease. Clin. Lab. 2015, 61, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, J.; Woo, H.G.; Ryu, D.R.; Oh, H.J.; Song, T.J. Plasma Fibroblast Growth Factor 23 Concentration Is Associated with Intracranial Cerebral Atherosclerosis in Acute Ischemic Stroke Patients. J. Clin. Neurol. 2020, 16, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; Lu, T.S.; Molostvov, G.; Lee, C.; Lam, F.T.; Zehnder, D.; Hsiao, L.L. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 2012, 125, 2243–2255. [Google Scholar] [CrossRef]
- Mencke, R.; Harms, G.; Mirkovic, K.; Struik, J.; Van Ark, J.; Van Loon, E.; Verkaik, M.; De Borst, M.H.; Zeebregts, C.J.; Hoenderop, J.G.; et al. Membrane-bound Klotho is not expressed endogenously in healthy or uraemic human vascular tissue. Cardiovasc. Res. 2015, 108, 220–231. [Google Scholar] [CrossRef]
- Kuro, O.M. The FGF23 and Klotho system beyond mineral metabolism. Clin. Exp. Nephrol. 2017, 21, 64–69. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Muros de Fuentes, M.; Mora-Fernandez, C.; Navarro-Gonzalez, J.F. Pathophysiological implications of fibroblast growth factor-23 and Klotho and their potential role as clinical biomarkers. Clin. Chem. 2014, 60, 933–940. [Google Scholar] [CrossRef]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate-Cardiovascular Toxins in CKD. Toxins 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Edmonston, D.; Grabner, A.; Wolf, M. FGF23 and klotho at the intersection of kidney and cardiovascular disease. Nat. Rev. Cardiol. 2024, 21, 11–24. [Google Scholar] [CrossRef]
- Marthi, A.; Donovan, K.; Haynes, R.; Wheeler, D.C.; Baigent, C.; Rooney, C.M.; Landray, M.J.; Moe, S.M.; Yang, J.; Holland, L.; et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. J. Am. Soc. Nephrol. 2018, 29, 2015–2027. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, M.E.; Alcala-Diaz, J.F.; Canalejo, A.; Torres-Pena, J.D.; Gomez-Delgado, F.; Munoz-Castaneda, J.R.; Delgado-Lista, J.; Rodriguez, M.; Lopez-Miranda, J.; Almaden, Y. Fibroblast growth factor 23 predicts carotid atherosclerosis in individuals without kidney disease. The CORDIOPREV study. Eur. J. Intern. Med. 2020, 74, 79–85. [Google Scholar] [CrossRef]
- Shah, N.H.; Dong, C.; Elkind, M.S.; Sacco, R.L.; Mendez, A.J.; Hudson, B.I.; Silverberg, S.; Wolf, M.; Rundek, T.; Wright, C.B. Fibroblast Growth Factor 23 Is Associated With Carotid Plaque Presence and Area: The Northern Manhattan Study. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2048–2053. [Google Scholar] [CrossRef]
- Biscetti, F.; Straface, G.; Porreca, C.F.; Bertoletti, G.; Vincenzoni, C.; Snider, F.; Stigliano, E.; Arena, V.; Angelini, F.; Pecorini, G.; et al. Increased FGF23 serum level is associated with unstable carotid plaque in type 2 diabetic subjects with internal carotid stenosis. Cardiovasc. Diabetol. 2015, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Del Porto, F.; Proietta, M.; di Gioia, C.; Cifani, N.; Dito, R.; Fantozzi, C.; Ferri, L.; Fabriani, L.; Rossi, M.; Tritapepe, L.; et al. FGF-23 levels in patients with critical carotid artery stenosis. Intern. Emerg. Med. 2015, 10, 437–444. [Google Scholar] [CrossRef]
- Zamani, M.; Skagen, K.; Lindberg, B.; Bjerkeli, V.; Aukrust, P.; Halvorsen, B.; Skjelland, M. Relationship between fibroblast growth factor in plasma and carotid plaque neovascularization: A pilot study. Front. Immunol. 2024, 15, 1385377. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Zheng, M.; Li, K.; Sun, S.; Zhang, Z.; Yan, N.; Li, X. Relationships of serum FGF23 and alpha-klotho with atherosclerosis in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 128. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Heart, L.; Institute, B. Study Quality Assessment Tools. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 18 September 2022).
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef]
- Gray-Weale, A.C.; Graham, J.C.; Burnett, J.R.; Byrne, K.; Lusby, R.J. Carotid artery atheroma: Comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J. Cardiovasc. Surg. 1988, 29, 676–681. [Google Scholar]
- Chua, W.; Cardoso, V.R.; Guasch, E.; Sinner, M.F.; Al-Taie, C.; Brady, P.; Casadei, B.; Crijns, H.; Dudink, E.; Hatem, S.N.; et al. An angiopoietin 2, FGF23, and BMP10 biomarker signature differentiates atrial fibrillation from other concomitant cardiovascular conditions. Sci. Rep. 2023, 13, 16743. [Google Scholar] [CrossRef]
- Donovan, K.; Herrington, W.G.; Pare, G.; Pigeyre, M.; Haynes, R.; Sardell, R.; Butterworth, A.S.; Folkersen, L.; Gustafsson, S.; Wang, Q.; et al. Fibroblast Growth Factor-23 and Risk of Cardiovascular Diseases: A Mendelian Randomization Study. Clin. J. Am. Soc. Nephrol. 2023, 18, 17–27. [Google Scholar] [CrossRef]
- Arnlov, J.; Carlsson, A.C.; Sundstrom, J.; Ingelsson, E.; Larsson, A.; Lind, L.; Larsson, T.E. Serum FGF23 and risk of cardiovascular events in relation to mineral metabolism and cardiovascular pathology. Clin. J. Am. Soc. Nephrol. 2013, 8, 781–786. [Google Scholar] [CrossRef]
- Hu, X.; Ma, X.; Luo, Y.; Xu, Y.; Xiong, Q.; Pan, X.; Bao, Y.; Jia, W. Contribution of fibroblast growth factor 23 to Framingham risk score for identifying subclinical atherosclerosis in Chinese men. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 147–153. [Google Scholar] [CrossRef]
- Parker, B.D.; Schurgers, L.J.; Brandenburg, V.M.; Christenson, R.H.; Vermeer, C.; Ketteler, M.; Shlipak, M.G.; Whooley, M.A.; Ix, J.H. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann. Intern. Med. 2010, 152, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Srivaths, P.R.; Goldstein, S.L.; Krishnamurthy, R.; Silverstein, D.M. High serum phosphorus and FGF 23 levels are associated with progression of coronary calcifications. Pediatr. Nephrol. 2014, 29, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Kaizu, Y.; Nagata, M.; Ura, Y.; Ikeda, H.; Shimamoto, S.; Kuma, K. Fibroblast growth factor 23 is associated with carotid artery calcification in chronic kidney disease patients not undergoing dialysis: A cross-sectional study. BMC Nephrol. 2013, 14, 22. [Google Scholar] [CrossRef]
- Lutsey, P.L.; Alonso, A.; Selvin, E.; Pankow, J.S.; Michos, E.D.; Agarwal, S.K.; Loehr, L.R.; Eckfeldt, J.H.; Coresh, J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: The Atherosclerosis Risk in Communities study. J. Am. Heart Assoc. 2014, 3, e000936. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.A.; Hansen, T.; Johansson, L.; Ahlstrom, H.; Larsson, A.; Lind, L.; Larsson, T.E. Relationship between circulating FGF23 and total body atherosclerosis in the community. Nephrol. Dial. Transpl. 2009, 24, 3125–3131. [Google Scholar] [CrossRef]
- Xiao, Y.; Peng, C.; Huang, W.; Zhang, J.; Xia, M.; Zhang, Y.; Ling, W. Circulating fibroblast growth factor 23 is associated with angiographic severity and extent of coronary artery disease. PLoS ONE 2013, 8, e72545. [Google Scholar] [CrossRef]
- Silswal, N.; Touchberry, C.D.; Daniel, D.R.; McCarthy, D.L.; Zhang, S.; Andresen, J.; Stubbs, J.R.; Wacker, M.J. FGF23 directly impairs endothelium-dependent vasorelaxation by increasing superoxide levels and reducing nitric oxide bioavailability. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E426–E436. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Saglam, M.; Yaman, H.; Kilic, S.; Demirkaya, E.; Eyileten, T.; Caglar, K.; Oguz, Y.; Vural, A.; et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney Int. 2010, 78, 679–685. [Google Scholar] [CrossRef]
- Munoz Mendoza, J.; Isakova, T.; Ricardo, A.C.; Xie, H.; Navaneethan, S.D.; Anderson, A.H.; Bazzano, L.A.; Xie, D.; Kretzler, M.; Nessel, L.; et al. Fibroblast growth factor 23 and Inflammation in CKD. Clin. J. Am. Soc. Nephrol. 2012, 7, 1155–1162. [Google Scholar] [CrossRef]
- Hasegawa, H.; Nagano, N.; Urakawa, I.; Yamazaki, Y.; Iijima, K.; Fujita, T.; Yamashita, T.; Fukumoto, S.; Shimada, T. Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int. 2010, 78, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Isakova, T.; Gutierrez, O.M.; Patel, N.M.; Andress, D.L.; Wolf, M.; Levin, A. Vitamin D deficiency, inflammation, and albuminuria in chronic kidney disease: Complex interactions. J. Ren. Nutr. 2011, 21, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Castell, J.V.; Gomez-Lechon, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef]
- Axelsson, J.; Rashid Qureshi, A.; Suliman, M.E.; Honda, H.; Pecoits-Filho, R.; Heimburger, O.; Lindholm, B.; Cederholm, T.; Stenvinkel, P. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am. J. Clin. Nutr. 2004, 80, 1222–1229. [Google Scholar] [CrossRef]
- Wisse, B.E. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Bonanno, E.; Sangiorgi, G.; Mauriello, A. Role of inflammation in atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef]
- Hanks, L.J.; Casazza, K.; Judd, S.E.; Jenny, N.S.; Gutierrez, O.M. Associations of fibroblast growth factor-23 with markers of inflammation, insulin resistance and obesity in adults. PLoS ONE 2015, 10, e0122885. [Google Scholar] [CrossRef]
- Dounousi, E.; Torino, C.; Pizzini, P.; Cutrupi, S.; Panuccio, V.; D’Arrigo, G.; Abd ElHafeez, S.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Intact FGF23 and alpha-Klotho during acute inflammation/sepsis in CKD patients. Eur. J. Clin. Investig. 2016, 46, 234–241. [Google Scholar] [CrossRef]
- Sato, H.; Kazama, J.J.; Murasawa, A.; Otani, H.; Abe, A.; Ito, S.; Ishikawa, H.; Nakazono, K.; Kuroda, T.; Nakano, M.; et al. Serum Fibroblast Growth Factor 23 (FGF23) in Patients with Rheumatoid Arthritis. Intern. Med. 2016, 55, 121–126. [Google Scholar] [CrossRef]
- Resende, A.L.; Elias, R.M.; Wolf, M.; Dos Reis, L.M.; Graciolli, F.G.; Santos, G.D.; Dias, C.B.; Jorgetti, V.; Woronik, V.; Moyses, R.M. Serum levels of fibroblast growth factor 23 are elevated in patients with active Lupus nephritis. Cytokine 2017, 91, 124–127. [Google Scholar] [CrossRef] [PubMed]
- David, V.; Martin, A.; Isakova, T.; Spaulding, C.; Qi, L.; Ramirez, V.; Zumbrennen-Bullough, K.B.; Sun, C.C.; Lin, H.Y.; Babitt, J.L.; et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016, 89, 135–146. [Google Scholar] [CrossRef]
- Yamazaki, M.; Kawai, M.; Miyagawa, K.; Ohata, Y.; Tachikawa, K.; Kinoshita, S.; Nishino, J.; Ozono, K.; Michigami, T. Interleukin-1-induced acute bone resorption facilitates the secretion of fibroblast growth factor 23 into the circulation. J. Bone Miner. Metab. 2015, 33, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Glosse, P.; Fajol, A.; Hirche, F.; Feger, M.; Voelkl, J.; Lang, F.; Stangl, G.I.; Foller, M. A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFalpha upregulation. Nutr. Diabetes 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Pathak, J.L.; Bakker, A.D.; Luyten, F.P.; Verschueren, P.; Lems, W.F.; Klein-Nulend, J.; Bravenboer, N. Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif. Tissue Int. 2016, 98, 596–608. [Google Scholar] [CrossRef]
- Ito, N.; Wijenayaka, A.R.; Prideaux, M.; Kogawa, M.; Ormsby, R.T.; Evdokiou, A.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol. Cell. Endocrinol. 2015, 399, 208–218. [Google Scholar] [CrossRef]
- Han, X.; Li, L.; Yang, J.; King, G.; Xiao, Z.; Quarles, L.D. Counter-regulatory paracrine actions of FGF-23 and 1,25(OH)2 D in macrophages. FEBS Lett. 2016, 590, 53–67. [Google Scholar] [CrossRef]
- Durlacher-Betzer, K.; Hassan, A.; Levi, R.; Axelrod, J.; Silver, J.; Naveh-Many, T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018, 94, 315–325. [Google Scholar] [CrossRef]
- Han, J.; Luo, L.; Marcelina, O.; Kasim, V.; Wu, S. Therapeutic angiogenesis-based strategy for peripheral artery disease. Theranostics 2022, 12, 5015–5033. [Google Scholar] [CrossRef]
- Clayton, J.A.; Chalothorn, D.; Faber, J.E. Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia. Circ. Res. 2008, 103, 1027–1036. [Google Scholar] [CrossRef]
- Shweiki, D.; Itin, A.; Soffer, D.; Keshet, E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992, 359, 843–845. [Google Scholar] [CrossRef]
- Wheeler, J.A.; Clinkenbeard, E.L. Regulation of Fibroblast Growth Factor 23 by Iron, EPO, and HIF. Curr. Mol. Biol. Rep. 2019, 5, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Francis, C.; David, V. Inflammation regulates fibroblast growth factor 23 production. Curr. Opin. Nephrol. Hypertens. 2016, 25, 325–332. [Google Scholar] [CrossRef]
- Zhang, Q.; Doucet, M.; Tomlinson, R.E.; Han, X.; Quarles, L.D.; Collins, M.T.; Clemens, T.L. The hypoxia-inducible factor-1alpha activates ectopic production of fibroblast growth factor 23 in tumor-induced osteomalacia. Bone Res. 2016, 4, 16011. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef]
- De Smet, F.; Segura, I.; De Bock, K.; Hohensinner, P.J.; Carmeliet, P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 639–649. [Google Scholar] [CrossRef]
- Schneeweis, C.; Grafe, M.; Bungenstock, A.; Spencer-Hansch, C.; Fleck, E.; Goetze, S. Chronic CRP-exposure inhibits VEGF-induced endothelial cell migration. J. Atheroscler. Thromb. 2010, 17, 203–212. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Gu, Z.; Wu, M.; Yang, Y.; Zhang, J.; Ou, J.; Zuo, Z.; Wang, J.; Chen, Y. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1alpha via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res. Ther. 2016, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical results in 1415 patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yoshimura, S.; Kawasaki, M.; Enomoto, Y.; Asano, T.; Hara, A.; Minatoguchi, S.; Iwama, T. Embolic complications after carotid artery stenting or carotid endarterectomy are associated with tissue characteristics of carotid plaques evaluated by magnetic resonance imaging. Atherosclerosis 2011, 215, 399–404. [Google Scholar] [CrossRef]
- Yoshimura, S.; Yamada, K.; Kawasaki, M.; Asano, T.; Kanematsu, M.; Miyai, M.; Enomoto, Y.; Egashira, Y.; Iwama, T. Selection of carotid artery stenting or endarterectomy based on magnetic resonance plaque imaging reduced periprocedural adverse events. J. Stroke Cerebrovasc. Dis. 2013, 22, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Perler, B.A.; Powell, R.J.; Rockman, C.B.; et al. The Society for Vascular Surgery implementation document for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 26S–98S. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef]
- Bonati, L.H.; Kakkos, S.; Berkefeld, J.; de Borst, G.J.; Bulbulia, R.; Halliday, A.; van Herzeele, I.; Koncar, I.; McCabe, D.J.; Lal, A.; et al. European Stroke Organisation guideline on endarterectomy and stenting for carotid artery stenosis. Eur. Stroke J. 2021, 6, I–XLVII. [Google Scholar] [CrossRef]
- Migdalski, A.; Jawien, A. New insight into biology, molecular diagnostics and treatment options of unstable carotid atherosclerotic plaque: A narrative review. Ann. Transl. Med. 2021, 9, 1207. [Google Scholar] [CrossRef]
- Paraskevas, K.I.; Veith, F.J.; Ricco, J.B. Best medical treatment alone may not be adequate for all patients with asymptomatic carotid artery stenosis. J. Vasc. Surg. 2018, 68, 572–575. [Google Scholar] [CrossRef]
- Hellings, W.E.; Peeters, W.; Moll, F.L.; Piers, S.R.; van Setten, J.; Van der Spek, P.J.; de Vries, J.P.; Seldenrijk, K.A.; De Bruin, P.C.; Vink, A.; et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: A prognostic study. Circulation 2010, 121, 1941–1950. [Google Scholar] [CrossRef]
| Study ID | ||||||
|---|---|---|---|---|---|---|
| Study | Publication Year | Journal | Study Center | Study Design | Sample | GRADE |
| Biscetti, F. et al. [22] | 2015 | Cardiovascular Diabetology | A. Gemelli University Hospital, Catholic University School of Medicine, Rome, Italy | Retrospective Observational Study | 959 | ** |
| Del Porto, F. et al. [23] | 2015 | Internal and Emergency Medicine–Official Journal of the Italian Society of Internal Medicine | Sant’ Andrea Hospital, “Sapienza” University of Rome, Italy | Cross-Sectional Observational Study | 51 | * |
| Zamani, M. et al. [24] | 2024 | Frontiers in Immunology | Oslo University Hospital, Oslo, Norway | Cross-Sectional Observational Study | 29 | * |
| Population | ||||||
| Study | Type of Participant | Recruitment Time | Age, Mean (Years) | Male, % | Number of CEA | Follow-Up |
| Biscetti, F. et al. [22] | DM2 | January 2009–February 2015 | 72.05 | 36.8 | 361 | - |
| Del Porto, F. et al. [23] | - | NA | 71.46 | 80.71 | 35 | - |
| Zamani, M. et al. [24] | - | Up to January 2019/January 2019–April 2019 | 72.5 | 62.1 | NA | - |
| Study | Hypertension, n (%) | Dyslipidemia, n (%) | Diabetes Mellitus, n (%) | Smoking History, n (%) | Coronary Artery Disease, n (%) | Carotid Territory Symptoms/Signs, n (%) |
|---|---|---|---|---|---|---|
| Biscetti, F. et al. [22] | 482 (50.3) | 460 (48.0) | 959 (100.0) | 761 (79.4) | 392 (40.9) | 174 (18.1) |
| Del Porto, F. et al. [23] | 35 (68.6) | 16 (31.4) | 9 (17.6) | 9 (17.6) | NA | 9 (17.6) |
| Zamani, M. et al. [24] | 18 (62.1) | 13 (44.8) | 4 (14.8) | 13 (44.8) | 11 (37.9) | 19 (65.5) |
| Study | Comparison Groups (Patients) | Serum Levels (Mean/Median ± SD/IQR) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FGF23 (pg/mL) | HsCRP (mg/L) | OPG (pmol/L) | IL-6 (pg/mL) | IL-8 (pg/mL) | MCP-1 (pg/mL) | VEGF (pg/mL) | |||
| Biscetti, F. et al. ‡ [22] | ICAS (n = 361) | 67.7 [59.5–77.8] | 7.95 [6.48–9.55] | 4.84 [3.52–5.95] | 62.3 [57.1–68.5] | NA | NA | NA | |
| Controls (n = 598) | 43.89 [37.5–50.4] | 3.95 [2.34–5.31] | 2.48 [1.69–3.35] | 39.1 [33.8–45.2] | NA | NA | NA | ||
| Unstable Plaque (n = 166) | 78.4 [68.4–87.5] | 9.31 [7.94–11.1] | 6.04 [4.65–7.34] | 71.5 [66.3–77.4] | NA | NA | NA | ||
| Stable Plaque (n = 195) | 34.7 [29.7–41.1] | 2.74 [1.92–4.12] | 2.12 [1.02–2.95] | 32.6 [28.8–36.6] | NA | NA | NA | ||
| Del Porto, F. et al. £ [23] | A † (n = 20) | t0 *: | 7.20 (7.45 ± 3.05) Δ | NA | NA | 114 ± 60.19 | 49.3 ± 9.71 | 391.9 ± 126.39 | 176.45 ± 239.28 |
| t1 **: | 11.18 (12.13 ± 5.80) Δ | NA | NA | NA | NA | NA | NA | ||
| B † (n = 15) | t0 *: | 1.80 (2.61 ± 1.76) Δ | NA | NA | 104.33 ± 50.59 | 46.56 ± 5.12 | 363.14 ± 121.17 | 270.7 ± 155.20 | |
| t1 **: | 3.35 (3.90 ± 2.52) Δ | NA | NA | NA | NA | NA | NA | ||
| C † (n = 16) | 1 (2.53 ± 3.3) | NA | NA | 3.99 ± 1.25 | |||||
| Zamani, M. et al. ¶ [24] | No IPN (n = 6) | 84.2 [55.8–136.2] | NA | NA | NA | NA | NA | NA | |
| Moderate IPN (n = 9) | 78.1 [58.5–112.7] | NA | NA | NA | NA | NA | NA | ||
| Extensive IPN (n = 14) | 132.8 [64.9–236.5] | NA | NA | NA | NA | NA | NA | ||
| Study | Comparison Groups (Patients) | Statistical Analysis (Serum Levels) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGF23 | HsCRP | OPG | IL-6 | IL-8 | MCP-1 | VEGF | TNF-α | b-FGF | CRP | |||
| Biscetti, F. et al. [22] | ICAS (n = 361) | p < 0.001 ‡ | p < 0.001 ‡ | p < 0.001 ‡ | p < 0.001 ‡ | NA | NA | NA | NA | NA | NA | |
| Controls (n = 598) | ||||||||||||
| Unstable Plaque (n = 166) | p < 0.001 ‡ | p < 0.001 ‡ | p < 0.001 ‡ | p < 0.001 ‡ | NA | NA | NA | NA | NA | NA | ||
| Stable Plaque (n = 195) | ||||||||||||
| Del Porto, F. et al. [23] | A † (n = 20) vs. B † (n = 15) | t0 *: | p < 0.05 Δ¤ | NA | NA | p > 0.05 ¤ | p > 0.05 ¤ | p > 0.05 ¤ | p > 0.05 ¤ | NA | NA | NA |
| t1 **: | p = 0.0047 Δ¤ | NA | NA | NA | NA | NA | NA | NA | NA | NA | ||
| A † (n = 20) vs. C † (n = 16) | t0 *: | p < 0.001 Δ¤ | NA | NA | p < 0.001 ¤ | p < 0.001 ¤ | p < 0.05 ¤ | p > 0.5 ¤ | NA | NA | NA | |
| B † (n = 15) vs. C † (n = 16) | t0 *: | p > 0.05 ¤ | NA | NA | p < 0.01 ¤ | p < 0.01 ¤ | p < 0.05 ¤ | p < 0.01 ¤ | NA | NA | NA | |
| A † (n = 20) | t0 *: | p = 0.0010 ¤ | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| t1 **: | ||||||||||||
| B † (n = 15) | t0 *: | p = 0.1563 ¤ | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| t1 **: | ||||||||||||
| Zamani, M. et al. [24] | No IPN (n = 6) | p = 0.016 § | NA | NA | p = 0.370, r = −0.173 œ | NA | NA | NA | p = 0.765, r = 0.058 œ | p = 0.577, r = −0.084 œ | p= 0.242, r= −0.224 œ | |
| Extensive IPN (n = 14) | ||||||||||||
| p = 0.052 § | ||||||||||||
| Moderate IPN (n = 9) | ||||||||||||
| Neovessel Count (n = 4.5 ¥) | p = 0.007, r = 0.491 œ | NA | NA | p = 0.482, r =−0.173 œ | NA | NA | NA | p = 0. 723, r = 0.069 œ | p = 0.598, r = 0.106 œ | p = 0.071, r = −0.340 œ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Sousa, J.; Fragão-Marques, M.; Duarte-Gamas, L.; Ribeiro, H.; Rocha-Neves, J. FGF-23 as a Biomarker for Carotid Plaque Vulnerability: A Systematic Review. Med. Sci. 2025, 13, 27. https://doi.org/10.3390/medsci13010027
Oliveira-Sousa J, Fragão-Marques M, Duarte-Gamas L, Ribeiro H, Rocha-Neves J. FGF-23 as a Biomarker for Carotid Plaque Vulnerability: A Systematic Review. Medical Sciences. 2025; 13(1):27. https://doi.org/10.3390/medsci13010027
Chicago/Turabian StyleOliveira-Sousa, Joana, Mariana Fragão-Marques, Luís Duarte-Gamas, Hugo Ribeiro, and João Rocha-Neves. 2025. "FGF-23 as a Biomarker for Carotid Plaque Vulnerability: A Systematic Review" Medical Sciences 13, no. 1: 27. https://doi.org/10.3390/medsci13010027
APA StyleOliveira-Sousa, J., Fragão-Marques, M., Duarte-Gamas, L., Ribeiro, H., & Rocha-Neves, J. (2025). FGF-23 as a Biomarker for Carotid Plaque Vulnerability: A Systematic Review. Medical Sciences, 13(1), 27. https://doi.org/10.3390/medsci13010027







