The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications
Abstract
1. Introduction
2. The Hypothesis
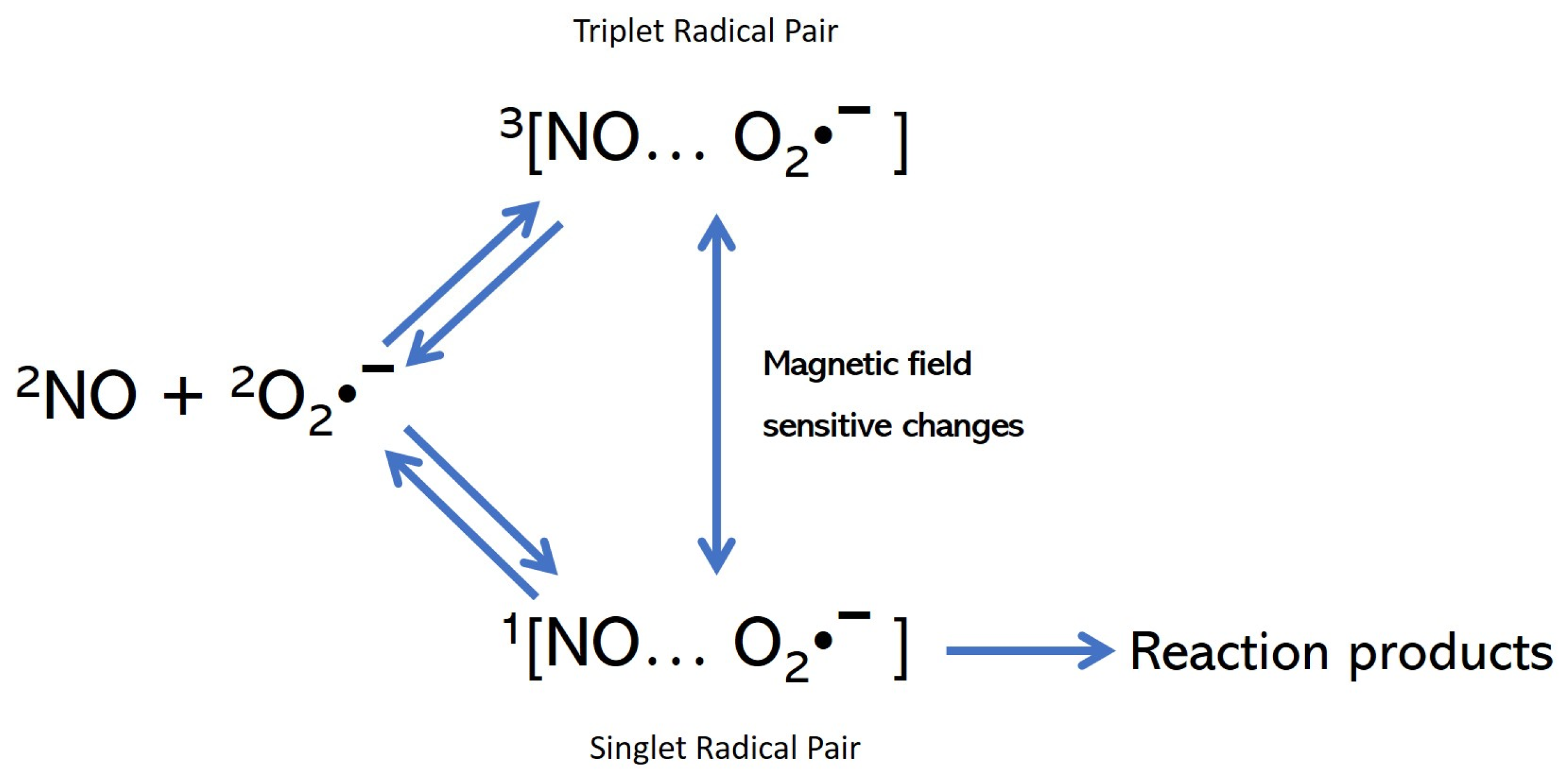
2.1. The Reversal of the Free Radicals (FRs) Negative Cycle
2.2. The Inflammatory Phase
2.3. The Anti-Inflammatory Phase
3. Cell Stress and Its Consequences Related to the Hypothesis
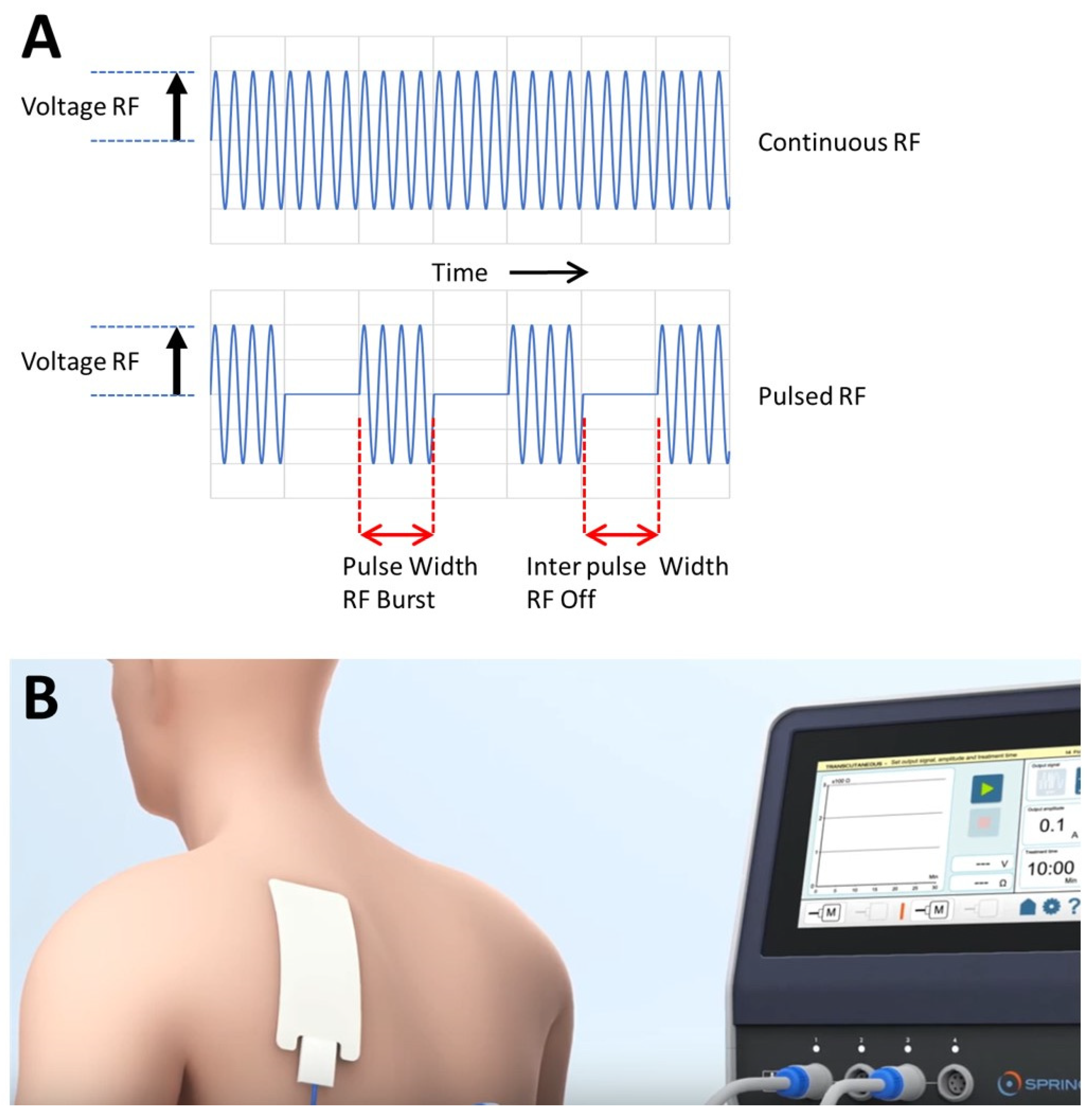
4. Some Notes on Pulsed Radiofrequency (PRF) Related to the Hypothesis
4.1. PRF Application in Inflammation Is Called RedoxPRF
4.2. Types of Treatment
4.3. Physical Aspects of RedoxPRF
4.4. General Information on RedoxPRF Treatment from Practice Observations and Considerations
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sluijter, M.E.; Cosman, E.; Rittman, W.; van Kleef, M. The effect of pulsed radiofrequency fields applied to the dorsal root ganglion—A preliminary report. Pain Clin. 1998, 11, 109–117. [Google Scholar]
- Vanneste, T.; Van Lantschoot, A.; Van Boxem, K.; Van Zundert, J. Pulsed radiofrequency in chronic pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.W.; Luthardt, F.; Staats, P.S. Radiofrequency treatment in the United States. Pain Pract. 2002, 2, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Heavner, J.E.; Boswell, M.V.; Racz, G.B. A comparison of pulsed radiofrequency and continuous radiofrequency on thermocoagulation of egg white in vitro. Pain Physician 2006, 9, 135–137. [Google Scholar] [PubMed]
- Ewertowska, E.; Mercadal, B.; Muñoz, V.; Ivorra, A.; Trujillo, M.; Berjano, E. Effect of applied voltage, duration and repetition frequency of RF pulses for pain relief on temperature spikes and electrical field: A computer modelling study. Int. J. Hyperth. 2018, 34, 112–121. [Google Scholar] [CrossRef]
- Sluijter, M.E.; Teixeira, A.; Serra, V.; Balogh, S.; Schianchi, P. Intra-articular application of Pulsed Radiofrequency for Arthrogenic Pain—Report of Six Cases. Pain Pract. 2008, 8, 57–61. [Google Scholar] [CrossRef]
- Teixeira, A.; Sluijter, M.E. Intravenous application of pulsed radiofrequency—4 case reports. Anesth Pain Med. 2013, 3, 219–222. [Google Scholar] [CrossRef]
- Rampersad, S. Distribution of E-Fields during Intravenous PRF. In Proceedings of the 5th International Symposium “Invasive Procedures in Motion”, Nottwil, Switzerland, 16–17 January 2015. [Google Scholar]
- Maretto, F.; Vennik, M.; Albers, K.I.; van Duijn, B. TNFα secretion of monocytes exposed to pulsed radiofrequency treatment: A possible working mechanism of PRF chronic pain management. Pain Pract. 2014, 14, 399–404. [Google Scholar] [CrossRef]
- Kumiko, T.; Shigeo, T.; Hiroki, I. Changes in the gene expression in mouse astrocytes induced by pulsed radiofrequency: A preliminary study. Neurosci. Lett. 2021, 742, 135536. [Google Scholar]
- Brasil, L.J.; Marroni, N.; Schemitt, E.; Colares, J. Effects of Pulsed Radiofrequency on a Standard Model of Muscle Injury in Rats. Anesth Pain Med. 2020, 10, e97372. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic signatures underlying inflammation: An interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.; Yousef, M.H.; Abdelnaser, A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021, 12, 606069. [Google Scholar] [CrossRef] [PubMed]
- Lytrivi, M.; Castell, A.L.; Poitout, V.; Cnop, M. Recent Insights Into Mechanisms of β-Cell Lipo- and Glucolipotoxicity in Type 2 Diabetes. J. Mol. Biol. 2020, 432, 1514–1534. [Google Scholar] [CrossRef] [PubMed]
- Grootjans, J.; Kaser, A.; Kaufman, R.J.; Blumberg, R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016, 16, 469–484. [Google Scholar] [CrossRef]
- Dandekar, A.; Mendez, R.; Zhang, K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015, 1292, 205–214. [Google Scholar] [PubMed]
- Barford, D. The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 2004, 14, 679–686. [Google Scholar] [CrossRef]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef]
- Biswas, S.K.; de Faria, J.B. Which comes first: Renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic. Res. 2007, 41, 216–224. [Google Scholar] [CrossRef]
- Wong, C.H.; Jenne, C.N.; Lee, W.Y.; Léger, C.; Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 2011, 334, 101–105. [Google Scholar] [CrossRef]
- Bellinger, D.L.; Millar, B.A.; Perez, S.; Carter, J.; Wood, C.; ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sympathetic modulation of immunity: Relevance to disease. Cell. Immunol. 2008, 252, 27–56. [Google Scholar] [CrossRef]
- Leitch, M.; Brown, R.; Macefield, V.G. Intramuscular stimulation of tibialis anterior in human subjects: The effects of discharge variability on force production and fatigue. Physiol. Rep. 2017, 5, e13326. [Google Scholar] [CrossRef]
- Van Gils, T.; Tiesinga, P.H.E.; Englitz, B.; Martens, M.B. Sensitivity to Stimulus Irregularity Is Inherent in Neural Networks. Neural Comput. 2019, 31, 1789–1824. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Shin, H.-W.; Shin, D.-G. Impact of Oxidative Stress on Long-Term Heart Rate Variability: Linear Versus Non-Linear Heart Rate Dynamics. Heart Lung Circ. 2020, 29, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. The antioxidant paradox: Less paradoxical now? Br. J. Clin. Pharmacol. 2013, 75, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Szent-Györgyi, A. An Introduction to Submolecular Biology; Academic Press: Cambridge, MA, USA, 1960. [Google Scholar]
- Eichwald, C.; Walleczek, J. Model for magnetic field effects on radical pair recombination in enzyme kinetics. Biophys. J. 1996, 71, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Walleczek, J. Low-Frequency-Dependent Magnetic Field Effects in Biological Systems and the Radical Pair Mechanism. In Electricity and Magnetism in Biology and Medicine; Bersani, F., Ed.; Springer: Boston, MA, USA, 1999. [Google Scholar] [CrossRef]
- Karogodina, T.Y.; Dranov, I.G.; Sergeeva, S.V.; Stass, D.V.; Steiner, U.E. Kinetic magnetic-field effect involving the small biologically relevant inorganic radicals NO and O2•−. ChemPhysChem 2011, 12, 1714–1728. [Google Scholar] [CrossRef]
- Zhao, H.; Han, Z.; Ji, X.; Luo, Y. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis. 2016, 7, 295–306. [Google Scholar] [CrossRef]
- Pradeep, H.; Diya, J.B.; Shashikumar, S.; Rajanikant, G.K. Oxidative stress-assassin behind the ischemic stroke. Folia Neuropathol. 2012, 50, 219–230. [Google Scholar] [CrossRef]
- Bikkad, M.D.; Somwanshi, S.D.; Ghuge, S.H.; Nagane, N.S. Oxidative Stress in Type II Diabetes Mellitus. Biomed. Res. 2014, 25, 84–87. [Google Scholar]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Blasiak, J.; Petrovski, G.; Veréb, Z.; Facskó, A.; Kaarniranta, K. Oxidative stress, hypoxia, and autophagy in the neovascular processes of age-related macular degeneration. Biomed. Res. Int. 2014, 2014, 768026. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Miller, A.H.; Raison, C.L. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016, 16, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Epel, E.S.; Mahan, L.; Rosser, R.; Wolkowitz, O.M.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic kidney disease influences multiple systems: Describing the relationship between oxidative stress, inflammation, kidney damage, and concomitant disease. Oxid. Med. Cell. Long. 2015, 2015, 806358. [Google Scholar] [CrossRef]
- Domej, W.; Oettl, K.; Renner, W. Oxidative stress and free radicals in COPD-implications and relevance for treatment. Int. J. Chronic Obstr. Pulm. Dis. 2014, 9, 1207–1224. [Google Scholar] [CrossRef]
- Kirkham, P.A.; Barnes, P.J. Oxidative stress in COPD. Chest 2013, 144, 266–273. [Google Scholar] [CrossRef]
- Portal-Núñez, S.; Esbrit, P.; Alcaraz, M.J.; Largo, R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem. Pharmacol. 2016, 108, 1–10. [Google Scholar] [CrossRef]
- Zuo, L.; Prather, E.R.; Stetskiv, M.; Garrison, D.E.; Meade, J.R.; Peace, T.I.; Zhou, T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019, 20, 4472. [Google Scholar] [CrossRef]
- Gow, J.W.; Hagan, S.; Herzyk, P.; Cannon, C.; Behan, P.O.; Chaudhuri, A. A gene signature for post-infectious chronic fatigue syndrome. BMC Med. Genom. 2009, 2, 38. [Google Scholar] [CrossRef] [PubMed]
- Zapatero-Solana, E.; García-Giménez, J.L.; Guerrero-Aspizua, S.; García, M.; Toll, A.; Baselga, E.; Durán-Moreno, M.; Markovic, J.; García-Verdugo, J.M.; Conti, C.J.; et al. Oxidative stress and mitochondrial dysfunction in Kindler syndrome. Orphanet J. Rare Dis. 2014, 9, 211. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, J.A.; Choi, Y.S.; Kim, S.H.; Lee, J.Y.; Kim, Y.E. Heart rate variability and length of survival in hospice cancer patients. J. Korean Med. Sci. 2010, 25, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.; Wilson, M.; Lee, G.; Kure, C.; Ou, R.; Braun, L.; de Haan, J. Oxidative stress in surgery in an ageing population: Pathophysiology and therapy. Exp. Gerontol. 2013, 48, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Mundi, S.; Pindiprolu, B.; Simunovic, N.; Bhandari, M. Similar mortality rates in hip fracture patients over the past 31 years. Acta Orthop. 2014, 85, 54–59. [Google Scholar] [CrossRef] [PubMed]
- d’Arsonval, A. Action physiologique des courants alternatifs a grande frequence. Arch. Physiol. Norm. Pathol. 1893, 5, 401–408. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sluijter, M.E.; Teixeira, A.; Vissers, K.; Brasil, L.J.; van Duijn, B. The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications. Med. Sci. 2023, 11, 58. https://doi.org/10.3390/medsci11030058
Sluijter ME, Teixeira A, Vissers K, Brasil LJ, van Duijn B. The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications. Medical Sciences. 2023; 11(3):58. https://doi.org/10.3390/medsci11030058
Chicago/Turabian StyleSluijter, Menno E., Alexandre Teixeira, Kris Vissers, Luis Josino Brasil, and Bert van Duijn. 2023. "The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications" Medical Sciences 11, no. 3: 58. https://doi.org/10.3390/medsci11030058
APA StyleSluijter, M. E., Teixeira, A., Vissers, K., Brasil, L. J., & van Duijn, B. (2023). The Anti-Inflammatory Action of Pulsed Radiofrequency—A Hypothesis and Potential Applications. Medical Sciences, 11(3), 58. https://doi.org/10.3390/medsci11030058





