Abstract
Background: Atrial fibrillation (AF) is the most common pathological arrhythmia, and its complications lead to significant morbidity and mortality. However, patients with AF can often go undetected, especially if they are asymptomatic or have a low burden of paroxysms. Identification of those at high risk of AF development may help refine screening and management strategies. Methods: PubMed and Embase databases were systematically searched for studies looking at electrocardiographic predictors of AF from inception to August 2021. Results: A total of 115 studies were reported which examined a combination of atrial and ventricular parameters that could be electrocardiographic predictors of AF. Atrial predictors include conduction parameters, such as the PR interval, p-wave index and dispersion, and partial interatrial or advanced interatrial block, or morphological parameters, such as p-wave axis, amplitude and terminal force. Ventricular predictors include abnormalities in QRS amplitude, morphology or duration, QT interval duration, r-wave progression and ST segment, i.e., t-wave abnormalities. Conclusions: There has been significant interest in electrocardiographic prediction of AF, especially in populations at high risk of atrial AF, such as those with an embolic stroke of undetermined source. This review highlights the breadth of possible predictive parameters, and possible pathological bases for the predictive role of each parameter are proposed.
1. Introduction
Atrial fibrillation (AF) is a supraventricular arrhythmia characterized by uncoordinated atrial electrical activation leading to ineffective atrial contraction and is the most common sustained pathological cardiac arrhythmia [1].
The estimated prevalence of AF in adults is 2–4% with an expected rise of at least two-fold by 2060 [2]. The prevalence of AF varies with sex and increases significantly with age, with those aged 80 or older having an estimated prevalence of 10–17% [3]. The lifetime risk of AF was estimated to be 25%, but this has now increased to 37% among adults over the age of 55 [1].
Whilst not inherently considered a life-threatening arrhythmia, the hemodynamic and thromboembolic complications of AF can lead to significant morbidity and mortality. Individuals with AF have a five-fold increased risk of stroke, and about 30% of embolic strokes of undetermined source (ESUS) are attributed to AF [4,5,6]. Moreover, AF appears to potentiate the impact of individual conditions, with the presence of AF post myocardial infarction being associated with greater mortality with a hazard ratio (HR) of 3.37 (95% confidence interval [CI]: 3.37–4.21) [7]. Furthermore, greater fatality and morbidity post-stroke were also seen in the European Community Stroke Project, where 33% of AF patients died within three months compared to 20% of those without AF [8].
While permanent AF is straightforward to identify on an electrocardiogram, paroxysmal AF (pAF) is considerably more difficult, especially in asymptomatic individuals. However, the risk of thromboembolic complications is considered the same in both conditions [9]. Thus, the detection of pAF is just as important, but is difficult if it is asymptomatic or infrequent. The aim, therefore, is to identify patients either prior to the onset of AF or early after the first paroxysm (even if asymptomatic) and risk stratify even asymptomatic patients for future AF development.
Multiple studies have suggested that AF occurs in the context of both electrical and anatomical abnormalities of the atria [10,11,12]. The 12-lead surface electrocardiogram (ECG) represents an easy, non-invasive approach to identify parameters that may represent electro-anatomical abnormalities that may either predict future AF or represent a pre-AF phenotype.
Developing a primary prevention approach to AF by identifying high-risk patients could potentially help with early identification of AF and appropriate therapy initiation, thereby reducing hospitalizations, AF-associated stroke incidence and the associated healthcare costs.
There are a number of potential markers of risk of AF development, including demographic, co-morbidity, electrocardiographic and echocardiographic data. We wished to focus on the electrocardiographic predictors of AF development in this systematic review.
2. Methods
We searched PubMed and Embase using three different keyword strings encompassing ‘atrial fibrillation’, ‘AF’, ‘atrial flutter’, ‘electrocardiography’, and ‘predictor’ for articles from inception to 31 August 2021. Eighteen thousand nine hundred and twenty-one records were identified and independently screened. One thousand six hundred and thirty-nine were identified by title as being possibly relevant to the review and underwent review of the full text or abstract. A total of 132 full text articles were assessed for eligibility and 115 were included in this section (Figure 1). Articles without a clear definition of how AF was detected were excluded. Studies examining predictors of AF recurrence in the post-operative setting were also excluded. Additionally, studies looking at the prevalence or incidence of AF only were also excluded.
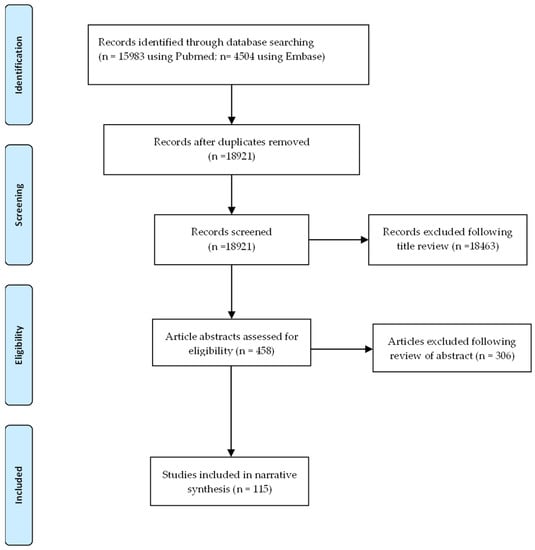
Figure 1.
PRISMA flow diagram of the study selection process.
3. Results
The 12-lead surface ECG is helpful not only in the diagnosis of AF but also in identifying parameters associated with an increased predictive value for subsequent AF detection [13]. These parameters can be conveniently divided into those that are atrial (Table 1 and Table 2) and those that are ventricular (Table 3).

Table 1.
Atrial conduction parameters predictive of atrial fibrillation.
3.1. Atrial Indices
When considering atrial indices, they can be further divided into those that reflect conduction abnormalities, morphological abnormalities and mixed parameters.
3.1.1. Atrial Conduction Parameters
P-Wave Duration and Partial Interatrial Block
P-wave duration represents the total time taken for a sinus impulse to propagate throughout the atria and is a surrogate for both intra- and interatrial conduction time. It is one of the most examined atrial indices, with respect to its predictive potential for AF. Prolongation of p-wave duration correlates with a slower conduction velocity within the atria, suggestive of atrial fibrosis, which could explain the association seen between prolonged p-wave duration and AF [77]. Differences in p-wave duration seen across leads can be a function of either differences in conduction velocities in different areas of the atria or of marked asymmetry of the atria themselves [41].
From a 12-lead ECG perspective, p-wave duration is measured from the first vertical deviation from the baseline (either upward or downward) to the return to baseline (Figure 2).
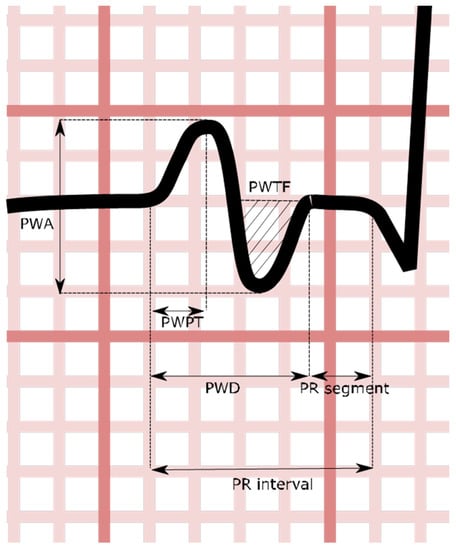
Figure 2.
Stylized V1 p-wave demonstrating certain atrial indices. PWA: p-wave amplitude; PWPT: p-wave peak time; PWD: p-wave duration; PWTF: p-wave terminal force.
Partial interatrial block (P-IAB) is a parameter defined in the literature as a p-wave duration greater than 120 ms. It is thought to reflect the precursor state of atrial fibrosis [78]. In view of the overlap with p-wave duration studies, for the purposes of this review, partial interatrial block studies have been combined with p-wave duration studies.
The literature refers to different measures of p-wave duration, including the minimum, maximum and dichotomous cut offs and mean or median p-wave duration across the 12 leads (Table 1).
A total of 37 studies looked at the predictive potential of p-wave duration or P-IAB, with respect to subsequent AF detection (Table 1). Nine large cohort studies across general populations were conducted. Considering ESUS patients, there have been three studies that primarily examined this group, whilst one included ESUS patients. The other 24 were smaller studies.
Eight studies were case-control studies. In the non-case-control studies, the majority utilized either 12-lead ECG, Holter monitoring or medical record analysis for the identification of the development of AF. Only three studies used any form of continuous monitoring, two used device-based intracardiac electrograms (EGM) [38,45] and one used an ILR-based study [14].
Across the nine large cohorts, eight showed an association between PR duration ≥ 120 ms and the subsequent detection of AF. The one study that did not replicate this finding was based on the PROSPER study for older adults, which considered p-wave duration as a continuous variable and did not demonstrate a statistically significant increase in the risk of AF for 20 ms incremental increases in p-wave duration [39].
Of the four studies that looked specifically at ESUS, no statistically significant risk ratio was demonstrated.
If studies that used a dichotomous cut off of 120 ms are considered, ten out of the fourteen studies suggested a predictive role for PR > 120 ms, detecting subsequent AF. If a more prolonged duration is considered, there appears to be a stronger predictive role, with Nielsen et al.’s analysis of the Copenhagen ECG study suggesting an odds ratio (OR) of 2.06 compared to 1.50 and with PR durations of >130 ms rather than >120 ms [30], whilst Edenborn’s assessment of heart failure with preserved EF (HfpEF) patients suggested an HR of 9.68 for p-wave durations over 175 ms [18].
If, instead, p-wave duration was considered a continuous variable, neither of the studies demonstrated a statistically significant risk statistic for either 10 ms or 20 ms increases in p-wave duration.
Only one study looked at short PR durations and their relationship with future AF development. This was the assessment of the Copenhagen ECG study. Here, p-wave duration < 89 milliseconds was found to have an HR of 1.60 (95% CI 1.41–1.81) for AF, compared to a reference group with a p-wave duration of 106–111 milliseconds [30].
Advanced Inter Atrial Block
Advanced interatrial block (A-IAB) further stratifies prolonged p-wave duration according to inferior lead p-wave morphology. It is defined as p-wave duration of ≥120 ms plus biphasic morphology in the inferior leads [78]. Pathological studies have related A-IAB to the presence of atrial fibrosis [79].
Fifteen studies investigated the association between the presence of A-IAB and AF (Table 1). Out of these, five studies looked at the presence of A-IAB or P-IAB and its association with AF. There were three large cohorts that investigated this association, namely two retrospective cohorts that included Finnish adults and primary care patients and a prospective cohort that involved ARIC participants [16,20,57]. One study specifically looked at patients with ESUS, whilst a different one included patients with ESUS, palpitations and syncope [14,51]. The remaining studies were small cohorts looking at specific sub-groups.
With regards to the methods of detection, three studies utilized prolonged continuous monitoring: one by ILR and the others by a pacemaker with different cut off values for AF duration, ranging from AHRE ≥ 30 s to >5 min. The rest used different methods, including ECG, Holter and the documentation of AF in medical records or according to ICD codes.
Data in the literature are largely consistent with regards to A-IAB. Out of the fifteen studies, thirteen reported that the presence of A-IAB was associated with AF, whilst only two studies failed to demonstrate such an association. The first one included 165 patients with chronic kidney disease (CKD) at stage 4 or 5, while the second one involved 240 healthy Italians aged 25–79 years [17,22].
The results from the three large cohorts were consistent, demonstrating a positive association between A-IAB and AF detection with an HR of up to 3.38 (95% CI 2.99–3.81) [16,20,57]. Similarly, among patients with ESUS, A-IAB appeared to be an independent predictor of AF [14,51].
Additionally, it worth noting that in 2018, Tse et al. conducted a meta-analysis to investigate whether IAB predicts new onset AF or AF recurrence. They included 16 studies and a total of 18,204 patients. They demonstrated that A-IAB was a significant predictor of new onset AF with a pooled HR of 2.58 (95% CI 1.35–4.96). However, the risk of new onset AF did not reach statistical significance for P-IAB [80].
Other P-Wave Duration Parameters
Perez et al. investigated the usefulness of p-wave index, defined as the standard deviation (SD) of p-wave durations. P-wave index > 35 ms was predictive of AF with an HR of 1.70 (95% CI 1.15–1.56) across a general population of over 40,000 individuals [41].
Two different groups examined the role of p-wave onset and p-wave peak. Both studies found a positive association with AF [25,60]. However, no association was seen between prolonged maximum p-wave peak and p-wave end [25].
P-Wave Dispersion
P-wave dispersion (PWD) represents the difference between the maximum and minimum p-wave duration on a 12-lead ECG. It is felt that different p-wave durations reflect regional delays in atrial depolarization and are a result of inhomogeneous and discontinuous atrial conduction due to anisotropic distribution of conduction between atrial myocardial fibers [81,82]. These regional delays may potentially act as a substrate for AF. Whilst less well studied than p-wave duration, there have been a number of smaller studies looking at its role in the development of AF.
Seventeen studies looked at PWD and its association with AF (Table 1), including the large cohort study by Perez et al., looking at over 40,000 patients who had had an ECG for any indication [41]. Two other studies specifically looked at patients with strokes, either acute ischemic strokes or ESUS [19,36]. The remaining studies were small cohorts looking at specific sub-groups or small case-control studies.
Two studies used prolonged continuous detection methods: one used an external loop recorder and the other used a pacemaker. The rest used a combination of inpatient monitoring, Holter monitoring, ECG and medical records and various criteria for AF duration. Six studies employed case-control methodologies, whereby patients were stratified by the presence of AF.
Thirteen studies reported that increased PWD was associated with AF, whilst four studies did not find an association. No studies suggested that increased PWD reduced the risk of AF.
The only large cohort study, which enrolled 42,571 patients, showed that a PWD > 80 ms had an HR of 1.95 (95% CI 1.70–2.30) when adjusted for age and sex, but not in multivariable analysis [41]. Considering patients from smaller cohorts, increased PWD demonstrated a positive association with AF in ten studies and no association in four. Both studies looking at stroke showed a positive association [19,36].
Of note, there was one study that reported an assessment of PWD, but on closer assessment of the text, the definition used was very different to that of the above-mentioned studies. PWD was defined as p-wave duration divided by p-wave vector magnitude (Pvm) (calculated by the square root of the sum of the squared p-wave magnitudes in leads V6, II and half of the p-wave amplitude in V2). This approach was based upon Kors’ quasi-orthogonal transformation [83]. They found that this parameter was associated with AF, with an HR of 2.02 (p = 0.010) [26].
PR Interval
The PR interval represents the time taken for an electrical impulse to be transmitted from the sinus node through the atrioventricular node to the Purkinje fibers. On the 12-lead ECG, this is measured from the time of p-wave onset to the initiation of the QRS segment. Both prolonged and short PR intervals have been associated with AF. Suspected degenerative alterations of the myocardium and the conduction system causing prolongation of PR interval [76] might explain the association between prolonged PR interval and AF, while the association of a short PR interval might be attributed to genetics, as both the genetic loci responsible for either shortening or prolonging the PR interval were associated with an increased risk of AF [84].
Twenty-one studies looked at the association between PR interval and AF (Table 1). Of these, 19 investigated the association between prolonged PR interval and AF, whilst two also investigated the association between short PR interval and AF. As described in Table 1, different groups used different measurements, including PR interval in lead II, maximum PR interval, median or mean PR interval across 12 leads. Ten studies looked at this association in large cohorts, three in ESUS, with the rest in subgroups.
With regards to the method of detection, only three studies used prolonged monitoring by an ILR with an AF duration cut off of 30 s. The remaining studies used different methods, mainly ECG, Holter monitoring and the documentation of AF in medical records or based on ICD codes.
Ten studies considered PR interval as a continuous variable and seven considered it to be dichotomous with a cut off value of 196 ms, defining a prolonged PR interval with values ranging from 120–129 ms for a short PR interval. The remaining four used PR interval as both a continuous and dichotomous variable.
Twelve studies showed that prolonged PR interval either as a continuous or dichotomous variable is associated with the development of AF. Seven studies failed to demonstrate such an association. Two studies showed different results according to whether PR interval was used as a continuous or dichotomous variable.
Considering participants from large cohorts, six showed a positive association between prolonged PR interval and AF, two did not show an association and two showed that increasing PR interval (per 1 SD) was associated with AF, but not when used as a dichotomous variable. Amongst studies in the ESUS population, two did not demonstrate any association between prolonged PR interval and AF, whilst data from CRYSTAL AF demonstrated that for every 10 ms increase in PR interval, the HR for AF detected by ILR was 1.30 (95% CI 1.20–1.40) [69]. Out of the eight studies examining different subgroups, such as patients with ESUS and chronic kidney disease (CKD), four showed a positive association, whilst four did not find a significant association.
Additionally, a meta-analysis performed in 2014 showed that amongst 328,932 individuals from prospective cohorts, prolonged PR interval was associated with AF with a pooled HR of 1.30 (95% CI 1.13–1.49) [85].
One small study looked at the utility of PR variation defined as PR interval maximum–minimum in patients with >100 supraventricular ectopics (SVEs) per day. Amongst 207 patients, greater PR variation was associated with AF detected by ECG or Holter monitor [67].
Two studies investigated the role of short PR interval in predicting AF. The Busselton Health Study showed a positive association for PR interval < 120 ms [73], whereas the Copenhagen ECG study showed this was only significant in women [75].
From the ARIC cohort, Smith et al. looked at the PR segment, defined as the time between the end of the p-wave and the start of the QRS complex, and found that PR segment prolongation was independently associated with subsequent AF detection [25].
3.1.2. P-Wave Morphological Parameters
P-Wave Axis
P-wave axis, a routinely reported measure on ECG represents atrial electrical activity. Abnormalities in this parameter are reflective of atrial pathology and possibly associated with an increased risk of AF development [86]. Mechanical and metabolic insults to the atria induce remodeling and abnormal electrical conduction, which results in abnormal p-axis and ultimately leads to AF [87,88].
P-wave axis is one of the better studied morphological p-wave features. There have been six studies assessing the relationship between p-wave axis and AF, all of them suggesting a positive predictive role of the abnormal p-wave axis and the subsequent development of AF (Table 2). A recent meta-analysis identified a pooled risk ratio of 2.12 for abnormal p-wave axis, and future AF detection from a total of 78,222 patients [89].
There have been four large retrospective studies looking at the ARIC, CHS and ACCORD populations and a general population of patients undergoing ECG. All four of these studies demonstrated a positive relationship between p-wave axis and the development of AF. One study looked specifically at p-wave axis amongst the population of ESUS patients, in which an abnormal p-wave axis was associated with an OR of 3.31 (95% CI 1.49–7.35) for AF [19].

Table 2.
Atrial morphological parameters predictive of atrial fibrillation.
Table 2.
Atrial morphological parameters predictive of atrial fibrillation.
| Authors, Year | Population (Size) | Study Type | Parameter Definition | Result | AF Detection |
|---|---|---|---|---|---|
| P-wave axis | |||||
| Dhaliwal et al., 2020 [90] | ACCORD (8965) | Retrospective | 0–75°—normal | HR 2.65 (95% CI 1.76–3.99) | ECG |
| Acampa et al., 2019 [19] | Cryptogenic stroke (222) | Prospective | 0–74°—normal | OR 3.31 (95% CI 1.49–7.35) | 7-day Holter |
| Maheshwari et al., 2017 [91] | ARIC population (15,102) | Retrospective | 0–75°—normal | RR 2.34 (95% CI 2.12–2.58) | ECG, Medical records |
| Rangel, O’Neal, and Soliman, 2016 [88] | CHS (4272) | Retrospective | 0–75°—normal | HR 1.17 (95% CI 1.03–1.33) | ECG, medical records |
| Hayashi et al., 2014 [65] | P-pulmonale (591) | Retrospective | <74°—normal | HR 2.55 (95% CI 1.20–5.41) | ECG |
| Perez et al., 2009 [41] | Patients that had an ECG for usual indications (42,751) | Retrospective | Not defined | HR 1.90 (95% CI 1.60–2.40) | ECG |
| P-wave terminal force | |||||
| Kreimer et al., 2021 [14] | ILR (366) | Retrospective | ≤−4000 µV·ms | HR 5.30 (95% CI 3.25–8.64) | ILR AF ≥ 30 s |
| Lehtonen et al., 2018 [21] | Hypertensives (665) | Retrospective | ≤−4 mV·ms | HR 0.85 (95% CI 0.66–1.09) | Medical records |
| Cortez et al., 2017 [26] | Ischemic stroke patients from LSR (n = 227) | Prospective | ≥0.04 mm·s | HR 1.00 (95% CI 1.00–1.00) | ECG |
| Goda et al., 2017 [92] | Ischemic stroke (226) | Retrospective | Per 0.01 mm·s | OR 1.61 (95% CI 1.24–2.09) | Inpatient monitoring |
| Sugiyama et al., 2017 [93] | Acute ischemic stroke (105) | Prospective | Continuous | OR 1.46 (95% CI 1.02–2.08) | 24 h Holter |
| Rasmussen et al., 2017 [94] | Copenhagen Holter study (678) | Prospective cohort | >4000 | HR 0.86 (95% CI 0.52–1.41) | ECG, inpatient monitoring, medical records |
| Baturova et al., 2016 [95] | Ischemic stroke with (55) and without AF (110) (165) | Case control | >40 mm·ms | OR 4.04 (95% CI 1.34–12.14) | Case control |
| Magnani et al., 2015 [29] | FHS (3110) ARIC (8254) | Prospective cohort | >4000 μV·ms | HR 1.00; 95% CI 0.71–1.40 HR 1.56; 95% CI 1.24–2.00 | Medical records |
| Francia et al., 2015 [31] | Hypertensive (88) | Case-control | Continuous | HR 1.03 (95% CI 0.91–1.15) | ECG, Holter |
| Kamel et al., 2014 [96] | 45–84 (6751) | Prospective cohort | Per 1 SD | HR 1.11 (95% CI 1.03–1.21) | ECG |
| Eranti et al., 2014 [97] | Middle-aged subjects (35–41 years) (10,647) | Prospective | ≥0.06 mm·s | HR 1.91 (95% CI 1.34–2.73) | Medical records |
| Nishi et al., 2013 [98] | Hemodialysis (299) | Retrospective | ≥0.04 mm·s | HR 4.89 (95% CI 2.54–9.90) | ECG |
| Hayashi et al., 2014 [65] | P-pulmonale (591) | Retrospective | Med free + >77 µV·ms | HR 2.22 (95% CI 0.70–8.31) | ECG |
| Soliman et al., 2009 [40] | General population (15,429) | Prospective cohort | >95th percentile | HR 1.22 (95% CI 1.14–1.31) | ECG |
| P-wave amplitude | |||||
| Yoshizawa et al., 2014 [34] | General population (136) | Retrospective | II | p = 0.032 p = 0.001 | ECG |
| Kreimer et al., 2021 [14] | Patients undergoing ILR for syncope, palpitations, ESUS ILR (366) | Retrospective | II < 0.1 mV | HR 2.11 (95% CI 1.30–3.44) | ILR |
| Altunkeser et al., 2003 [46] | Patients with structural heart disease and LAD ≤ 5.0 cm with AF (n = 37) and without AF (n = 38) (75) | Case control | P-wave amplitude max P-wave amplitude min P-wave dispersion (amplitude) | p < 0.001 NS in multivariable analysis p < 0.01 | Case-control study |
| Other morphological parameters | |||||
| Lentz et al., 2019 [99] | Patients on ibrutinib (168) | Retrospective | (1) Lead II-bifid p-wave, with 40 ms between peaks for ≥ 2.5 mm wide ≥ 100 msec in duration, (2) Lead V1-biphasic p-wave with terminal portion ≥ 40 msec in duration or terminal portion ≥ 1 mm deep or (3) PR interval ≥ 200 msec (intra-atrial conduction delay) | HR 5.40 (95% CI 1–9–15.4) | ECG, medical records |
| Hayashi and Horie, 2015 [32] | Patients with biphasic p-wave in lead II (141) | Retrospective | Amplitude of initial p-wave portion in lead II ≥ 73 (μV) Amplitude of terminal p-wave portion in lead III ≥ 48 (μV) Duration of initial p-wave portion in lead III ≥ 71 (ms) | HR 1.22 (95% CI 0.50–2.88) HR 1.60 (95% CI 0.68–3.72) HR 2.90 (95% CI 1.16–7.11) | ECG |
| van Diepen et al., 2010 [100] | Patients on pexelizumab with (315) and without AF (315) (630) | Case-control | M-shaped, W-shaped, irregular or notched p-waves | OR 1.68 (95% CI 1.03–2.73) | Case control (ECG, medical records) |
| Compound conduction and morphological parameters | |||||
| Rasmussen et al., 2020 [15] | Copenhagen Holter study (632) | Retrospective | P-wave area/duration index | HR 2.80 (95% CI 1.64–4.79) | ECG, inpatient monitoring |
| Tse et al., 2020 [101] | Mitral stenosis (59) | Retrospective | Mean p-wave area in V3 | OR 1.08 (95% CI 1.01–1.16) | 2 ECGs (persistent or permanent AF) |
| Hellman et al., 2020 [17] | CKD 4/5–non-dialysis (165) | Prospective | PWD ≥ 120 ms in lead II ± > 1 biphasic p-waves in leads II, III or aVF; or duration of terminal negative portion of p-wave > 40 ms or depth of terminal negative portion of p-wave > 1 mm in lead V1 | Not significant | ECG, 24 Holter |
| Soliman et al., 2009 [40] | ARIC participants (15,429) | Prospective cohort | Maximum p-wave area Mean p-wave area | HR 1.13 (95% CI 1.05–1.23) HR 1.11 (95% CI 1.02–1.20) | ECG |
| De Bacquer, Willekens, and De Backer, 2007 [102] | 55–74 years old with AF (40) and age-matched and gender-matched controls (120) | Nested case control | Maximum p-wave duration and notched or deflected p-wave morphology | OR 13.4 (95% CI 3.30–46.60) | Case control |
ARIC, atherosclerosis risk in communities; ACCORD, Action to Control Cardiovascular Risk in Diabetes; CHS, Canadian Health Study; CABG, coronary artery bypass graft; MADIT, multicenter automatic defibrillator implantation trial; PPM, permanent pacemaker; PWD, p-wave duration; SND, sinus node disease; RR; HR, hazard ratio; OR, odds ratio; IPN, interpeak notch.
P-Wave Terminal Force
P-wave terminal force (PTFV1) has garnered significant interest as a possible predictor of AF. PTFV1 is the duration of the terminal (negative) part of the p-wave in lead V1 multiplied by the depth. If the p-wave terminal part is positive, then the interval extending from the first notch to the wave end must be considered [103] Commonly, it is considered abnormal when it is greater than 0.04 μV·ms, which is considered a marker of LA abnormality or enlargement [103,104].
One of the most pertinent criticisms of its use came from Jaroszynski et al. [105], who argued that it was particularly susceptible to lead position variation.
PTFV1 has been examined in 16 separate primary studies (Table 2), 12 of which were summarized in Huang et al.’s 2020 meta-analysis. This demonstrated a pooled odds ratio of 1.39 (95% CI 1.08–1.79) [104].
Considering the sixteen primary studies, five of them did not demonstrate a significant predictive role of PTFV1 in the prediction of AF. Four studies examined PTFV1 specifically in ischemic stroke, all of them demonstrating a positive result.
Only one study utilized continuous ILR monitoring for AF identification, while the rest utilized a mix of ECG, Holter monitoring or patient records [14].
P-Wave Amplitude
P-wave amplitude refers to the height of the p-wave in different ECG leads. Different groups have assessed its role as a predictor of AF by considering p-wave amplitude in different leads.
There have been four studies looking at its utilization as a predictor for AF (Table 2), all of which have suggested that increased p-wave amplitude is associated with AF detection. Only one study demonstrated that maximum p-wave amplitude, but not minimum p-wave amplitude, was significant [46].
Other P-Wave Morphological Parameters
There have been other p-wave morphological parameters studied in three small cohorts, as described in Table 2. The parameters are varied and use composite measures based on the shape of the p-wave in different leads. The exact parameter definition in each paper is summarized in the table. These have shown promising results for the possible prediction of AF, but more research is required, especially in larger general populations.
Compound Conduction and Morphological Parameters
There have been a number of studies that have combined p-wave conduction and morphology parameters. Generally, these have been smaller studies looking at populations that include individuals with mitral stenosis and non-dialysis CKD4/5 patients; however, there was a larger study that looked at p-wave area across 15,429 patients. In the two studies that examined p-wave areas, the mean area in lead III, as well as the overall mean and maximal p-wave areas, have all shown promise as possible AF predictors.
3.2. Ventricular Parameters
It is conceptually more difficult to associate changes in ventricular electrocardiographic parameters with a pre-AF or AF risk phenotype. Nevertheless, a number of studies have shown relationships between specific parameters and the risk of developing AF (Table 3).

Table 3.
Ventricular parameters predictive of atrial fibrillation.
Table 3.
Ventricular parameters predictive of atrial fibrillation.
| Author (Year) | Population and Size | Study Type | Parameter Definition | Result | AF Detection |
|---|---|---|---|---|---|
| Left Ventricular Hypertrophy | |||||
| Lehtonen et al., 2018 [21] | Hypertensive (2665) Non-hypertensive (3148) (5813) | Retrospective | Sokolov criteria Cornell | HR 1.51 (95% CI 1.14–2.01) HR 1.26 (95% CI 0.94–1.69) | Medical records |
| Patel et al., 2017 [106] | CHS (4904) | Retrospective | Minnesota | HR 1.50 (95% CI 1.18–1.90) | ECG |
| Chrispin et al., 2014 [107] | MESA (4942) | Retrospective | Sokolov product Cornell Framingham adjusted Cornell Minnesota Lewis Gubner and Ungerleider Sokolow voltage Cornell product Romhilt-Estes Perugia | HR 1.83 (95% CI 1.06–3.14) HR 1.36 (95% CI 0.72–2.58) HR 1.36 (95% CI 0.76–2.58) HR 1.26 (95% CI 0.76–2.08) HR 0.72 (95% CI 0.47–1.11) HR 1.02 (95% CI 0.62–1.68) HR 1.37 (95% CI 0.92–2.07) HR 1.69 (95% CI 0.94–2.31) HR 1.48 (95% CI 0.64–3.39) HR 1.35 (95% CI 0.79–2.28) | Medical records |
| Knuiman et al., 2014 [73] | Busselton Health Study participants (4267) | Prospective | LVH Minnesota code | HR 0.33; 95% CI 0.08–1.33) | ICD codes |
| Macfarlane et al., 2011 [39] | Older patients on pravastatin (5804) | Retrospective | LVH Minnesota code Definite Probable Possible | HR 2.13 (95% CI 1.38–3.28) HR 2.21 (95% CI 1.49–3.28) HR 1.30 (95% CI 1.03–1.64) | ECG |
| Perez et al., 2009 [41] | Patients that had an ECG for usual indications (42,751) | Retrospective | LVH Romhilt Estes criteria | HR 1.30 (95% CI 1.00–1.70, p = 0.046) | ECG |
| Watanabe et al., 2006 [108] | Niigata study (63,386) | Retrospective | LVH Sokolov- Lyon criteria | OR 1.39 (95% CI 1.1–1.75) | ECG |
| QT interval | |||||
| Patel et al., 2018 [109] | CHS (4181) | Retrospective | Prolonged > 95th percentile Per 1-SD increase | HR 1.50 (95% CI 1.20–1.88 HR 1.07 (95% CI 1.01–1.13 | ECG, medical records |
| Lehtonen et al., 2018 [21] | Hypertensive (2665) Non-hypertensive (3148) (5813) | Retrospective | 1 SD increment in QTc (Bazzet’s) Prolonged QTc > 450 ms (men), >460 ms (women) | HR 1.11 (95% CI 1.01–1.22) HR 1.26 (95% CI 0.78–2.03) | ECG |
| Nguyen et al., 2016 [110] | CHS (4696) | Retrospective | Prolonged QTc (Framingham) | HR 2.50 (95% CI 1.40–4.30) | ECG, medical records |
| Baturova et al., 2016 [95] | Ischemic stroke patients with AF (55) and without AF (110) (165) | Retrospective | QTc (Bazzet’s) | NS in multivariable analysis | Case control |
| Hoshino et al., 2015 [111] | Stroke (972) | Retrospective | QTc (per 10 ms increase) | OR 1.41 (95% CI 1.24–1.61) | Inpatient monitoring, 24 h Holter |
| Baturova et al., 2015 [112] | Ischemic stroke with (454) | Retrospective | QTc (Bazzet’s) | NS in multivariable analysis | ECG, medical records |
| Hayashi et al., 2014 [65] | Patients with p-pulmonale (591) | Retrospective | QT interval > 353 ms | HR 0.89 (95% CI 0.34–2.31) | ECG |
| Shulman et al., 2015 [70] | African American, Hispanic and non- Hispanic white (n = 50870) | Retrospective | QTc (per 10 ms increase) | HR 1.00 (95% CI 1.00–1.01, p < 0.001) | ECG |
| Mandyam et al., 2013 [113] | ARIC (14,538) + CHS (4745) + 2396 (Health ABC) | Retrospective | 10 ms increase in QTc (Framingham) | HR 2.05 (95% CI 1.42–2.96) | ECG, medical records |
| Nielsen et al., 2013 [114] | Copenhagen (281,277) | Retrospective | QTc ≤ 372 ms QTc ≥ 464 ms QTc ≥ 458 ms | HR 1.45 (95% CI 1.14–1.84) HR 1.44 (95% CI 1.24–1.66) HR 2.32 (95% CI 1.52–3.54) | Medical records |
| Macfarlane et al., 2011 [39] | Older patients on pravastatin (5804) | Retrospective | Prolonged QTc (Hodges) (per 30 ms increase) | HR 1.21 (95% CI 1.11–1.32) | ECG |
| QRS duration | |||||
| Patel et al., 2018 [109] | CHS (4181) | Retrospective | Prolonged Per 1-SD | HR 1.00 (95% CI 0.77–1.30) HR 0.99 (95% CI 0.94–1.06) | ECG, medical records |
| Aeschbacher et al., 2018 [115] | ARIC (15314) | Retrospective | QRS 100–119 ms QRS ≥ 120 ms Per 1-SD increase | HR 1.13 (95% CI 1.02–1.26) HR 1.35 (95% CI 1.08–1.68) HR 1.11 (95% CI 1.07–1.15) | ECG, medical records |
| Cortez et al., 2017 [26] | Ischemic stroke patients from LSR (227) | Prospective | QRS duration (continuous) | HR 1.01 (95% CI 1.00 to 1.02, p = 0.354) | ECG |
| Baturova et al., 2015 [112] | Ischemic stroke (454) | Retrospective | QRS duration (continuous) | HR 1.02 (95% CI 1.00–1.03) | ECG, medical records |
| Shulman et al., 2015 [70] | African American, Hispanic and non-Hispanic white (50,870) | Retrospective | QRS duration (per 10 ms increase) | HR 1.00 (95% CI 1.00–1.00; p = 0.092) | ECG |
| Macfarlane et al., 2011 [39] | Older patients on pravastatin (5804) | Retrospective | QRS (per 20 ms) | HR 1.07 (95% CI 0.98–1.16; p = 0.14) | ECG |
| El-Chami et al., 2010 [116] | ADVANCENT (25,268) | Retrospective | QRS duration (continuous) | OR 1.20 (95% CI 1.14–1.25) | Medical records |
| LBBB, RBBB, LAFB | |||||
| Uhm et al., 2020 [117] | Patients that had ECG (n = 107,838) | Retrospective | NIVCD ≥ 110 ms | HR 2.57 (95% CI 1.07–6.16) | ECG, medical records |
| Nguyen et al., 2016 [110] | CHS (4696) | Retrospective | LAFB | HR 2.10 (95% CI 1.10–3.90) | ECG, medical records |
| Frontera et al., 2015 [71] | ILR implanted for syncope or palpitations (n = 200) | Retrospective | LBBB | OR 1.05 (95% CI 0.18–4.70) | ILR AF > 30 s |
| Frontera et al., 2015 [71] | ILR for syncope or palpitations (n = 200) | Retrospective | RBBB iRBBB | OR 3.60 (95% CI 0.84–14.99) OR 9.04 (95% CI 1.40–10.24) | ILR AF > 30 s |
| Knuiman et al., 2014 [73] | Busselton Health Study participants (n = 4267) | Prospective | LBBB | HR 1.84 (95% CI 0.90–3.74) | ICD codes |
| Perez et al., 2009 [41] | 42,751 | Retrospective | LBBB | HR 1.70 (95% CI 1.20–2.50) | ECG |
| Watanabe et al., 2006 [108] | Niigata study (63,386) | Retrospective | LBBB | OR 0.98 (95% CI 0.13–7.23; p = 0.98) | ECG |
| Watanabe et al., 2006 [108] | Niigata study (63,386) | Retrospective | RBBB | OR 0.84 (95% CI 0.46–1.53) | ECG |
| Fragmented QRS | |||||
| Hellman et al., 2020 [17] | CKD 4/5—non-dialysis (165) | Prospective | Notched R or S wave or the presence of ≥1 additional r-waves (R’) or in the presence of a wide QRS complex (>120 ms), >2 notches in R or S waves in two contiguous leads corresponding to a myocardial region, | Not significant | ECG, 24 h Holter |
| Yesin et al., 2018 [61] | STEMI (171) | Prospective | Various RSR’ patterns | OR 3.24 (95% CI 1.02–10.25) | Inpatient monitoring |
| Poor R- wave progression | |||||
| Lehtonen et al., 2018 [21] | Hypertensive (2665) Non-hypertensive (3148) (5813) | Retrospective | Poor r-wave progression | HR 1.49 (95% CI 1.01–2.20) | ECG |
| Frontal QRS-T angle | |||||
| Jogu et al., 2017 [118] | CHS (4282) | Retrospective | >Sex specific 95th percentile Per 10° increase | HR 1.55 (95% CI 1.23–1.97) HR 1.03 (95% CI 1.01–1.05) | ECG, medical records |
| ST-T segment abnormalities | |||||
| Lehtonen et al., 2018 [21] | Hypertensive (2665) Non-hypertensive (3148) (5813) | Retrospective | Negative t-wave in I and V6 Positive t-wave in aVR | HR 2.10 (95% CI 1.40–3.13) HR 3.47 (95% CI 1.16–10.34) | ECG |
| Bachmann et al., 2016 [119] | Copenhagen ECG study (138,404) | Retrospective | T peak- T end lead V5 < 5th % (58–77 ms) lead V5 < 95th % (116–140 ms) | HR 1.18 (95% CI 1.06–1.32) HR 1.09 (95% CI 0.99–1.22) | Medical records |
| Macfarlane et al., 2011 [39] | Older patients on pravastatin (5804) | Retrospective | Minnesota code 5-1 or 5-2 Minnesota code 4-1 or 4-2 See Supplementary Table S3 | HR 1.69 (95% CI 1.34–2.13) HR 1.70 (95% CI 1.32–2.20) | ECG |
| Watanabe et al., 2006 [108] | Niigata study (63,386) | Retrospective | Mild ST abnormality Severe ST abnormality | OR 1.66 (95% CI 1.13–2.43) OR 5.12 (95% CI 2.30–11.38) | ECG |
AF, atrial fibrillation; ARIC, atherosclerosis risk in communities study; CHS, Canadian Health Study; CI, confidence interval; CKD, chronic kidney disease; CRT, cardiac resynchronization therapy; ECG, electrocardiogram; HF, heart failure; HR, hazard ratio; ILR, implantable loop recorder; LAFB, left anterior fascicular block; LBBB; left bundle branch block; MESA, multi-ethnic study of atherosclerosis; ms, millisecond; NIVCD, non-specific intraventricular conduction delay; NS, non-significant; OR, odds ratio; RBBB, right bundle branch block; RWP, r-wave progression; SD, standard deviation; STEMI, ST-elevation myocardial infarction.
3.2.1. Left Ventricular Hypertrophy
Left ventricular hypertrophy (LVH) can be diagnosed from a 12-lead ECG. Different criteria exist and have been used in different studies (Supplementary Table S1).
There have been seven large cohorts assessing LVH and AF. A variety of ECG LVH scores have been assessed, but the most common ones are the Minnesota code, Sokolow–Lyon and Cornell criteria. There were five large cohort studies that assessed general populations and two large studies which used specifically older patient cohorts or hypertensive individuals.
With respect to the method of AF detection, all of the studies used a combination of ECG, or AF present on medical records or ICD codes.
Of the seven studies, four demonstrated a consistently positive predictive association between ECG-defined LVH and AF, with one not showing any association and two studies providing mixed results across the different LVH criteria.
3.2.2. QT Interval
Congenital abnormalities of the QT interval (short or long QT syndrome) are known to be associated with a high incidence of AF [120,121]. QT interval corrected (QTc) can be calculated using the Bazett, Hodges, Framingham and Fridericia formula (Supplementary Table S2). The QT interval reflects cardiac ventricular repolarization. It has been thought that the QT interval might be a marker of cardiomyocyte refractoriness [110,122].
The QT interval has had a reasonable amount of interest as a possible predictor of AF, with eleven studies examining the relationship between QT interval and AF detection. Of the six large cohort studies, five demonstrated a positive risk ratio AF.
Within the ischemic stroke population, there has been one case-control study, and two cohort studies. Only one of the cohort studies demonstrated a predictive role of the QT interval, with Hoshino et al.’s analysis of 972 stroke patients suggesting an OR of 1.41 (95% CI 1.24–1.61).
One study assessed a short QT interval [75], which was also noted to have a statistically significant predictive role in AF development.
None of the studies utilized any form of continuous monitoring, with retrospective medical record analysis and ECG assessment being the techniques used.
Zhang et al. performed a meta-analysis and found that when Bazett correction was utilized alongside a dichotomous cut off, there was a statistically significant predictive role, with a pooled HR of 1.16 (95% CI 1.09–1.24). If the QT interval was instead considered a continuous variable, each 10 ms prolongation was associated with an HR of 1.17 (95% CI 1.09–1.25).
3.2.3. QRS Duration
The QRS duration is a simple-to-measure electrocardiographic parameter defined by the duration of time between the start of the QRS complex and the end (Figure 3). With QRS duration prolongation being associated with structural heart disease, it has been suggested that it may act as a proxy for left atrial disease [115].
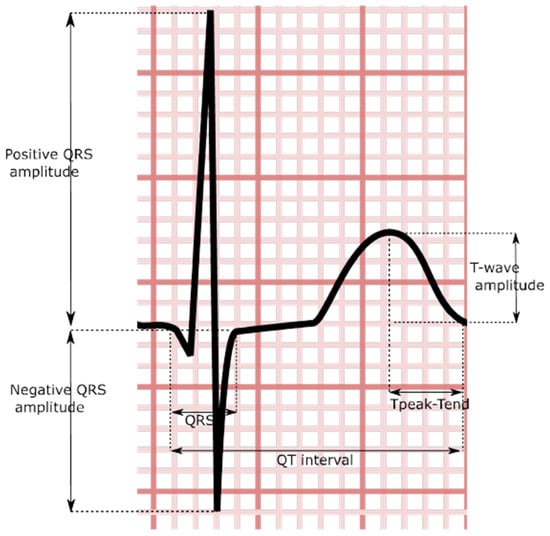
Figure 3.
Stylized QRS and t-wave demonstrating certain ventricular indices.
Seven studies have investigated the role of prolonged QRS duration as a predictor of AF. Several of these were large cohort studies. The results are somewhat variable, with four studies suggesting a minor predictive role for QRS prolongation, either as a continuous variable or with dichotomous cut offs.
In the two studies looking specifically at prediction within stroke populations, the HRs were 1.02 and 1.01. Studies utilized sporadic 12-lead ECGs and patient records for the detection of AF development.
3.2.4. Bundle Brunch Block (BBB)
Bundle branch block (BBB) is a marker of conduction disease. Autopsy reports have shown that conduction disease is due to fibrosis in the conduction system [123], which could be associated with myocardial fibrosis and might explain the rationale behind the association between AF and BBB. There is a degree of overlap between conduction disease and QRS duration prolongation. A variety of studies have looked at the presence of right (RBBB) and left bundle branch block (LBBB), left anterior fascicular block (LAFB) and non-specific interventricular conduction delay (NIVCD).
There have been six studies that look at a variety of different manifestations of conduction disease.
Only one of the three LBBB studies demonstrated a positive predictive relationship for AF. Neither of the RBBB studies suggested any association with future AF; however, interestingly, Frontera’s et al., 2015 study suggested a strong relationship between the presence of incomplete RBBB and future AF. Two studies have suggested roles for LAFB and NIVCD in AF detection.
3.2.5. QRS Fragmentation
Fragmented QRS (fQRS) is defined as the presence of various RSR patterns with or without q waves on 12-lead ECG. The presence of fQRS in ECG is a sign of delay in ventricular conduction, associated with myocardial scarring, ischemia and fibrosis [124].
Two studies have looked at fQRS in the CKD population and the STEMI population. The former was non-significant, whilst the latter suggested a possible role as a predictor, albeit in a small population.
3.2.6. Poor R-Wave Progression
One study of 5813 patients suggested a positive, but weak association between the presence of poor r-wave progression and AF, with an HR of 1.49 (CI 1.01–2.20).
3.2.7. Frontal QRS-T Angle
The interest in AF predictors has meant slightly more niche ECG parameters have been examined. The frontal QRS-T angle, representing the difference between the QRS and t-wave axis, has gained increasing interest recently as an ECG parameter, although it is not routinely measured by ECG machines. It has been studied in the context of 4282 participants within the CHS, where 1276 participants with an abnormal frontal QRS-T angle were shown to have an HR of 1.55 (95% CI 1.23–1.97) for the development of AF [118].
3.2.8. ST Segment—T-Wave Abnormalities
ST-T changes have also been linked to AF. ST segment abnormalities may reflect underlying myocardial changes, including hypertrophy and or/overload that can cause AF, but not severe enough to precipitate other cardiac diseases [108].
Four large cohort studies all demonstrated a statistically significant association between a variety of ST segment and t-wave abnormalities. One of the studies utilized the Minnesota criteria to objectively define ST segment and t-wave abnormalities (Supplementary Table S3). None of the studies utilized continuous methods of rhythm monitoring, instead relying on sporadic follow-up ECGs or retrospective assessment of medical records.
The Tpeak-Tend interval as a specific component of the ventricular repolarization waveform has also been assessed within the Copenhagen ECG study. Here, a U-shaped relationship between the parameter and detection of AF was suggested with values outside of 98–103 ms with an HR of 1.18 (95% CI 1.06–1.32) for the development of AF [119].
4. Discussion
4.1. Summary of Findings
This study fills an important gap in the current literature. There have been two previous review articles of ECG predictors of AF [13,125], the most recent of which was in 2017. Neither of these studies were systematic in their approach to identifying relevant studies, and they specifically focused on large population studies. This study provides a comprehensive analysis of the current state of the field, with consideration of smaller studies of at-risk or important populations, such as individuals who have hypertrophic cardiomyopathy or stroke.
When considering the utility of individual parameters as predictors for AF, the combination of ease of calculation, reliability and strength as a predictor are all important facets. Figure 4 provides a summary of the identified predictors. The present review has highlighted that atrial parameters are particularly useful, and there exists a reasonable amount of evidence for A-IAB, PWTFV1 and PWD as being useful AF predictors. All of these predictors require further assessment of the ECG beyond the numerical values that are calculated. P-wave axis and p-wave amplitude have both shown consistent promising results, but in a limited number of studies. Ventricular parameters were generally not as useful as predictors. Indeed, it is not clear if the predictive power of the ventricular parameters is wholly independent of the atrial parameters.
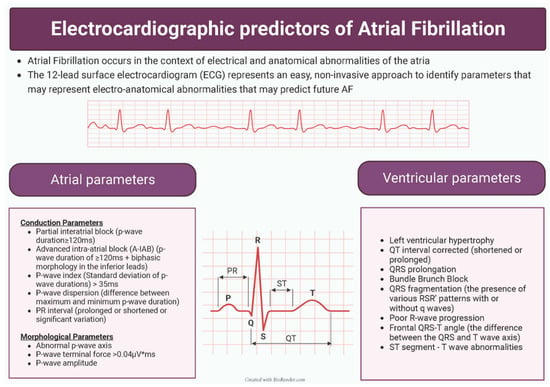
Figure 4.
Summary of identified electrocardiographic predictors of atrial fibrillation.
As alluded to by Smith et al., there is an overlap between components (different components of p-wave) [25]. Disentangling this overlap is important as it facilitates a greater understanding of the parameters that are most useful as AF predictors and potentially provides understanding regarding the mechanistic reasons as to why these parameters are useful.
The reproducibility of measurements both at a single time point and across a period of time has not been examined fully. Composite measures, such as PTFV1, have been critiqued as being particularly susceptible to lead position variation.
Table 1, Table 2 and Table 3 all demonstrate that there are a multitude of different approaches used across studies to detect AF. The most common approaches are ad hoc ECGs and Holter monitors, as well as retrospective assessment of patient notes, registry data and death certificates. These approaches have obvious limitations. The former risks missing paroxysms between recordings, whilst the latter is limited by the accuracy of coding, as demonstrated by Shah et al [126].
A limited number of studies have utilized device EGMs, which have the advantage of providing a continuous rhythm recording from the point of device implantation, and the rise of ILRs has furthered interest in this. Of note, the cut off duration for diagnosing AF was variable across these studies.
4.2. The Logistics of AF Prediction
One unstudied aspect of ECG prediction of AF is the temporal evolution of ECG parameters. It is not clear if it is the change in a parameter or the absolute value of the parameter that is critical in the development of AF. Longitudinal studies would be useful here as it would be possible to evaluate the pattern of change in a parameter (if it exists) as a predictor of AF.
The digitization of patient records and ECGs has created a particularly rich data resource. Hospital-wide ECG analysis programs already exist, whereby any individual who undergoes an ECG is specifically screened for AF. With the advent of machine learning and artificial intelligence, more sophisticated screening approaches could be used, utilizing some of the above identified parameters, mainly p-wave indices, to help identify patients at risk of developing AF at the earliest possible stage.
4.3. The Role of AF Prediction
AF is endemic within the older population and is associated with significant morbidity and mortality. Its early prediction could offer several possible avenues for further management.
If it is not possible to prevent the development of AF, avoiding its consequences, including stroke, would also be of significant interest. Given the simplicity of administration and improved safety profile of direct oral anticoagulants, targeted use of anticoagulation in high-risk groups could potentially help reduce the incidence of stroke. Indeed, within the ESUS group, AF prediction could be used to target those patients who would benefit most from targeted longer-term cardiac monitoring approaches, or empirical anticoagulation.
4.4. Multi Dimension Risk Prediction
Combining ECG parameters may help to maximize AF prediction. This was neatly demonstrated by both Alexander et al. and Yoshizawa et al [34,127]. The former used a morphology–voltage–p-wave duration-based risk model, which had an OR of 2.1 and 2.4 for the intermediate and high-risk groups, respectively, based on a cohort of 676 patients undergoing coronary angiography. The latter used a p-wave amplitude in II and V1 and a p-wave dispersion-based score, with less promising results.
Of course, the 12-lead ECG is not the only parameter which provides data for AF risk. Much work has been conducted on biochemical, Holter, clinical and echocardiographic parameters to aid in AF prediction [68,128,129,130]. Creating a multi-dimension model of risk prediction would provide a more holistic and hopefully accurate model for stratifying AF risk. This could be valuable in the stroke population not only in targeting populations that may benefit the most from invasive monitoring, but also creating stroke primary prevention strategies.
4.5. The Role of Artificial Intelligence and Consumer-Facing Devices in AF Prediction
The rise of artificial intelligence (AI) technologies and consumer-facing wearable devices are providing exciting new avenues for AF prediction. Groups from America [131] and Sweden [132] have created machine learning algorithms for the prediction of AF based on a 12-lead ECG and a single-lead ECG, respectively. AI-based models have been shown to have a comparative performance to conventional risk scores, such as the CHARGE-AF score, without the need for significant data extraction [133]. The utilization of feature visualization techniques has yielded analysis of AI-based algorithms to identify which areas the algorithms focus on for AF prediction. Unsurprisingly, algorithms appear to focus on the p-wave for AF prediction, although there also appears to be a contribution from the initial component of the QRS complex [134]. The primary limitation of AI-based algorithms, similar to any AF prediction approach, remains the provenance of the data input and the approach to AF identification. Highly curated ILR-based datasets remain uncommon, with AF diagnoses for training datasets usually based on medical record analysis. Moreover, input data require individuals to have had an ECG at some point, and thus they may not provide a full representation of a general population.
Consumer-facing wearable devices have provided the potential for data from wider cohorts to be assessed, as well as for longitudinal analyses to be performed. Whilst not applied to AF as of yet, the Mayo group have demonstrated the utility of AI assessment of smart watch data to predict left ventricular dysfunction [135]. Algorithms that can work across the different modalities of consumer-facing devices will be of particular use, given the growing number of devices that are available to both consumers and physicians.
This does raise the question as to whether there remains a role for conventional analysis of ECG parameters. As mentioned, identification of key ECG parameters that predict future AF may help facilitate improved understanding of the pathogenesis of AF, and this process may be aided by feature visualization of AI algorithms.
5. Conclusions
We have systematically reviewed evidence for the use of different surface ECG parameters as predictors for AF. This is an area of increasing interest, with several parameters showing association with pAF. More work is required to help refine these parameters and the relative predictive risk to each other, to understand their pathophysiological basis in the development of AF and to maximize their use in identifying this group of patients early, particularly in combination with other variables.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medsci11020030/s1, Table S1: ECG criteria for LVH; Table S2: QT correction formulae, Table S3: Minnesota code ST-segment abnormalities [136,137,138,139,140,141,142,143].
Author Contributions
Conceptualization, P.A.C., V.S.V. and P.J.P.; methodology, P.A.C. and R.C.; data analysis and investigation, P.A.C. and R.C.; writing—original draft preparation, P.A.C. and R.C.; writing—review and editing, P.A.C., R.C., V.T., V.S.V. and P.J.P.; supervision, V.S.V. and P.J.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to it being a systematic review of already published data.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Krijthe, B.P.; Kunst, A.; Benjamin, E.J.; Lip, G.Y.H.; Franco, O.H.; Hofman, A.; Witteman, J.; Stricker, B.H.; Heeringa, J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur. Heart J. 2013, 34, 2746–2751. [Google Scholar] [CrossRef]
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.E.; Martin, P.J.; Ring, L.; Warburton, E.A.; Belham, M.; Pugh, P.J. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013, 80, 1546–1550. [Google Scholar] [CrossRef]
- Jabre, P.; Jouven, X.; Adnet, F.; Thabut, G.; Bielinski, S.J.; Weston, S.A.; Roger, V.L. Atrial Fibrillation and Death After Myocardial Infarction. Circulation 2011, 123, 2094–2100. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Lamassa, M.; Baldereschi, M.; Pracucci, G.; Consoli, D.; Wolfe, C.D.A.; Giroud, M.; Rudd, A.; Burger, I.; Ghetti, A.; et al. Risk factors and outcome of subtypes of ischemic stroke. Data from a multicenter multinational hospital-based registry. Eur. Commun. Stroke Project J. Neurol. Sci. 2006, 244, 143–150. [Google Scholar]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. Subclinical Atrial Fibrillation and the Risk of Stroke. N. Engl. J. Med. 2012, 366, 120–129. [Google Scholar] [CrossRef]
- Kottkamp, H. Human atrial fibrillation substrate: Towards a specific fibrotic atrial cardiomyopathy. Eur. Heart J. 2013, 34, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Nakano, Y.; Hidaka, T.; Oda, N.; Kajihara, K.; Tokuyama, T.; Uchimura, Y.; Sairaku, A.; Motoda, C.; Fujiwara, M.; et al. Mechanical and substrate abnormalities of the left atrium assessed by 3-dimensional speckle-tracking echocardiography and electroanatomic mapping system in patients with paroxysmal atrial fibrillation. Heart Rhythm 2015, 12, 490–497. [Google Scholar] [CrossRef]
- Teh, A.W.; Kistler, P.M.; Lee, G.; Medi, C.; Heck, P.M.; Spence, S.J.; Sparks, P.B.; Morton, J.B.; Kalman, J.M. Electroanatomic remodeling of the left atrium in paroxysmal and persistent atrial fibrillation patients without structural heart disease. J. Cardiovasc. Electrophysiol. 2012, 23, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, Y.; Watanabe, H.; Okumura, K. Electrocardiogram (ECG) for the Prediction of Incident Atrial Fibrillation: An Overview. J. Atr. Fibrillation 2017, 10, 1724. [Google Scholar] [CrossRef]
- Kreimer, F.; Aweimer, A.; Pflaumbaum, A.; Mügge, A.; Gotzmann, M. Impact of P-wave indices in prediction of atrial fibrillation—Insight from loop recorder analysis. Ann. Noninvasive Electrocardiol. 2021, 26, e12854. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M.U.; Kumarathurai, P.; Fabricius-Bjerre, A.; Larsen, B.S.; Domínguez, H.; Davidsen, U.; Gerds, T.A.; Kanters, J.K.; Sajadieh, A. P-wave indices as predictors of atrial fibrillation. Ann. Noninvasive Electrocardiol. 2020, 25, e12751. [Google Scholar] [CrossRef] [PubMed]
- Istolahti, T.; Eranti, A.; Huhtala, H.; Lyytikäinen, L.P.; Kähönen, M.; Lehtimäki, T.; Eskola, M.; Anttila, I.; Jula, A.; Antoni, B.; et al. The prevalence and prognostic significance of interatrial block in the general population. Ann. Med. 2020, 52, 63–73. [Google Scholar] [CrossRef]
- Hellman, T.; Hakamäki, M.; Lankinen, R.; Koivuviita, N.; Pärkkä, J.; Kallio, P.; Kiviniemi, T.; Airaksinen, K.; Järvisalo, M.; Metsärinne, K. Interatrial block, P terminal force or fragmented QRS do not predict new-onset atrial fibrillation in patients with severe chronic kidney disease. BMC Cardiovasc. Disord. 2020, 20, 437. [Google Scholar] [CrossRef]
- Müller-Edenborn, B.; Minners, J.; Kocher, S.; Chen, J.; Zeh, W.; Lehrmann, H.; Allgeier, J.; Neumann, F.; Arentz, T.; Jadidi, A. Amplified P-wave duration predicts new-onset atrial fibrillation in patients with heart failure with preserved ejection fraction. Clin. Res. Cardiol. 2019, 109, 978–987. [Google Scholar] [CrossRef]
- Acampa, M.; Lazzerini, P.E.; Guideri, F.; Tassi, R.; Andreini, I.; Domenichelli, C.; Cartocci, A.; Martini, G. Electrocardiographic Predictors of Silent Atrial Fibrillation in Cryptogenic Stroke. Heart Lung Circ. 2019, 28, 1664–1669. [Google Scholar] [CrossRef]
- Skov, M.W.; Ghouse, J.; Kühl, J.T.; Platonov, P.G.; Graff, C.; Fuchs, A.; Rasmussen, P.V.; Pietersen, A.; Nordestgaard, B.G.; Torp-Pedersen, C.; et al. Risk prediction of atrial fibrillation based on electrocardiographic interatrial block. J. Am. Heart Assoc. 2018, 7, e008247. [Google Scholar] [CrossRef]
- Lehtonen, A.O.; Langén, V.L.; Porthan, K.; Kähönen, M.; Nieminen, M.S.; Jula, A.M.; Niiranen, T.J. Electrocardiographic predictors of atrial fibrillation in nonhypertensive and hypertensive individuals. J. Hypertens. 2018, 1, 1874–1881. [Google Scholar] [CrossRef] [PubMed]
- Massó-van Roessel, A.; Escobar-Robledo, L.A.; Dégano, I.R.; Grau, M.; Sala, J.; Ramos, R.; Marrugat, J.; Bayés de Luna, A.; Elosua, R. Analysis of the Association Between Electrocardiographic P-wave Characteristics and Atrial Fibrillation in the REGICOR Study. Rev. Esp. Cardiol. Engl. Ed. 2017, 70, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.; MacHaalany, J.; Lam, B.; van Rooy, H.; Haseeb, S.; Kuchtaruk, A.; Glover, B.; Bayés de Luna, A.; Baranchuk, A. Comparison of the Extent of Coronary Artery Disease in Patients with Versus Without Interatrial Block and Implications for New-Onset Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Luca, A.; Yazdani, S.; Caputo, M.L.; Regoli, F.; Moccetti, T.; Kappenberger, L.; Vesin, J.-M.; Auricchio, A. Usefulness of P-Wave Duration and Morphologic Variability to Identify Patients Prone to Paroxysmal Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W.; O’Neal, W.T.; Shoemaker, M.B.; Chen, L.Y.; Alonso, A.; Whalen, S.P.; Soliman, E.Z. PR-Interval Components and Atrial Fibrillation Risk (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2017, 119, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Cortez, D.; Baturova, M.; Lindgren, A.; Carlson, J.; Shubik, Y.V.; Olsson, B.; Platonov, P.G. Atrial time and voltage dispersion are both needed to predict new-onset atrial fibrillation in ischemic stroke patients. BMC Cardiovasc. Disord. 2017, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Çinier, G.; Tekkeşin, A.İ.; Genç, D.; Yıldız, U.; Parsova, E.; Pay, L.; Alexander, B.; Bozbeyoğlu, E.; Türkkan, C.; Alper, A.T.; et al. Interatrial block as a predictor of atrial fibrillation in patients with ST-segment elevation myocardial infarction. Clin. Cardiol. 2018, 41, 1232–1237. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Wang, S.L.; Chu, Y.J.; Long, D.Y.; Dong, J.Z.; Fan, X.W.; Yang, H.-T.; Duan, H.-Y.; Yan, L.-J.; Qian, P.; et al. Usefulness of a Combination of Interatrial Block and a High CHADS2 Score to Predict New Onset Atrial Fibrillation. Int. Heart J. 2016, 57, 580–585. [Google Scholar] [CrossRef]
- Magnani, J.W.; Zhu, L.; Lopez, F.; Pencina, M.J.; Agarwal, S.K.; Soliman, E.Z.; Benjamin, E.J.; Alonso, A. P-wave indices and atrial fibrillation: Cross-cohort assessments from the Framingham Heart Study (FHS) and Atherosclerosis Risk in Communities (ARIC) study. Am. Heart J. 2015, 169, 53–61.e1. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Kühl, J.T.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Sinner, M.F.; Bachmann, T.N.; Haunsø, S.; et al. P-wave duration and the risk of atrial fibrillation: Results from the Copenhagen ECG Study. Heart Rhythm 2015, 12, 1887–1895. [Google Scholar] [CrossRef]
- Francia, P.; Ricotta, A.; Balla, C.; Adduci, C.; Semprini, L.; Frattari, A.; Modestino, A.; Mercanti, F.; Sensini, I.; Caprinozzi, M.; et al. P-wave duration in lead aVR and the risk of atrial fibrillation in hypertension. Ann. Noninvasive Electrocardiol. 2015, 20, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Horie, M. Biphasic P wave in inferior leads and the development of atrial fibrillation. J. Arrhythmia 2015, 31, 376–380. [Google Scholar] [CrossRef]
- Chang, I.C.Y.; Austin, E.; Krishnan, B.; Benditt, D.G.; Quay, C.N.; Ling, L.H.; Chen, L.Y. Shorter minimum p-wave duration is associated with paroxysmal lone atrial fibrillation. J. Electrocardiol. 2014, 47, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Niwano, S.; Niwano, H.; Igarashi, T.; Fujiishi, T.; Ishizue, N.; Oikawa, J.; Satoh, A.; Kurokawa, S.; Hatakeyama, Y.; et al. Prediction of new onset atrial fibrillation through P wave analysis in 12 lead ECG. Int. Heart J. 2014, 55, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Girasis, C.; Vassilikos, V.; Efthimiadis, G.K.; Papadopoulou, S.L.; Dakos, G.; Dalamaga, E.G.; Chouvarda, I.; Giannakoulas, G.; Kamperidis, V.; Paraskevaidis, S.; et al. Patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation: Advanced echocardiographic evaluation of the left atrium combined with non-invasive P-wave analysis. Eur. Heart J.-Cardiovasc. Imaging 2013, 14, 425–434. [Google Scholar] [CrossRef]
- Dogan, U.; Dogan, E.A.; Tekinalp, M.; Tokgoz, O.S.; Aribas, A.; Akilli, H.; Ozdemir, K.; Gok, H.; Yuruten, B. P-wave dispersion for predicting paroxysmal atrial fibrillation in acute ischemic stroke. Int. J. Med. Sci. 2011, 9, 108–114. [Google Scholar] [CrossRef]
- Magnani, J.W.; Johnson, V.M.; Sullivan, L.M.; Gorodeski, E.Z.; Schnabel, R.B.; Lubitz, S.A.; Levy, D.; Ellinor, P.T.; Benjamin, E.J. P wave duration and risk of longitudinal atrial fibrillation in persons ≥60 years old (from the Framingham Heart Study). Am. J. Cardiol. 2011, 107, 917–921.e1. [Google Scholar] [CrossRef]
- Radeljić, V.; Pavlović, N.; Manola, Š.; Delić-Brkljačić, D.; Pintarić, H.; Petrač, D. Incidence and predictors of asymptomatic atrial fibrillation in patients older than 70 years with complete atrioventricular block and dual chamber pacemaker implantation. Croat. Med. J. 2011, 52, 61–67. [Google Scholar] [CrossRef]
- Macfarlane, P.W.; Murray, H.; Sattar, N.; Stott, D.J.; Ford, I.; Buckley, B.; Jukema, J.W.; Westendorp, R.G.J.; Shepherd, J. The incidence and risk factors for new onset atrial fibrillation in the PROSPER study. Europace 2011, 13, 634–639. [Google Scholar] [CrossRef]
- Soliman, E.Z.; Prineas, R.J.; Case, L.D.; Zhang, Z.M.; Goff, D.C. Ethnic Distribution of ECG Predictors of Atrial Fibrillation and Its Impact on Understanding the Ethnic Distribution of Ischemic Stroke in the Atherosclerosis Risk in Communities (ARIC) Study. Stroke 2009, 40, 1204–1211. [Google Scholar] [CrossRef]
- Perez, M.V.; Dewey, F.E.; Marcus, R.; Ashley, E.A.; Al-Ahmad, A.A.; Wang, P.J.; Froelicher, V.F. Electrocardiographic predictors of atrial fibrillation. Am. Heart J. 2009, 158, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Ariyarajah, V.; Apiyasawat, S.; Fernandes, J.; Kranis, M.; Spodick, D.H. Association of atrial fibrillation in patients with interatrial block over prospectively followed controls with comparable echocardiographic parameters. Am. J. Cardiol. 2007, 99, 390–392. [Google Scholar] [CrossRef]
- Ozdemir, O.; Soylu, M.; Demir, A.D.; Topaloglu, S.; Alyan, O.; Turhan, H.; Bicer, A.; Kutuk, E. P-wave durations as a predictor for atrial fibrillation development in patients with hypertrophic cardiomyopathy. Int. J. Cardiol. 2004, 94, 163–166. [Google Scholar] [CrossRef]
- Aras, D.; Maden, O.; Ozdemir, O.; Aras, S.; Topaloglu, S.; Yetkin, E.; Demir, A.D.; Soylu, M.O.; Erdogan, M.F.; Kisacik, H.L. Simple electrocardiographic markers for the prediction of paroxysmal atrial fibrillation in hyperthyroidism. Int. J. Cardiol. 2005, 99, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.; Nielsen, J.C.; Mortensen, P.T.; Christensen, P.D.; Vesterlund, T.; Pedersen, A.K.; Andersen, H.R. Sinus and paced P wave duration and dispersion as predictors of atrial fibrillation after pacemaker implantation in patients with isolated sick sinus syndrome. Pacing Clin. Electrophysiol. PACE 2004, 27, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Altunkeser, B.B.; Ozdemir, K.; Gök, H.; Temizhan, A.; Tokaç, M.; Karabağ, T. Can P wave parameters obtained from 12-lead surface electrocardiogram be a predictor for atrial fibrillation in patients who have structural heart disease? Angiology 2003, 54, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Köse, S.; Aytemir, K.; Sade, E.; Can, I.; Ozer, N.; Amasyali, B.; Aksöyek, S.; Ovünç, K.; Ozmen, F.; Atalar, E.; et al. Detection of patients with hypertrophic cardiomyopathy at risk for paroxysmal atrial fibrillation during sinus rhythm by P-wave dispersion. Clin. Cardiol. 2003, 26, 431–434. [Google Scholar] [CrossRef]
- Aytemir, K.; Ozer, N.; Atalar, E.; Sade, E.; Aksöyek, S.; Ovünç, K.; Oto, A.; Ozmen, F.; Kes, S. P wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. PACE 2000, 23, 1109–1112. [Google Scholar] [CrossRef]
- Ozer, N.; Aytemir, K.; Atalar, E.; Sade, E.; Aksöyek, S.; Ovünç, K.; Açýl, T.; Nazlý, N.; Ozmen, F.; Oto, A.; et al. P wave dispersion in hypertensive patients with paroxysmal atrial fibrillation. Pacing Clin. Electrophysiol. PACE 2000, 23, 1859–1862. [Google Scholar] [CrossRef]
- Dilaveris, P.E.; Gialafos, E.J.; Sideris, S.K.; Theopistou, A.M.; Andrikopoulos, G.K.; Kyriakidis, M.; Gialafos, J.E.; Toutouzas, P.K. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am. Heart J. 1998, 135, 733–738. [Google Scholar] [CrossRef]
- Mendieta, G.; Guasch, E.; Weir, D.; Aristizabal, D.; Escobar-Robledo, L.A.; Llull, L.; Mont, L.; Bayés de Luna, A.; Sitges, M. Advanced interatrial block: A predictor of covert atrial fibrillation in embolic stroke of undetermined source. J. Electrocardiol. 2020, 58, 113–118. [Google Scholar] [CrossRef]
- Boccanelli, A.; Mureddu, G.F.; Cesaroni, G.; Prati, F.; Rangoni, F.; Agabiti, N.; Davoli, M.; Scardovi, A.B.; Latini, R. Predictive value of interatrial block for atrial fibrillation in elderly subjects enrolled in the PREDICTOR study. J. Electrocardiol. 2019, 54, 22–27. [Google Scholar] [CrossRef]
- Alexander, B.; Baranchuk, A.; Haseeb, S.; van Rooy, H.; Kuchtaruk, A.; Hopman, W.; Çinier, G.; Hetu, M.-F.; Li, T.Y.; Johri, A.M. Interatrial block predicts atrial fibrillation in patients with carotid and coronary artery disease. J. Thorac. Dis. 2018, 10, 4328–4334. [Google Scholar] [CrossRef]
- Escobar-Robledo, L.A.; Bayés-de-Luna, A.; Lupón, J.; Baranchuk, A.; Moliner, P.; Martínez-Sellés, M.; Zamora, E.; de Antonio, M.; Domingo, M.; Cediel, G.; et al. Advanced interatrial block predicts new-onset atrial fibrillation and ischemic stroke in patients with heart failure: The “Bayes’ Syndrome-HF” study. Int. J. Cardiol. 2018, 271, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Papa, A.A.; Rago, A.; Ciardiello, C.; Marano, M.; Proietti, R.; Politano, L.; Nigro, G. Interatrial block to predict atrial fibrillation in myotonic dystrophy type 1. Neuromuscul. Disord. 2018, 28, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Tekkesin, A.I.; Çinier, G.; Cakilli, Y.; Hayıroğlu, M.İ.; Alper, A.T. Interatrial block predicts atrial high rate episodes detected by cardiac implantable electronic devices. J. Electrocardiol. 2017, 50, 234–237. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Zhang, Z.M.; Loehr, L.R.; Chen, L.Y.; Alonso, A.; Soliman, E.Z. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am. J. Cardiol. 2016, 117, 1755–1759. [Google Scholar] [CrossRef]
- Sadiq Ali, F.; Enriquez, A.; Conde, D.; Redfearn, D.; Michael, K.; Simpson, C.; Abdollah, H.; Bayés de Luna, A.; Hopman, W.; Baranchuk, A. Advanced Interatrial Block Predicts New Onset Atrial Fibrillation in Patients with Severe Heart Failure and Cardiac Resynchronization Therapy. Ann. Noninvasive Electrocardiol. 2015, 20, 586–591. [Google Scholar] [CrossRef]
- Bayés de Luna, A.; Cladellas, M.; Oter, R.; Torner, P.; Guindo, J.; Martí, V.; Rivera, I.; Iturralde, P. Interatrial conduction block and retrograde activation of the left atrium and paroxysmal supraventricular tachyarrhythmia. Eur. Heart J. 1988, 9, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Öz, A.; Cinar, T.; Klzllto Güler, C.; Efe, S.Ç.; Emre, U.; Karaba, T.; Ayça, B. Novel electrocardiography parameter for paroxysmal atrial fibrillation in acute ischaemic stroke patients: P wave peak time. Postgrad. Med. J. 2020, 96, 584–588. [Google Scholar] [CrossRef]
- Yesin, M.; Kalçık, M.; Çağdaş, M.; Karabağ, Y.; Rencüzoğulları, İ.; Gürsoy, M.O.; Efe, S.Ç.; Karakoyun, S. Fragmented QRS may predict new onset atrial fibrillation in patients with ST-segment elevation myocardial infarction. J. Electrocardiol. 2018, 51, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Rago, A.; Russo, V.; Papa, A.A.; Ciardiello, C.; Pannone, B.; Mayer, M.C.; Cimmino, G.; Nigro, G. The role of the atrial electromechanical delay in predicting atrial fibrillation in beta-thalassemia major patients. J. Interv. Card. Electrophysiol. Int. J. Arrhythmia Pacing 2017, 48, 147–157. [Google Scholar] [CrossRef]
- Tuluce, K.; Yakar Tuluce, S.; Kahya Eren, N.; Kocabas, U.; Akyildiz Akcay, F.; Gunduz, R.; Akyildiz, Z.I.; Ergene, O. Predictors of Future Atrial Fibrillation Development in Patients with Hypertrophic Cardiomyopathy: A Prospective Follow-Up Study. Echocardiography 2016, 33, 379–385. [Google Scholar] [CrossRef]
- Tükek, T.; Yildiz, P.; Akkaya, V.; Karan, M.A.; Atilgan, D.; Yilmaz, V.; Korkut, F. Factors associated with the development of atrial fibrillation in COPD patients: The role of P-wave dispersion. Ann. Noninvasive Electrocardiol. 2002, 7, 222–227. [Google Scholar] [CrossRef]
- Hayashi, H.; Miyamoto, A.; Kawaguchi, T.; Naiki, N.; Xue, J.Q.; Matsumoto, T.; Murakami, Y.; Horie, M. P-pulmonale and the development of atrial fibrillation. Circ. J. Off. J. Jpn. Circ. Soc. 2014, 78, 329–337. [Google Scholar] [CrossRef]
- Chun, K.J.; Hwang, J.K.; Choi, S.R.; Park, S.J.; On, Y.K.; Kim, J.S.; Park, K.-M. Electrocardiogram PR Interval Is a Surrogate Marker to Predict New Occurrence of Atrial Fibrillation in Patients with Frequent Premature Atrial Contractions. J. Korean Med. Sci. 2016, 31, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.J.; Hwang, J.K.; Park, S.J.; On, Y.K.; Kim, J.S.; Park, K.M. Electrical PR Interval Variation Predicts New Occurrence of Atrial Fibrillation in Patients with Frequent Premature Atrial Contractions. Medicine 2016, 95, e3249. [Google Scholar] [CrossRef]
- Cabrera, S.; Vallès, E.; Benito, B.; Alcalde, Ó.; Jiménez, J.; Fan, R.; Martí-Almor, J. Simple predictors for new onset atrial fibrillation. Int. J. Cardiol. 2016, 221, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Thijs, V.N.; Brachmann, J.; Morillo, C.A.; Passman, R.S.; Sanna, T.; Bernstein, R.A.; Diener, H.-C.; Di Lazzaro, V.; Rymer, M.M.; Hogge, L.; et al. Predictors for atrial fibrillation detection after cryptogenic stroke. Neurology 2016, 86, 261–269. [Google Scholar] [CrossRef]
- Shulman, E.; Aagaard, P.; Kargoli, F.; Hoch, E.; Zheng, L.; Di Biase, L.; Fisher, J.; Gross, J.; Kim, S.; Ferrick, K.; et al. Validation of PR interval length as a criterion for development of atrial fibrillation in non-Hispanic whites, African Americans and Hispanics. J. Electrocardiol. 2015, 48, 703–709. [Google Scholar] [CrossRef]
- Frontera, A.; Carpenter, A.; Ahmed, N.; Fasiolo, M.; Nelson, M.; Diab, I.; Cripps, T.; Thomas, G.; Duncan, E. Demographic and Clinical Characteristics to Predict Paroxysmal Atrial Fibrillation: Insights from an Implantable Loop Recorder Population. Pacing Clin. Electrophysiol. 2015, 38, 1217–1222. [Google Scholar] [CrossRef]
- Aro, A.L.; Anttonen, O.; Kerola, T.; Junttila, M.J.; Tikkanen, J.T.; Rissanen, H.A.; Reunanen, A.; Huikuri, H.V. Prognostic significance of prolonged PR interval in the general population. Eur. Heart J. 2014, 35, 123–129. [Google Scholar] [CrossRef]
- Knuiman, M.; Briffa, T.; Divitini, M.; Chew, D.; Eikelboom, J.; McQuillan, B.; Hung, J. A cohort study examination of established and emerging risk factors for atrial fibrillation: The Busselton Health Study. Eur. J. Epidemiol. 2014, 29, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Magnani, J.W.; Wang, N.; Nelson, K.P.; Connelly, S.; Deo, R.; Rodondi, N.; Schelbert, E.B.; Garcia, M.E.; Phillips, C.L.; Shlipak, M.G.; et al. Electrocardiographic PR Interval and Adverse Outcomes in Older Adults. Circ. Arrhythmia Electrophysiol. 2013, 6, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Pietersen, A.; Graff, C.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Haunsø, S.; Gerds, T.A.; Ellinor, P.T.; Køber, L.; et al. Risk of atrial fibrillation as a function of the electrocardiographic PR interval: Results from the Copenhagen ECG Study. Heart Rhythm 2013, 10, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Keyes, M.J.; Larson, M.G.; McCabe, E.L.; Newton-Cheh, C.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Wang, T.J. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA-J. Am. Med. Assoc. 2009, 301, 2571–2577. [Google Scholar] [CrossRef]
- Josephson, M.E.; Kastor, J.A.; Morganroth, J. Electrocardiographic left atrial enlargement electrophysiologic, echocardiographic and hemodynamic correlates. Am. J. Cardiol. 1977, 39, 967–971. [Google Scholar] [CrossRef]
- Bayés de Luna, A.; Martínez-Sellés, M.; Bayés-Genís, A.; Elosua, R.; Baranchuk, A. Surface ECG interatrial block-guided treatment for stroke prevention: Rationale for an attractive hypothesis. BMC Cardiovasc. Disord. 2017, 17, 211. [Google Scholar] [CrossRef]
- Legato, M.J.; Bull, M.B.; Ferrer, M.I. Atrial ultrastructure in patients with fixed intra-atrial block. Chest 1974, 65, 252–261. [Google Scholar] [CrossRef]
- Tse, G.; Wong, C.W.; Gong, M.; Wong, W.T.; Bazoukis, G.; Wong, S.H.; Li, G.; Wu, W.K.K.; Tse, L.A.; Lampropoulos, K.; et al. Predictive value of inter-atrial block for new onset or recurrent atrial fibrillation: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 250, 152–156. [Google Scholar] [CrossRef]
- Okutucu, S.; Aytemir, K.; Oto, A. P-wave dispersion: What we know till now? JRSM Cardiovasc. Dis. 2016, 5, 2048004016639443. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Riera, A.R.; de Abreu, L.C.; Barbosa-Barros, R.; Grindler, J.; Fernandes-Cardoso, A.; Baranchuk, A. P-wave dispersion: An update. Indian Pacing Electrophysiol. J. 2016, 16, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Jaros, R.; Martinek, R.; Danys, L. Comparison of Different Electrocardiography with Vectorcardiography Transformations. Sensors 2019, 19, 3072. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Gudbjartsson, D.F.; Arnar, D.O.; Thorleifsson, G.; Thorgeirsson, G.; Stefansdottir, H.; Gudjonsson, S.A.; Jonasdottir, A.; Mathiesen, E.B.; Njølstad, I.; et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat. Genet. 2010, 42, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Lu, X.; Huang, J.; Zhang, S.; Gu, D. Electrocardiographic PR prolongation and atrial fibrillation risk: A meta-analysis of prospective cohort studies. J. Cardiovasc. Electrophysiol. 2015, 26, 36–41. [Google Scholar] [CrossRef]
- Li, Y.; Shah, A.J.; Soliman, E.Z. Effect of electrocardiographic p-wave axis on mortality. Am. J. Cardiol. 2014, 113, 372–376. [Google Scholar] [CrossRef]
- Spach, M.S. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circ. Res. 2007, 101, 743–745. [Google Scholar] [CrossRef]
- Rangel, M.O.; O’Neal, W.T.; Soliman, E.Z. Usefulness of the Electrocardiographic P-Wave Axis as a Predictor of Atrial Fibrillation. Am. J. Cardiol. 2016, 117, 100–104. [Google Scholar] [CrossRef]
- Chattopadhyay, R.K.; Chousou, P.A.; Mukherjee, T.; Pugh, P.J.; Vassiliou, V.S. The predictive value of abnormal P-wave axis for the detection of incident atrial fibrillation: A systematic review with meta-analysis. PLoS ONE 2022, 17, e0278527. [Google Scholar] [CrossRef]
- Dhaliwal, K.K.; Upadhya, B.; Soliman, E.Z.; Beaty, E.H.; Yeboah, J.; Bhave, P.D.; Whalen, S.P.; Singleton, M.J. Association of P-Wave Axis with Incident Atrial Fibrillation in Diabetes Mellitus (from the ACCORD Trial). Am. J. Cardiol. 2020, 128, 191–195. [Google Scholar] [CrossRef]
- Maheshwari, A.; Norby, F.L.; Soliman, E.Z.; Koene, R.; Rooney, M.; O’Neal, W.T.; Alonso, A.; Chen, L.Y. Refining Prediction of Atrial Fibrillation Risk in the General Population with Analysis of P-Wave Axis (from the Atherosclerosis Risk in Communities Study). Am. J. Cardiol. 2017, 120, 1980–1984. [Google Scholar] [CrossRef]
- Goda, T.; Sugiyama, Y.; Ohara, N.; Ikegami, T.; Watanabe, K.; Kobayashi, J.; Takahashi, D. P-Wave Terminal Force in Lead V1 Predicts Paroxysmal Atrial Fibrillation in Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2017, 26, 1912–1915. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Ohara, N.; Watanabe, K.; Kobayashi, J.; Takahashi, D. Abstract WMP64: Utility of Left Atrial Abnormality on Admission Electrocardiography in Acute Ischemic Stroke. Stroke 2017, 48 (Suppl. S1), AWMP64. [Google Scholar] [CrossRef]
- Rasmussen, M.U.; Kumarathurai, P.; Fabricius-Bjerre, A.; Davidsen, U.; Sajadieh, A. P1723P-wave indices as markers of development of atrial fibrillation in Copenhagen Holter Study. Eur. Heart J. 2017, 38 (Suppl. S1), ehx502.P1723. [Google Scholar] [CrossRef]
- Baturova, M.A.; Sheldon, S.H.; Carlson, J.; Brady, P.A.; Lin, G.; Rabinstein, A.A.; Friedman, P.A.; Platonov, P.G. Electrocardiographic and Echocardiographic predictors of paroxysmal atrial fibrillation detected after ischemic stroke. BMC Cardiovasc. Disord. 2016, 16, 209. [Google Scholar] [CrossRef]
- Kamel, H.; Soliman, E.Z.; Heckbert, S.R.; Kronmal, R.A.; Longstreth, W.T.; Nazarian, S.; Okin, P.M. P-Wave Morphology and the Risk of Incident Ischemic Stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke 2014, 45, 2786–2788. [Google Scholar] [CrossRef]
- Eranti, A.; Aro, A.L.; Kerola, T.; Anttonen, O.; Rissanen, H.A.; Tikkanen, J.T.; Junttila, M.J.; Kenttä, T.V.; Knekt, P.; Huikuri, H.V. Prevalence and prognostic significance of abnormal P terminal force in lead V1 of the ECG in the general population. Circ. Arrhythmia Electrophysiol. 2014, 7, 1116–1121. [Google Scholar] [CrossRef]
- Nishi, K.; Fujimoto, S.; Hisanaga, S.; Ogawa, O.; Kitamura, K. Electrocardiographic assessment of incident atrial fibrillation in hemodialysis patients. Ther. Apher. Dial. 2013, 17, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.; Feinglass, J.; Ma, S.; Akhter, N. Risk factors for the development of atrial fibrillation on ibrutinib treatment. Leuk. Lymphoma 2019, 60, 1447–1453. [Google Scholar] [CrossRef]
- Van Diepen, S.; Siha, H.; Fu, Y.; Westerhout, C.M.; Lopes, R.D.; Granger, C.B.; Armstrong, P.W.; APEX AMI Investigators. Do baseline atrial electrocardiographic and infarction patterns predict new-onset atrial fibrillation after ST-elevation myocardial infarction? Insights from the Assessment of Pexelizumab in Acute Myocardial Infarction Trial. J. Electrocardiol. 2010, 43, 351–358. [Google Scholar] [CrossRef]
- Tse, G.; Lakhani, I.; Zhou, J.; Li, K.H.C.; Lee, S.; Liu, Y.; Leung, K.S.K.; Liu, T.; Baranchuk, A.; Zhang, Q. P-Wave Area Predicts New Onset Atrial Fibrillation in Mitral Stenosis: A Machine Learning Approach. Front. Bioeng. Biotechnol. 2020, 8, 479. [Google Scholar] [CrossRef] [PubMed]
- De Bacquer, D.; Willekens, J.; De Backer, G. Long-term prognostic value of p-wave characteristics for the development of atrial fibrillation in subjects aged 55 to 74 years at baseline. Am. J. Cardiol. 2007, 100, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Poli, S.; Barbaro, V.; Bartolini, P.; Calcagnini, G.; Censi, F. Prediction of atrial fibrillation from surface ECG: Review of methods and algorithms. Ann. Ist. Super Sanita 2003, 39, 195–203. [Google Scholar] [PubMed]
- Huang, Z.; Zheng, Z.; Wu, B.; Tang, L.; Xie, X.; Dong, R.; Luo, Y.; Li, S.; Zhu, J.; Liu, J. Predictive value of P wave terminal force in lead V1 for atrial fibrillation: A meta-analysis. Ann. Noninvasive Electrocardiol. 2020, 25, e12739. [Google Scholar] [CrossRef]
- Jaroszyński, A.; Jaroszyńska, A.; Dąbrowski, W.; Zaborowski, T.; Stepulak, A.; Iłżecki, M.; Zubilewicz, T. Factors influencing P terminal force in lead V1 of the ECG in hemodialysis patients. Arch. Med. Sci. 2018, 14, 257–264. [Google Scholar] [CrossRef]
- Patel, N.; O’Neal, W.T.; Whalen, S.P.; Soliman, E.Z. Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Ann. Noninvasive Electrocardiol. 2017, 22, 1–5. [Google Scholar] [CrossRef]
- Chrispin, J.; Jain, A.; Soliman, E.Z.; Guallar, E.; Alonso, A.; Heckbert, S.R.; Bluemke, D.A.; Lima, J.A.C.; Nazarian, S. Association of electrocardiographic and imaging surrogates of left ventricular hypertrophy with incident atrial fibrillation: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll Cardiol. 2014, 63, 2007–2013. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanabe, N.; Makiyama, Y.; Chopra, S.S.; Okura, Y.; Suzuki, H.; Matsui, K.; Watanabe, T.; Kurashina, Y.; Aizawa, Y. ST-segment abnormalities and premature complexes are predictors of new-onset atrial fibrillation: The Niigata Preventive Medicine Study. Am. Heart J. 2006, 152, 731–735. [Google Scholar] [CrossRef]
- Patel, N.; O’Neal, W.T.; Whalen, S.P.; Soliman, E.Z. The association of QT interval components with atrial fibrillation. Ann. Noninvasive Electrocardiol. 2018, 23, e12467. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Vittinghoff, E.; Dewland, T.A.; Mandyam, M.C.; Stein, P.K.; Soliman, E.Z.; Heckbert, S.R.; Marcus, G.M. Electrocardiographic Predictors of Incident Atrial Fibrillation. Am. J. Cardiol. 2016, 118, 714–719. [Google Scholar] [CrossRef]
- Hoshino, T.; Nagao, T.; Shiga, T.; Maruyama, K.; Toi, S.; Mizuno, S.; Ishizuka, K.; Shimizu, S.; Uchiyama, S.; Kitagawa, K. Prolonged QTc interval predicts poststroke paroxysmal atrial fibrillation. Stroke 2015, 46, 71–76. [Google Scholar] [CrossRef]
- Baturova, M.A.; Lindgren, A.; Carlson, J.; Shubik, Y.V.; Olsson, S.B.; Platonov, P.G. Predictors of new onset atrial fibrillation during 10-year follow-up after first-ever ischemic stroke. Int. J. Cardiol. 2015, 199, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Mandyam, M.C.; Soliman, E.Z.; Alonso, A.; Dewland, T.A.; Heckbert, S.R.; Vittinghoff, E.; Cummings, S.R.; Ellinor, P.T.; Chaitman, B.R.; Stocke, K.; et al. The QT interval and risk of incident atrial fibrillation. Heart Rhythm 2013, 10, 1562–1568. [Google Scholar] [CrossRef]
- Nielsen, J.B.; Graff, C.; Pietersen, A.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Haunsø, S.; Gerds, T.A.; Svendsen, J.H.; Køber, L.; et al. J-Shaped Association Between QTc Interval Duration and the Risk of Atrial Fibrillation. J. Am. Coll. Cardiol. 2013, 61, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Aeschbacher, S.; O’Neal, W.T.; Krisai, P.; Loehr, L.; Chen, L.Y.; Alonso, A.; Soliman, E.Z.; Conen, D. Relationship between QRS duration and incident atrial fibrillation. Int. J. Cardiol. 2018, 266, 84–88. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Brancato, C.; Langberg, J.; Delurgio, D.B.; Bush, H.; Brosius, L.; Leon, A.R. QRS duration is associated with atrial fibrillation in patients with left ventricular dysfunction. Clin. Cardiol. 2010, 33, 132–138. [Google Scholar] [CrossRef]
- Uhm, J.S.; Lee, Y.; Roh, Y.H.; Lee, J.; Kang, D.; Jin, M.N.; Kim, I.S.; Yu, H.T.; Kim, T.H.; Kim, J.Y.; et al. Nonspecific intraventricular conduction delay is associated with future occurrence of atrial fibrillation in patients with structurally normal heart. Eur. J. Intern. Med. 2020, 72, 67–72. [Google Scholar] [CrossRef]
- Jogu, H.R.; O’Neal, W.T.; Broughton, S.T.; Shah, A.J.; Zhang, Z.M.; Soliman, E.Z. Frontal QRS-T Angle and the Risk of Atrial Fibrillation in the Elderly. Ann. Noninvasive Electrocardiol. 2017, 22, e12388. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, T.N.; Skov, M.W.; Rasmussen, P.V.; Graff, C.; Pietersen, A.; Lind, B.; Struijk, J.J.; Olesen, M.S.; Haunsø, S.; Køber, L.; et al. Electrocardiographic Tpeak-Tend interval and risk of cardiovascular morbidity and mortality: Results from the Copenhagen ECG study. Heart Rhythm 2016, 13, 915–924. [Google Scholar] [CrossRef] [PubMed]
- Giustetto, C.; Schimpf, R.; Mazzanti, A.; Scrocco, C.; Maury, P.; Anttonen, O.; Probst, V.; Blanc, J.-J.; Sbragia, P.; Dalmasso, P.; et al. Long-Term Follow-Up of Patients with Short QT Syndrome. J. Am. Coll. Cardiol. 2011, 58, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.N.; Tester, D.J.; Perry, J.; Salisbury, B.A.; Reed, C.R.; Ackerman, M.J. Prevalence of early-onset atrial fibrillation in congenital long QT syndrome. Heart Rhythm 2008, 5, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Eckardt, L.; Franz, M.R.; Mönnig, G.; Peter, L.; Wedekind, H.; Schulze-Bahr, E.; Breithardt, G.; Haverkamp, W. Prolonged Atrial Action Potential Durations and Polymorphic Atrial Tachyarrhythmias in Patients with Long QT Syndrome. J. Cardiovasc. Electrophysiol. 2003, 14, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Demoulin, J.C.; Simar, L.J.; Kulbertus, H.E. Quantitative study of left bundle branch fibrosis in left anterior hemiblock: A stereologic approach. Am. J. Cardiol. 1975, 36, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Pietrasik, G.; Zareba, W. QRS fragmentation: Diagnostic and prognostic significance. Cardiol. J. 2012, 19, 114–121. [Google Scholar] [CrossRef] [PubMed]
- German, D.M.; Kabir, M.M.; Dewland, T.A.; Henrikson, C.A.; Tereshchenko, L.G. Atrial Fibrillation Predictors: Importance of the Electrocardiogram. Ann. Noninvasive Electrocardiol. 2015, 21, 20–29. [Google Scholar] [CrossRef]
- Shah, R.U.; Mukherjee, R.; Zhang, Y.; Jones, A.E.; Springer, J.; Hackett, I.; Steinberg, B.A.; Lloyd-Jones, D.M.; Chapman, W.W. Impact of Different Electronic Cohort Definitions to Identify Patients with Atrial Fibrillation from the Electronic Medical Record. J. Am. Heart Assoc. 2020, 9, e014527. [Google Scholar] [CrossRef]
- Alexander, B.; Milden, J.; Hazim, B.; Haseeb, S.; Bayes-Genis, A.; Elosua, R.; Martínez-Sellés, M.; Yeung, C.; Hopman, W.; Bayes de Luna, A.; et al. New electrocardiographic score for the prediction of atrial fibrillation: The MVP ECG risk score (morphology-voltage-P-wave duration). Ann. Noninvasive Electrocardiol. 2019, 24, e12669. [Google Scholar] [CrossRef]
- Svennberg, E.; Henriksson, P.; Engdahl, J.; Hijazi, Z.; Al-Khalili, F.; Friberg, L.; Frykman, V. N-terminal pro B-type natriuretic peptide in systematic screening for atrial fibrillation. Heart 2017, 103, 1271–1277. [Google Scholar] [CrossRef]
- Alonso, A.; Krijthe, B.P.; Aspelund, T.; Stepas, K.A.; Pencina, M.J.; Moser, C.B.; Sinner, M.F.; Sotoodehnia, N.; Fontes, J.D.; Janssens, A.C.J.W.; et al. Simple Risk Model Predicts Incidence of Atrial Fibrillation in a Racially and Geographically Diverse Population: The CHARGE-AF Consortium. J. Am. Heart Assoc. 2013, 2, e000102. [Google Scholar] [CrossRef]
- Olsen, F.J.; Møgelvang, R.; Jensen, G.B.; Jensen, J.S.; Biering-Sørensen, T. Relationship Between Left Atrial Functional Measures and Incident Atrial Fibrillation in the General Population. JACC Cardiovasc. Imaging 2018, 12, 981–989. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Hygrell, T.; Viberg, F.; Dahlberg, E.; Charlton, P.H.; Kemp Gudmundsdottir, K.; Mant, J.; Lindman Hörnlund, J.; Svennberg, E. An artificial intelligence–based model for prediction of atrial fibrillation from single-lead sinus rhythm electrocardiograms facilitating screening. Europace 2023, euad036. [Google Scholar] [CrossRef]
- Christopoulos, G.; Graff-Radford, J.; Lopez, C.L.; Yao, X.; Attia, Z.I.; Rabinstein, A.A.; Petersen, R.C.; Knopman, D.S.; Mielke, M.M.; Kremers, W.; et al. Artificial Intelligence–Electrocardiography to Predict Incident Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2020, 13, e009355. [Google Scholar] [CrossRef]
- Melzi, P.; Tolosana, R.; Cecconi, A.; Sanz-Garcia, A.; Ortega, G.J.; Jimenez-Borreguero, L.J.; Vera-Rodriguez, R. Analyzing artificial intelligence systems for the prediction of atrial fibrillation from sinus-rhythm ECGs including demographics and feature visualization. Sci. Rep. 2021, 11, 22786. [Google Scholar] [CrossRef]
- Attia, Z.I.; Harmon, D.M.; Dugan, J.; Manka, L.; Lopez-Jimenez, F.; Lerman, A.; Siontis, K.C.; Noseworthy, P.A.; Yao, X.; Klavetter, E.W.; et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat. Med. 2022, 28, 2497–2503. [Google Scholar] [CrossRef]
- Sokolow, M.; Lyon, T.P. the ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am. Heart J. 1949, 37, 161–186. [Google Scholar] [CrossRef]
- Romhilt, D.W.; Estes, E.H. A point-score system for the ecg diagnosis of left ventricular hypertrophy. Am. Heart J. 1968, 75, 752–758. [Google Scholar] [CrossRef]
- Molloy, T.J.; Okin, P.M.; Devereux, R.B.; Kligfield, P. Electrocardiographic detection of left ventricular hypertrophy by the simple qrs voltage-duration product. J. Am. Coll. Cardiol. 1992, 20, 1180–1186. [Google Scholar] [CrossRef]
- Macfarlane, P.W.; Latif, S. Automated serial ecg comparison based on the minnesota code. J. Electrocardiol. 1996, 29, 29–34. [Google Scholar] [CrossRef]
- Bazzett, H.C. An analysis of the time-relations of the electrocardiograms. Heart 1920, 7, 353–370. [Google Scholar] [CrossRef]
- Hodges, M.S.; Salerno, D.E.D. Bazett’s qt correction reviewed: Evidence that a linear qt correction for heart rate is better. J. Am. Coll. Cardiol 1983, 1, 694. [Google Scholar]
- Sagie, A.; Larson, M.G.; Goldberg, R.J.; Bengtson, J.R.; Levy, D. An improved method for adjusting the qt interval for heart rate (the framingham heart study). Am. J. Cardiol. 1992, 70, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Fredericia, L.S. Die systolendauer im elektrokardiogramm bei normalen menschen und bei herzkranken. Acta Med. Scand. 1920, 53, 469–486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).