17β-estradiol Enhances 5-Fluorouracil Anti-Cancer Activities in Colon Cancer Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Reagents
2.2. Colon Cancer Cell Lines and Culture Conditions
2.3. Treatment Regimes
2.4. Cell Cycle Analysis Using PI Staining and Flow Cytometry
2.5. Cell Death Analysis Using Annexin V-FITC/PI Staining and Flow Cytometry
2.6. Statistical Analysis
2.6.1. Analysis of Monotherapies Effect
2.6.2. Analysis of Dual Therapy Effect
3. Results
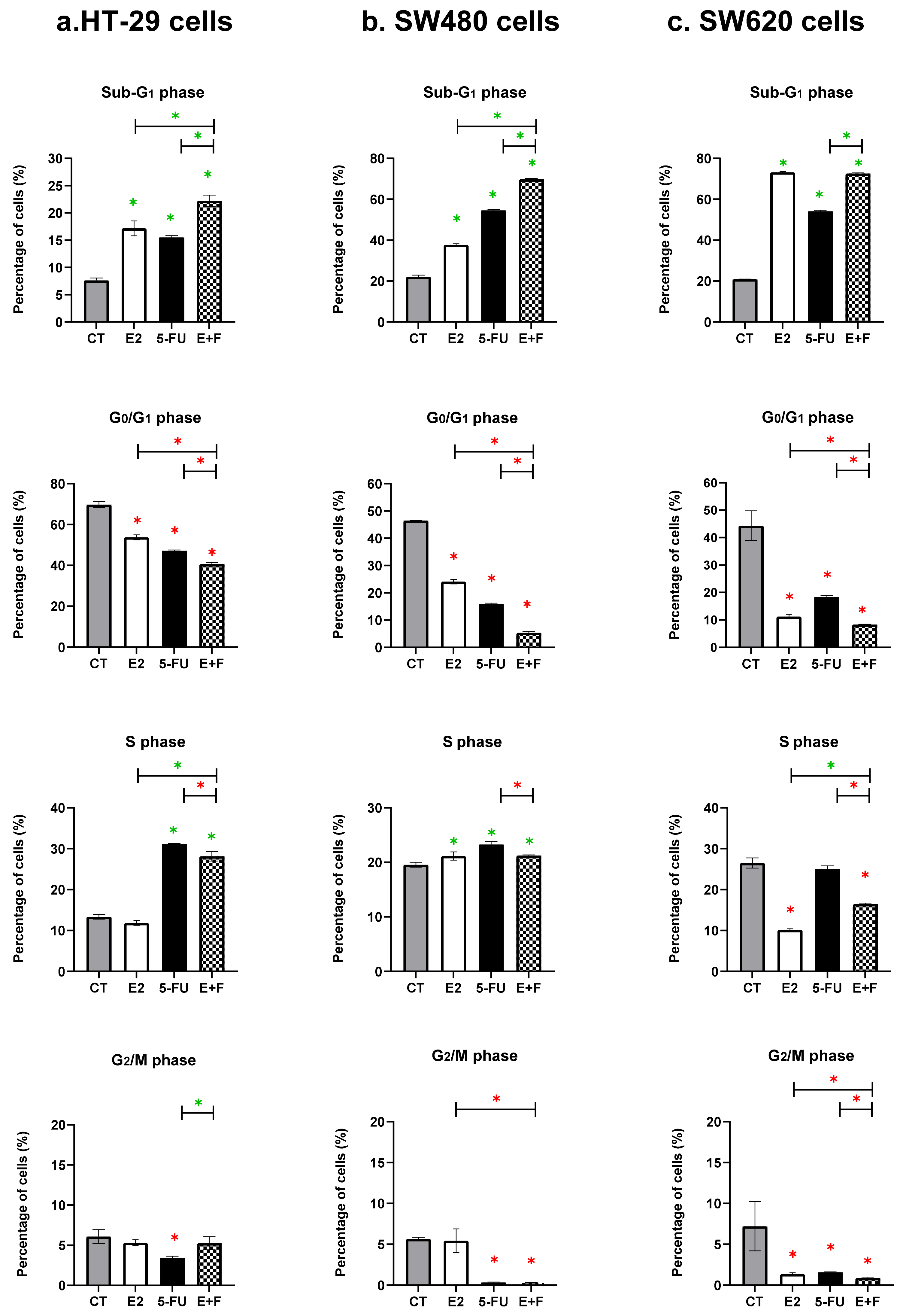
3.1. Cell Cycle Results
3.1.1. The Monotherapy Effect of 7β-estradiol
3.1.2. The Monotherapy Effect of 5-Fluorouracil
3.1.3. The Dual Therapy Effect of 7β-estradiol and 5-Fluorouracil
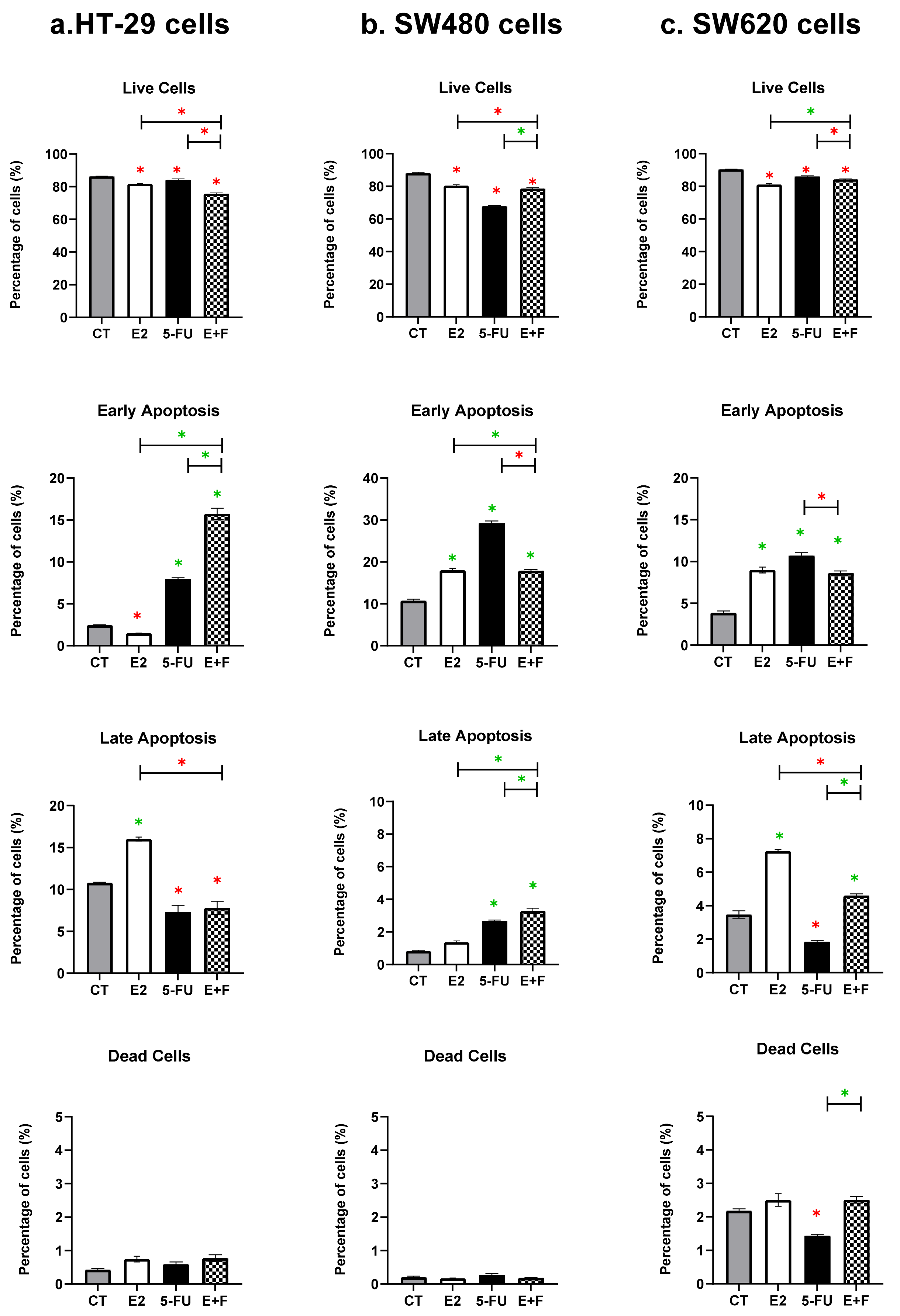
3.2. Cell Death Results
3.2.1. The Monotherapy Effect of 7β-estradiol
3.2.2. The Monotherapy Effect of 5-Fluorouracil
3.2.3. The Dual Therapy Effect of 7β-estradiol and 5-Fluorouracil
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide 2020. Available online: https://gco.iarc.fr/today/%20data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 15 September 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Filipits, M.; Pirker, R.; Dunant, A.; Lantuejoul, S.; Schmid, K.; Huynh, A.; Haddad, V.; Andre, F.; Stahel, R.; Pignon, J.-P.; et al. Cell Cycle Regulators and Outcome of Adjuvant Cisplatin-Based Chemotherapy in Completely Resected Non–Small-Cell Lung Cancer: The International Adjuvant Lung Cancer Trial Biologic Program. J. Clin. Oncol. 2007, 25, 2735–2740. [Google Scholar] [CrossRef]
- Brough, R.; Bajrami, I.; Vatcheva, R.; Natrajan, R.; Reis-Filho, J.S.; Lord, C.J.; Ashworth, A. APRIN is a cell cycle specific BRCA2-interacting protein required for genome integrity and a predictor of outcome after chemotherapy in breast cancer. EMBO J. 2012, 31, 1160–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, J.; Xu, C.; Zhao, Z.; You, T. Prognostic significance of cyclin D1 expression in colorectal cancer: A meta-analysis of observational studies. PLoS ONE 2014, 9, e94508. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, K.; Pryczynicz, A.; Dymicka-Piekarska, V.; Famulski, W.; Guzińska-Ustymowicz, K. Immunohistochemical expression and serum level of survivin protein in colorectal cancer patients. Oncol. Lett. 2016, 12, 3591–3597. [Google Scholar] [CrossRef] [PubMed]
- Al-Maghrabi, J.; Al-Ahwal, M.; Buhmeida, A.; Syrjänen, K.; Sibyani, A.; Emam, E.; Ghanim, A.; Al-Qahtani, M. Expression of Cell Cycle Regulators P21 and P27 as Predictors of Disease Outcome in Colorectal Carcinoma. J. Gastrointest. Cancer 2011, 43, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Simpson, K.L.; Cawthorne, C.; Zhou, C.; Hodgkinson, C.L.; Walker, M.J.; Trapani, F.; Kadirvel, M.; Brown, G.; Dawson, M.J.; Macfarlane, M.; et al. A caspase-3 ‘death-switch’ in colorectal cancer cells for induced and synchronous tumor apoptosis in vitro and in vivo facilitates the development of minimally invasive cell death biomarkers. Cell Death Dis. 2013, 4, e613. [Google Scholar] [CrossRef]
- Oh, H.J.; Bae, J.M.; Wen, X.; Jung, S.; Kim, Y.; Kim, K.J.; Cho, N.-Y.; Kim, J.H.; Han, S.-W.; Kim, T.-Y.; et al. p53 expression status is associated with cancer-specific survival in stage III and high-risk stage II colorectal cancer patients treated with oxaliplatin-based adjuvant chemotherapy. Br. J. Cancer 2019, 120, 797–805. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef]
- Katona, C.; Kralovánszky, J.; Rosta, A.; Pandi, E.; Fónyad, G.; Tóth, K.; Jeney, A. Putative Role of Dihydropyrimidine Dehydrogenase in the Toxic Side Effect of 5-Fluorouracil in Colorectal Cancer Patients. Oncology 1998, 55, 468–474. [Google Scholar] [CrossRef]
- Abdel Latif, Y.; El-Bana, M.; Hussein, J.; El-Khayat, Z.; Farrag, A.R. Effects of resveratrol in combination with 5-fluorouracil on N-methylnitrosourea-induced colon cancer in rats. Comp. Clin. Pathol. 2019, 28, 1351–1362. [Google Scholar] [CrossRef]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil resistance mechanisms in colorectal cancer: From classical pathways to promising processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 142. [Google Scholar] [CrossRef]
- Kim, J.H. Chemotherapy for colorectal cancer in the elderly. World J. Gastroenterol. WJG 2015, 21, 5158–5166. [Google Scholar] [CrossRef]
- Yaffee, P.; Osipov, A.; Tan, C.; Tuli, R.; Hendifar, A. Review of systemic therapies for locally advanced and metastatic rectal cancer. J. Gastrointest. Oncol. 2015, 6, 185–200. [Google Scholar] [CrossRef]
- Jang, Y.; Huang, H.; Leung, C.Y. Association of hormone replacement therapy with mortality in colorectal cancer survivor: A systematic review and meta-analysis. BMC Cancer 2019, 19, 1199. [Google Scholar] [CrossRef]
- Schmuck, R.; Gerken, M.; Teegen, E.; Krebs, I.; Klinkhammer-Schalke, M.; Aigner, F.; Pratschke, J.; Rau, B.; Benz, S. Gender comparison of clinical, histopathological, therapeutic and outcome factors in 185,967 colon cancer patients. Langenbecks Arch. Surg. 2020, 405, 71–80. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef]
- Maingi, J.W.; Tang, S.; Liu, S.; Ngenya, W.; Bao, E. Targeting estrogen receptors in colorectal cancer. Mol. Biol. Rep. 2020, 47, 4087–4091. [Google Scholar] [CrossRef]
- Almatroudi, A. The Incidence Rate of Colorectal Cancer in Saudi Arabia: An Observational Descriptive Epidemiological Analysis. Int. J. Gen. Med. 2020, 13, 977–990. [Google Scholar] [CrossRef]
- Mosli, M.H.; Al-Ahwal, M.S. Colorectal Cancer in the Kingdom of Saudi Arabia: Need for Screening. Asian Pac. J. Cancer Prev. 2012, 13, 3809–3813. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahwal, M.S.; Shafik, Y.H.; Al-Ahwal, H.M. First national survival data for colorectal cancer among Saudis between 1994 and 2004: What’s next? BMC Public Health 2013, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Alsanea, N.; Abduljabbar, A.S.; Alhomoud, S.; Ashari, L.H.; Hibbert, D.; Bazarbashi, S. Colorectal cancer in Saudi Arabia: Incidence, survival, demographics and implications for national policies. Ann. Saudi Med. 2015, 35, 196–202. [Google Scholar] [CrossRef]
- Chen, C.; Gong, X.; Yang, X.; Shang, X.; Du, Q.; Liao, Q.; Xie, R.; Chen, Y.; Xu, J. The roles of estrogen and estrogen receptors in gastrointestinal disease (Review). Oncol. Lett. 2019, 18, 5673–5680. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Zhang, Y.; Wang, L.; Huang, Y.; Yu, Q.; Guo, P.; Li, K. Risk of colorectal cancer with hysterectomy and oophorectomy: A systematic review and meta-analysis. Int. J. Surg. 2016, 34, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Koch, T.; Therming Jørgensen, J.; Christensen, J.; Duun-Henriksen, A.K.; Priskorn, L.; Kildevæld Simonsen, M.; Dehlendorff, C.; Andersen, Z.J.; Juul, A.; Bräuner, E.V.; et al. Bilateral oophorectomy and rate of colorectal cancer: A prospective cohort study. Int. J. Cancer 2022, 150, 38–46. [Google Scholar] [CrossRef]
- Labadie, J.D.; Harrison, T.A.; Banbury, B.; Amtay, E.L.; Bernd, S.; Brenner, H.; Buchanan, D.D.; Campbell, P.T.; Cao, Y.; Chan, A.T.; et al. Postmenopausal Hormone Therapy and Colorectal Cancer Risk by Molecularly Defined Subtypes and Tumor Location. JNCI Cancer Spectr. 2020, 4, pkaa042. [Google Scholar] [CrossRef]
- Grodstein, F.; Newcomb, P.A.; Stampfer, M.J. Postmenopausal hormone therapy and the risk of colorectal cancer: A review and meta-analysis. Am. J. Med. 1999, 106, 574–582. [Google Scholar] [CrossRef]
- Prihartono, N.; Palmer, J.R.; Louik, C.; Shapiro, S.; Rosenberg, L. A Case-Control Study of Use of Postmenopausal Female Hormone Supplements in Relation to the Risk of Large Bowel Cancer. Cancer Epidemiol. Biomark. Prev. 2000, 9, 443–447. [Google Scholar]
- Woodson, K.; Lanza, E.; Tangrea, J.A.; Albert, P.S.; Slattery, M.; Pinsky, J.; Caan, B.; Paskett, E.; Iber, F.; Kikendall, J.W.; et al. Hormone Replacement Therapy and Colorectal Adenoma Recurrence Among Women in the Polyp Prevention Trial. J. Natl. Cancer Inst. 2001, 93, 1799–1805. [Google Scholar] [CrossRef][Green Version]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Anderson, G.L.; Limacher, M.; Assaf, A.R.; Bassford, T.; Beresford, S.A.A.; Black, H.; Bonds, D.; Brunner, R.; Brzyski, R.; Caan, B.; et al. Effects of Conjugated Equine Estrogen in Postmenopausal Women with Hysterectomy: The Women’s Health Initiative Randomized Controlled Trial. JAMA 2004, 291, 1701–1712. [Google Scholar] [CrossRef]
- Newcomb, P.A.; Zheng, Y.; Chia, V.M.; Morimoto, L.M.; Doria-Rose, V.P.; Templeton, A.; Thibodeau, S.N.; Potter, J.D. Estrogen Plus Progestin Use, Microsatellite Instability, and the Risk of Colorectal Cancer in Women. Cancer Res. 2007, 67, 7534–7539. [Google Scholar] [CrossRef]
- Hartman, J.; Edvardsson, K.; Lindberg, K.; Zhao, C.; Willimas, S.C.; Strom, A.; Gustafsson, J.A. Tumor Repressive Functions of Estrogen Receptor β in SW480 Colon Cancer Cells. Cancer Res. 2009, 69, 6100–6106. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, C.; Wu, H.; Huang, J. Estrogen Receptor Beta (ERβ) Mediated-CyclinD1 Degradation via Autophagy Plays an AntiProliferation Role in Colon Cells. Int. J. Biol. Sci. 2019, 15, 942–952. [Google Scholar] [CrossRef]
- Topi, G.; Satapathy, S.R.; Dash, P.; Fred Mehrabi, S.; Ehrnström, R.; Olsson, R.; Lydrup, M.-L.; Sjölander, A. Tumour-suppressive effect of oestrogen receptor β in colorectal cancer patients, colon cancer cells, and a zebrafish model. J. Pathol. 2020, 251, 297–309. [Google Scholar] [CrossRef]
- Fiocchetti, M.; Camilli, G.; Acconcia, F.; Leone, S.; Ascenzi, P.; Marino, M. ERβ-dependent neuroglobin up-regulation impairs 17β-estradiol-induced apoptosis in DLD-1 colon cancer cells upon oxidative stress injury. J. Steroid Biochem. Mol. Biol. 2015, 149, 128–137. [Google Scholar] [CrossRef]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef]
- Escajadillo, T.; Wang, H.; Li, L.; Li, D.; Sewer, M.B. Oxysterol-related-binding-protein related Protein-2 (ORP2) regulates cortisol biosynthesis and cholesterol homeostasis. Mol. Cell. Endocrinol. 2016, 427, 73–85. [Google Scholar] [CrossRef]
- Slattery, M.L.; Sweeney, C.; Murtaugh, M.; Ma, K.N.; Wolff, R.K.; Potter, J.D.; Caan, B.J.; Samowitz, W. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2936–2942. [Google Scholar] [CrossRef]
- Check, J.H.; Dix, E.; Cohen, R.; Check, D.; Wilson, C. Efficacy of the Progesterone Receptor Antagonist Mifepristone for Palliative Therapy of Patients with a Variety of Advanced Cancer Types. Anticancer Res. 2010, 30, 623–628. [Google Scholar] [PubMed]
- Gan, L.; He, J.; Zhang, X.; Zhang, Y.; Yu, G.; Chen, Y.; Pan, J.; Wang, J.-J.; Wang, X. Expression profile and prognostic role of sex hormone receptors in gastric cancer. BMC Cancer 2012, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Thompson, M.; Lynch, I.J.; Bhardwaj, B. Expression of estrogen receptor (ER) subtypes and ERbeta isoforms in colon cancer. Cancer Res. 2001, 61, 632–640. [Google Scholar] [PubMed]
- Di Leo, A.; Barone, M.; Maiorano, E.; Tanzi, S.; Piscitelli, D.; Marangi, S.; Lofano, K.; Ierardi, E.; Principi, M.; Francavilla, A. ER-β expression in large bowel adenomas: Implications in colon carcinogenesis. Dig. Liver Dis. 2007, 40, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.D.; Tzelepi, V.; Sotiropoulou-Bonikou, G.; Kefalopoulou, Z.; Papavassiliou, A.G.; Kalofonos, H. Expression of ERalpha, ERbeta and co-regulator PELP1/MNAR in colorectal cancer: Prognostic significance and clinicopathologic correlations. Cell. Oncol. 2009, 31, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, V.; Grivas, P.; Kefalopoulou, Z.; Kalofonos, H.; Varakis, J.N.; Melachrinou, M.; Sotiropoulou-Bonikou, G. Estrogen signaling in colorectal carcinoma microenvironment: Expression of ERbeta1, AIB-1, and TIF-2 is upregulated in cancer-associated myofibroblasts and correlates with disease progression. Virchows Arch. Int. J. Pathol. 2009, 454, 389–399. [Google Scholar] [CrossRef]
- Foley, E.F.; Jazaeri, A.A.; Shupnik, M.A.; Jazaeri, O.; Rice, L.W. Selective loss of estrogen receptor β in malignant human colon. Cancer Res. 2000, 60, 245–248. [Google Scholar]
- Castiglione, F.; Taddei, A.; Degl’Innocenti, D.; Buccoliero, A.; Bechi, P.; Garbini, F.; Chiara, F.G.; Moncini, D.; Cavallina, G.; Marascio, L.; et al. Expression of Estrogen Receptor β in Colon Cancer Progression. Diagn. Mol. Pathol. 2008, 17, 231–236. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, Z.; Wang, F.; Wu, X.; Li, L.; Zhang, L.; Pan, Z.-Z.; Wan, D.-S. Prognostic impact of ERβ and MMP7 expression on overall survival in colon cancer. Tumor Biol. 2010, 31, 651–658. [Google Scholar] [CrossRef]
- Koehler, K.F.; Helguero, L.A.; Haldosén, L.; Warner, M.; Gustafsson, J. Reflections on the Discovery and Significance of Estrogen Receptor β. Endocr. Rev. 2005, 26, 465–478. [Google Scholar] [CrossRef]
- Hsu, H.; Cheng, S.; Wu, C.; Chu, C.; Weng, Y.; Lin, C.; Lee, S.; Wu, H.; Huang, C.; Kuo, W. Apoptotic Effects of Over-Expressed Estrogen Receptor-β on LoVo Colon Cancer Cell is Mediated by p53 Signalings in a Ligand-Dependent Manner. Chin. J. Physiol. 2006, 49, 110–116. [Google Scholar]
- Mahbub, A.A. Therapeutic Strategies and Potential Actions of Female Sex Steroid Hormones and Their Receptors in Colon Cancer Based on Preclinical Studies. Life 2022, 12, 605. [Google Scholar] [CrossRef]
- Filgueiras, M.d.C.; Morrot, A.; Soares, P.M.G.; Costa, M.L.; Mermelstein, C. Effects of 5-fluorouracil in nuclear and cellular morphology, proliferation, cell cycle, apoptosis, cytoskeletal and caveolar distribution in primary cultures of smooth muscle cells. PLoS ONE 2013, 8, e63177. [Google Scholar] [CrossRef]
- Borralho, P.M.; Moreira da Silva, I.B.; Aranha, M.M.; Albuquerque, C.; Nobre Leitão, C.; Steer, C.J.; Rodrigues, C.M. Inhibition of Fas expression by RNAi modulates 5-fluorouracil-induced apoptosis in HCT116 cells expressing wild-type p53. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2007, 1772, 40–47. [Google Scholar] [CrossRef][Green Version]
- Jung, Y.; Qian, Y.; Chen, X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell. Signal. 2010, 22, 1003–1012. [Google Scholar] [CrossRef]
- Aslam, A.; Ahmad, J.; Baghdadi, M.A.; Idris, S.; Almaimani, R.; Alsaegh, A.; Alhadrami, M.; Refaat, B. Chemopreventive effects of vitamin D3 and its analogue, paricalcitol, in combination with 5-fluorouracil against colorectal cancer: The role of calcium signalling molecules. Biochim. Biophys. Acta. Mol. Basis Dis. 2021, 1867, 166040. [Google Scholar] [CrossRef] [PubMed]
- Fiorelli, G.; Picariello, L.; Martineti, V.; Tonelli, F.; Brandi, M.L. Functional Estrogen Receptor β in Colon Cancer Cells. Biochem. Biophys. Res. Commun. 1999, 261, 521–527. [Google Scholar] [CrossRef]
- Marino, M.; Galluzzo, P.; Leone, S.; Acconcia, F.; Ascenzi, P. Nitric oxide impairs the 17β-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr.-Relat. Cancer 2006, 13, 559–569. [Google Scholar] [CrossRef]
- Bolli, A.; Bulzomi, P.; Galluzzo, P.; Acconcia, F.; Marino, M. Bisphenol A impairs estradiol-induced protective effects against DLD-1 colon cancer cell growth. IUBMB Life 2010, 62, 684–687. [Google Scholar] [CrossRef]
- Galluzzo, P.; Caiazza, F.; Moreno, S.; Marino, M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr.-Relat. Cancer 2007, 14, 153–167. [Google Scholar] [CrossRef]
- Arai, N.; Ström, A.; Rafter, J.J.; Gustafsson, J. Estrogen Receptor β mRNA in Colon Cancer Cells: Growth Effects of Estrogen and Genistein. Biochem. Biophys. Res. Commun. 2000, 270, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Waters, C.; Lewis, A.; Langman, M.; Eggo, M. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J. Endocrinol. 2002, 174, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ditonno, I.; Losurdo, G.; Rendina, M.; Pricci, M.; Girardi, B.; Ierardi, E.; Di Leo, A. Estrogen Receptors in Colorectal Cancer: Facts, Novelties and Perspectives. Curr. Oncol. 2021, 28, 4256–4263. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, F.; Galluzzo, P.; Lorenzetti, S.; Marino, M. 17Beta-estradiol induces ERbeta up-regulation via p38/MAPK activation in colon cancer cells. Biochem. Biophys. Res. Commun. 2007, 359, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Ström, A.; Jonsson, P.; Gustafsson, J.A.; Williams, C. Estrogen receptor β induced anti-inflammatory and antitumorogenic networks in colon cancer cells. Mol. Endocrinol. 2011, 25, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Nguyen-Vu, T.; Kalasekar, S.M.; Pontén, F.; Gustafsson, J.; Williams, C. Estrogen receptor β expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis 2013, 34, 1431–1441. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, X.; Guo, X.; Huang, S.; Wang, T.; Zhou, P.; Li, W.; Zhou, L.-F.; Hu, Y.-H. Progesterone suppresses the progression of colonic carcinoma by increasing the activity of the GADD45α/JNK/c-Jun signalling pathway. Oncol. Rep. 2021, 45, 95. [Google Scholar] [CrossRef]
- Ngabire, D.; Seong, Y.A.; Patil, M.P.; Niyonizigiye, I.; Seo, Y.B.; Kim, G.D. Induction of apoptosis and G1 phase cell cycle arrest by Aste. Int. J. Oncol. 2018, 53, 2300–2308. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen receptors alpha (ERα) and beta (ERβ): Subtype-selective ligands and clinical potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef]
- Rennert, G. Reproductive factors, hormones and colorectal cancer—Still unresolved. Br. J. Cancer 2017, 116, 1–3. [Google Scholar] [CrossRef][Green Version]
- Mohammed, H.; Russell, I.A.; Stark, R.; Rueda, O.M.; Hickey, T.E.; Tarulli, G.A.; Serandour, A.A.A.; Birrell, S.N.; Bruna, A.; Saadi, A.; et al. Progesterone receptor modulates ERα action in breast cancer. Nature 2015, 523, 313–317. [Google Scholar] [CrossRef]
| Colon Cancer Cell Lines | Monotherapies Compared to CT Only | Dual Therapy Compared to CT & Monotherapies | |
|---|---|---|---|
| E2 | 5-FU | E+F | |
| HT-29 cells | Sub-G1 phase Significant arrest vs. CT, p = 0.0007 | Sub-G1 phase Significant arrest vs. CT, p = 0.0133 S phase Significant arrest vs. CT, p < 0.0001 | Interactive effect at Sub-G1 phase Significant arrest vs. CT, p < 0.0001 Significant arrest vs. E2, p = 0.0133 Significant arrest vs. 5-FU, p = 0.0007 |
| SW480 cells | Sub-G1 phase Significant arrest vs. CT, p = 0.0063 S phase Significant arrest vs. CT, p = 0.0166 | Sub-G1 phase Significant arrest vs. CT, p = 0.0002 S phase Significant arrest vs. CT, p = 0.0001 | Interactive effect at Sub-G1 phase Significant arrest vs. CT, p < 0.0001 Significant arrest vs. E2, p < 0.0001 Significant arrest vs. 5-FU, p = 0.0063 |
| SW620 cells | Sub-G1 phase Significant arrest vs. CT, p < 0.0001 | Sub-G1 phase Significant arrest vs. CT, p = 0.0063 | Non-interactive effect at Sub-G1 phase Significant arrest vs. CT, p < 0.0001 No significant arrest vs. E2, p = 0.2017 Significant arrest vs. 5-FU, p = 0.0063 |
| Colon Cancer Cell Lines | Monotherapies Compared to CT Only | Dual Therapy Compared to CT & Monotherapies | |
|---|---|---|---|
| E2 | 5-FU | E+F | |
| HT-29 cells | Significant reduction vs. CT, p < 0.0001 | Significant reduction vs. CT, p = 0.0063 | Interactive effect on live cells Significant reduction vs. CT, p = 0.0063 Significant reduction vs. E2, p = 0.0063 Significant reduction vs. 5-FU, p < 0.0001 |
| SW480 cells | Significant reduction vs. CT, p = 0.0063 | Significant reduction vs. CT, p < 0.0001 | Non-interactive effect on live cells Significant reduction vs. CT, p < 0.0001 Significant reduction vs. E2, p = 0.0063 Significant increase vs. 5-FU, p = 0.0063 |
| SW620 cells | Significant reduction vs. CT, p < 0.0001 | Significant reduction vs. CT, p = 0.0063 | Non-interactive effect on live cells Significant reduction vs. CT, p < 0.0001 Significant increase vs. E2, p = 0.0063 Significant reduction vs. 5-FU, p = 0.0052 |
| Colon Cancer Cell Lines | Monotherapies Compared to CT Only | Dual Therapy Compared to CT & Monotherapies | |
|---|---|---|---|
| E2 | 5-FU | E+F | |
| HT-29 cells | Late apoptosis Significant induction vs. CT, p = 0.0372 | Early apoptosis Significant induction vs. CT, p = 0.0063 | Interactive effect on early apoptosis Significant induction vs. CT, p < 0.0001 Significant induction vs. E2, p < 0.0001 Significant induction vs. 5-FU, p = 0.0063 |
| SW480 cells | Early apoptosis Significant induction vs. CT, p = 0.0047 | Early apoptosis Significant induction vs. CT, p = 0.0001 Late apoptosis Significant induction vs. CT, p < 0.0001 | Interactive effect on late apoptosis Significant induction vs. CT, p < 0.0001 Significant induction vs. E2, p < 0.0001 Significant induction vs. 5-FU, p = 0.0064 |
| SW620 cells | Early apoptosis Significant induction vs. CT, p = 0.0007 Late apoptosis Significant induction vs. CT, p < 0.0001 | Early apoptosis Significant induction vs. CT, p < 0.0001 | Non-interactive effect on early apoptosis Significant induction vs. CT only, p = 0.0133 No significant induction vs. E2, p = 0.0578 Significant reduction vs. 5-FU, p = 0.0007 Non-interactive effect on late apoptosis Significant induction vs. CT, p = 0.0063 Significant induction vs. E2, p = 0.0063 Significant reduction vs. 5-FU, p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahbub, A.A. 17β-estradiol Enhances 5-Fluorouracil Anti-Cancer Activities in Colon Cancer Cell Lines. Med. Sci. 2022, 10, 62. https://doi.org/10.3390/medsci10040062
Mahbub AA. 17β-estradiol Enhances 5-Fluorouracil Anti-Cancer Activities in Colon Cancer Cell Lines. Medical Sciences. 2022; 10(4):62. https://doi.org/10.3390/medsci10040062
Chicago/Turabian StyleMahbub, Amani A. 2022. "17β-estradiol Enhances 5-Fluorouracil Anti-Cancer Activities in Colon Cancer Cell Lines" Medical Sciences 10, no. 4: 62. https://doi.org/10.3390/medsci10040062
APA StyleMahbub, A. A. (2022). 17β-estradiol Enhances 5-Fluorouracil Anti-Cancer Activities in Colon Cancer Cell Lines. Medical Sciences, 10(4), 62. https://doi.org/10.3390/medsci10040062






